Abstract
Ternary metal dichalcogenides alloys exhibit compositionally tunable optical properties and electronic structure and therefore, band gap engineering by controllable doping would provide a powerful approach to promote their physical and chemical properties. Herein we obtained ternary SnS2−xSex alloys with tunable chemical compositions and optical properties via a simple one-step solvothermal process. Raman scattering and UV-vis-NIR absorption spectra reveal the composition-related optical features and the band gaps can be discretely modulated from 2.23 to 1.29 eV with the increase of Se content. The variation tendency of band gap was also confirmed by first-principles calculations. The change of composition results in the difference of crystal structure as well as morphology for SnS2−xSex solid solution, namely, nanosheets assemblies or nanosheet. The photoelectrochemical measurements indicate that the performance of ternary SnS2−xSex alloys depends on their band structures and morphology characteristics. Furthermore, SnS2−xSex photodetectors present high photoresponsivity with a maximum of 35 mA W−1 and good light stability in a wide range of spectral response from ultraviolet to visible light, which renders them promising candidates for a variety of optoelectronic applications.
Similar content being viewed by others
Introduction
Two-dimensional (2D) layered metal dichalcogenides nanomaterials are attracting intense interest due to their fascinating properties and potential applications in optics, optoelectrinics, catalysis, energy conversion and storage, etc1,2,3,4,5,6,7,8. The dichalcogenides possess individual sandwiched X−M−X layer structure with weak out-of-plane van der Waals forces between molecular layers and strong in-plane chemical bonding within the layers. Unlike graphene with zero band gap, metal dichalcogenides 2D layered structures own sizable band gaps and exhibit strong light-matter interaction, which are promising for electronic and optoelectronic devices9. It is required to modify pristine materials to promote their physical and chemical properties. Chemical doping is a high-efficiency approach to fine-tune the structures and optical features of these layered materials. In view of the similar atomic structure of congeners, it is possible to construct a mixed alloy system (MSSe, M is metal atom) with tunable composition and continuously tuned band gap, which has been widely studied for applications in nanoelectronics and nanophotonics10,11,12,13. For example, optical band gap modulations have been reported via a broad range of Se doping of atomic thin MoS2 on SiO2 by chemical vapor deposition (CVD) method. The band gaps of ternary alloys could be finely tuned between 1.85 and 1.60 eV with the change of Se concentration14. The photoelectric properties of monolayer MoS2(1−x)Se2x devices have been studied, which largely depends on the chemical composition of ternary alloys. Compared with S-rich ones with decreased diffusion approach of photogenerated carriers, the photocurrents present significant decrease for Se-rich alloys15. Theoretical calculations have proved that mixing energy of transition metal dichalcogenides (TMDs) ternary alloys is low and mixed MoS2/MoSe2/MoTe2 compounds are thermodynamically stable at room temperature. Moreover, their compositions and band gaps could be continuously tuned between the constituent limits, indicating the benefit of band gap engineering for optoelectronic applications16.
As narrow-gap IV–VI semiconductors, tin dichalcogenides have been widely studied for electronic and optoelectronic applications17,18,19,20. Their valence bands (VB) primarily depend on the p orbital of chalcogens, while the conduction bands (CB) are hybridized orbitals generated by the interaction of p orbital of chalcogens and s orbital of tin atom21. Layered SnS2 and SnSe2 are both isostructural with typical CdI2−type structure and possess indirect band gaps of 2.18−2.44 eV and 1−2 eV, respectively19,22. Consequently, it is feasible to form a solid solution of isostructural SnS2−xSex alloys. This might be an efficient approach to the strong incorporation and homogeneous distribution of different atoms, which are favorable for the separation of photo-generated charges. The band gap engineering would offer an efficient platform for changing the electronic performance of tin dichalcogenides. For example, SnS2−xSex single crystals have been obtained via chemical vapor transport with iodine as transport agent. Increasing Se content could lead to increase of dielectric constant and the decrease of donor ionization energy and suppress the gate-modulated drain-source current in field effect transistors (FETs)21. Hadjiev et al. reported the variations of Raman phonon frequency and line-width with the change of Se content in mixed crystals SnSxSe2−x via experimental method and density functional perturbation theory (DFPT) first-principle calculations. The absence of overlapping of the corresponding phonon dispersion bands in SnS2 and SnSe2 results in the two-mode behavior (2MB) of A1g and Eg vibrations of Se (S) atoms23. The modified materials based on 2D SnS2 crystals would provide diversified strategies for electronic structure engineering and efficient device applications in electronics and optoelectronics.
Previous works about 2D MSSe are mainly focusing on CVD growth24,25,26, however, there have been only a few works on controlled synthesis of MSxSey alloys based on solution approach, which provides a efficient method for large-scale preparation and promising application. In addition, the photoelectronic properties of SnS2−xSex alloys with different chemical compositions have been rarely reported according to our knowledge. In this work, composition-tunable SnS2−xSex alloys were successfully prepared by a one-step solvothermal procedure. Upon Se doping, the variations of crystal structures and morphologies of SnS2 nanosheets were investigated. Raman scattering, UV-vis-NIR absorption spectra and first-principles calculations were carried out to reveal their composition-dependent optical properties. The photoelectrochemical performances of SnS2−xSex alloys under the irradiation of green light (λ = 550 nm) were examined. We fabricated SnS2−xSex devices to study their optoelectronic properties as photodetectors. The electrical properties of different devices were characterized. The photoresponsivity of SnS0.44Se1.56 films was found to be strongly dependent on incident light power and wavelength.
Results
We first examined the chemical compositions of SnS2−xSex alloys according to EDS analysis, as shown in Supplementary Fig. S1 and Table 1. The actual concentrations of S and Se atoms in SnS2−xSex alloys were close to the nominal concentration. Moreover, the ratios of (S + Se)/Sn in SnS2−xSex samples were close to the stoichiometry of 2. EDS elemental mapping of SnS0.82Se1.18 alloy (in Supplementary Fig. S2) clearly reveals homogenous composition distribution of Sn, S and Se elements.
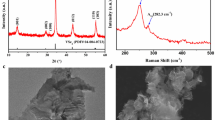
XRD analysis for SnS2−xSex alloys with different Se contents was performed to examine the change of crystal structure upon Se doping. As shown in Fig. 1, SnSe2 can be indexed with hexagonal CdI2-type unit cells (JCPDS no. 23-0602). The lattice constants of hexagonal SnS2 are a = b = 3.649 Å and c = 5.899 Å (JCPDS no. 23-0677) and the values of SnSe2 are a = b = 3.81 Å and c = 6.14 Å. As expected, the main peak positions of SnS2−xSex alloys gradually shift toward lower angles with increasing Se content (Fig. 1b), indicating the increase of lattice constants and formation of solid solution rather than the mechanical mixture of two pure phases27,28. The continuous peak shifting (lattice expanding) of ternary alloys might rule out the phase separation or separated nucleation of SnS2 or SnSe2 nanomaterials29,30. As shown in Supplementary Fig. S3 and Table 1, the change of lattice parameter a in SnS2−xSex alloys is in linear with the change of Se content. According to Végard’s Law, the variation of lattice parameters of ternary alloys would present a linear relationship with composition in the absence of strong electronic effects28. Consequently, the variation tendency in SnS2−xSex is in agreement with the Végard’s Law and demonstrates the formation of homogeneous alloy structure31. In additional, the crystallite dimensions of all the samples were calculated by Scherrer equation, which were 12.4 nm, 9.9 nm, 11.5 nm, 12.0 nm, 7.9 nm and 23.6 nm with the increase of Se contents, respectively (Supplementary Table S1).
The morphology variation of SnS2−xSex alloys with Se contents was shown in Fig. 2 and the corresponding AFM and height curves were provided in Supplementary Fig. S4. Similar to our previous work32, pure SnS2 presented typical nanosheets structure with lateral sizes of ca. 0.8−1 μm and thicknesses of ca. 22 nm. The introduction of Se element would have a large affect on the morphology of the samples. Upon Se doping, nanosheets and nanosheet assemblies are formed, the later one of which consists of building block of nanosheets. When low content of Se element was introduced (x = 0.34), small NSs structure were obtained with lateral dimensions of ca. 80−160 nm and thicknesses of ca. 10−20 nm. With the increase of Se concentration, SnS1.22Se0.78 showed nanosheet shape with lateral sizes of around 400−600 nm and thicknesses of around 20−30 nm. When the value of x was 1.18, the sample would form into stacked structure (1−2 μm) composed of numerous 2D nanosheets. Interestingly, SnS0.44Se1.56 alloy owned ultrathin nanosheets structure with diameters of ca. 1.8−2.5 μm and thicknesses of ca. 8 nm with further increase of Se content (x = 1.56). The pure SnSe2 sample showed 2D layered plates structure with large sizes of several micrometers and heights of hundreds of nanometers. As shown in Fig. 2f and Supplementary Fig. S4g, SnSe2 plates were assembled by tens of individual nanosheets and the thickness of nanosheet was determined to be around 25.4 nm. The thickness variation tendency of SnS2−xSex nanosheets was approximately consistent with the crystallite sizes derived from Scherrer equation. The TEM images and SAED patterns were provided in Supplementary Fig. S5. The nanosheets structure of SnS1.22Se0.78, SnS0.44Se1.56 and SnSe2 were in good agreement with the SEM and AFM results. Remarkably, the pure SnS232 and SnSe2 are single crystalline and own 2D layered structure with hexagonal symmetry. However, the diffraction rings of polycrystalline would appear with the introduction of Se element. The tunable composition may provide a good candidate for photodector applications. As we know, the crystal growth habits and environmental factors would play a critical role in crystallization process33. Layered SnS2 and SnSe2 are both isostructural with typical CdI2−type structure. According to our previous work32, the synthesis approach in this work would provide a favorable environmental to induce tin dichalcogenides to grow along lateral direction and expose (001) facets. Consequently, we believe the ternary SnS2−xSex alloys would prefer to grow and form 2D nanosheets structure. However, the practical growth environment may affect the self-assembling behavior. The (001) orientation is preferentially oriented for pure SnS232 and that of SnSe2 is (101) facet19. The different crystal orientation might result in synergistic effect on the crystal growth of ternary alloys. The competition phenomenon was especially obvious in SnS0.82Se1.18 nanosheets assemblies, which owned nearly equal S and Se concentrations in the initial stage of chemical reaction (in view of the incomplete dissolution of add Se). Before the solvothermal reaction, all of the reactants were dissolved in the TEG. However, it is difficult to understand the exact reaction mechanism during such a fast reaction. The detailed mechanism of nanosheets and nanosheets assemblies is still under investigation.
Raman spectra were used to examine the composition-dependent vibration modes of SnS2−xSex ternary alloys. Figure 3 presents the normalized Raman spectra of as-prepared SnS2−xSex samples with the increase of Se content. For SnS2 NSs32, only A1g(S−Sn) mode was detected at 313.4 cm−1. The absence of intra-layer Eg(S−Sn) can be ascribed to the weak rejection of Rayleigh scattered radiation or the choice principle for scattering geometry in SnS2 nanosheets18,34. In contrast, two prominent vibration peaks in SnSe2 plates assigned to A1g(Se−Sn) mode at 177.4 cm−1 and Eg(Se−Sn) mode at 99.2 cm−1 are observed. Because of the low concentration of Se in SnS1.66Se0.34 alloy, the vibration peaks of SnSe2-like modes are not strong enough to be observed. Similarly, the peaks of SnS2-like modes are also not obvious in SnS0.44Se1.56 with low S content. As shown in Fig. 3, the intensity of SnS2-like A1g mode would decrease until completely disappear, while the SnSe2-like A1g and Eg modes come into appearance and gradually enhance with increasing Se content. The peak patterns of pure material (SnS232 or SnSe2) are simple and sharp, while that of the alloys are broad and complex. In addition, all the vibration modes shift to low frequency. The transitions of molecular vibration modes exhibit strong dependence on increasing Se concentrations in the composition-dependent SnS2−xSex alloys.
X-ray photoelectron spectroscopy (XPS) was used to measure the changes of binding energy of Sn, S and Se in SnS2−xSex alloys. As shown in Supplementary Fig. S6a, the binding energies of Sn 3d5/2 at 486.4 eV and Sn 3d3/2 at 494.8 eV in SnSe2 plates were close to that of pure SnS2 NSs32 (Sn 3d5/2 at 486.2 eV and Sn 3d3/2 at 494.6 eV). Compared with SnSe2, the peaks of Sn 3d5/2 and Sn 3d3/2 in SnS0.44Se1.56 present a shift of about 0.5 eV to 486.9 and 495.3 eV. As shown in Supplementary Fig. S6b, Se 3d5/2 and Se 3d3/2 peaks locate at the same peak positions of 54.2 and 54.9 eV for SnS0.44Se1.56 and SnSe2. The binding energies of S 2p3/2 (160.5 eV) and S 2p1/2 (161.6 eV) in SnS0.44Se1.56 (Supplementary Fig. S6c) were lower than that of pure SnS2 with S 2p3/2 at 161.1 eV and S 2p1/2 at 162.5 eV32. Meanwhile, the peaks from Se 3p3/2 at 158.9 eV and Se 3p1/2 at 166.4 eV become dominant, which is in agreement with previous work14. The obvious shift of binding energies in SnS2−xSex alloy indicated the formation of solid solutions, which was attributed to the easy electron transfer in SnS2−xSex alloy because of strong combination between different atoms35.
The band gaps could be tuned by controlling the chemical compositions of SnS2−xSex alloys. Figure 4a shows the UV-vis-NIR absorption spectra of SnS2−xSex with different Se contents. The absorption edge exhibits a red-shift with the increase of Se content, indicating enhanced optical absorption property. The band gap of semiconductor could be calculated by extrapolating straight line of the plot (αhν)1/2 vs. hν based on the equation: αhν = A(hν–Eg)n/2 and the estimated data were shown in Table 1. SnS2−xSex alloys own the band gap ranging from 2.23 eV for SnS2 to 1.92, 1.81, 1.74, 1.39 and 1.29 eV with increasing Se content. The empirical relation between band gap and composition ratio has been predicted according to the extended Vegard’s Law. Figure 4b presents the composition-dependent band gaps of SnS2−xSex alloys. The solid line represents the fitted values for the band gap relation of ternary semiconductor alloys according to the generalized equation9,36,37:

where b is the optical bowing constant. In this work, y equals to x/2. The best fit yields b = 0.03, which was found to be in the range of 0 to 0.6530. And the small b value demonstrates that SnS2 and SnSe2 have a good miscibility24,38. The non-linear relationship is ascribed to the alteration of band structure caused by the volume deformation in SnS2−xSex alloys and the change of electron distribution due to the electronegativity difference of different atoms35,39.
Discussion
For further theoretical study, we employed first principles calculations to obtain the band gap structures and DOS curves, which are benefit to analyze the electronic structures of SnS2−xSex alloys and possible affecting factors. Figure 5 shows the first-principles calculations results of SnS0.44Se1.56 alloy. The corresponding band structure is shown in Fig. 5a, which clearly demonstrates SnS0.44Se1.56 is an indirect band gap semiconductor. The band gap of SnS0.44Se1.56 is calculated to be 1.421 eV, which is in good accordance with the experimental value of 1.39 eV. Additionally, the band gaps of pure SnS2 and SnSe2 were estimated to be 2.461 and 1.402 eV, respectively (Supplementary Fig. S7), which are close to the experimental values of 2.23 and 1.29 eV. The calculated results roughly reveal the variation tendency of band gaps with the increase of Se concentration in SnS2−xSex crystals, which might be ascribed to the replacement of S and Se, affecting electronic structure distribution in the alloy system37. The total and partial density of states (TDOS and PDOS) of SnS0.44Se1.56 are provided in Fig. 5b and the energy zero is defined as Fermi energy level. From Fig. 5b, we could conclude the contribution of different orbitals to VB and CB SnS0.44Se1.56 alloy. PDOS curves actually presented different tendencies in the regions close to the VB and CB. The states near VB are dominated by the S 3p and Se 4p orbitals, while CB is mainly composed of hybridized states of Sn 5s, S 3p and Se 4p orbitals. The difference of constituting orbitals in VB and CB would result in the dissimilarities of band structure in SnS2−xSex alloys with the change of S/Se ratio.
In order to explore the potential applications of SnS2−xSex alloys in optoelectronic devices, the photoelectrochemical performances were carried out to study the separation and transmission efficiency of photogenerated electrons and holes. The photocurrent densities are 2.1 μA cm−2 for SnS2, 1.6 μA cm−2 for SnS1.66Se0.34, 3.3 μA cm−2 for SnS1.22Se0.78, 4.3 μA cm−2 for SnS0.82Se1.18, 27.2 μA cm−2 for SnS0.44Se1.56 and 14.1 μA cm−2 for SnSe2, respectively (see Table 1). As shown in Supplementary Fig. S8a, the photocurrent values presented negligible variation under continued irradiation of monochromatic green light (λ = 550 nm), clearly revealing the good photostability of SnS2−xSex alloys. After the incorporation of Se element, SnS2−xSex alloys presented gradually enhanced absorption regime with increasing Se doping (Fig. 4). Correspondingly, the photocurrent values of ternary materials presented significant and regular increase. The current density of SnS0.44Se1.56 still remained at 27.2 μA cm−2 after 500 s of irradiation. As is well known, the morphology, size and spatial arrangement of materials have an important effect on their properties. From previous reports2,40, 2D configuration would endow semiconductor material a more convenient transmission approach for photogenerated electrons and holes and a much better grain boundary connectivity, which benefits to enhance electron−hole pairs transport/separation efficiency and prevents their recombination. Compared with SnS0.82Se1.18, the photocurrent of SnS0.44Se1.56 NSs presented tremendous enhancement from 4.31 μA cm−2 to 27.2 μA cm−2. Furthermore, the value is much larger than that of SnSe2 plates (14.1 μA cm−2), which owns narrower band gap than SnS0.44Se1.56 NSs. The stacked structures (SnS0.82Se1.18 and SnSe2) would provide a long approach to the surface for photogenerated electrons and holes. And before collected, inner carriers were easier to be lost because of recombination, which needed to take longer time to arrive at the surface than those generated near the surface41,42. Noteworthily, the current value of SnS2 (2.1 μA cm−2) is higher than that of SnS1.66Se0.34 (1.6 μA cm−2), which might be ascribed to the dense stacking of small nanosheets on ITO during the preparation of photoelectrode. The repeated ON/OFF switching measurements were carried out to examine the sensitivity of materials to incident light. As shown in Supplementary Fig. S9, the photoresponse currents of sheet-based materials would reach steady state with a short time, however, the stacked structures needed a longer response time. This delay phenomenon is particularly apparent for SnS0.82Se1.18. That could be attributed to the large contact area and thin thickness of 2D materials, which enable them to capture visible light efficiently and encourage electron-hole pairs to transfer fast. Accordingly, the recombination probability of photogenerated electrons and holes would be reduced to a low level. The corresponding electrochemical impedance spectra (Supplementary Fig. S8b) present analogous variation tendency to the I–t curves. Lower EIS means smaller interfacial charge–transfer resistance. SnS0.44Se1.56 NSs exhibit the lowest EIS, which greatly benefits to carrier transport efficiency in the electrode.
Tin-based chalcogenides have been widely studied as the building blocks for nanoelectronics21,43,44,45, which would provide great potentials for next-generation electronic applications. For further extending the optoelectronic applications, we measured the optoelectronic response of as-prepared SnS2−xSex in a wide range from ultraviolet to visible light. A schematic depiction of the devices structure is shown in Supplementary Fig. S10 and the results of electrical and photodetector properties are presented in Fig. 6 and Table 1. At low source-drain voltage, the IDS–VDS curves of all devices are symmetric and linear, indicating the Ohmic contacts between Au electrodes and SnS2−xSex films. The currents present significant enhancement with the increasing Se content, which might be attributed to the synergistic influence of morphology and tunable electronic structure. The SnS0.44Se1.56 film shows highest current values. Its unique 2D configuration (large dimension of 1.8−2.5 μm and thin thickness of ca. 8 nm) would provide more active sites and shorter route in electronic transfer process. Furthermore, the photoresponse measurements for SnS0.44Se1.56 alloy were carried out. Figure 6b provides IDS vs VDS curves of SnS0.44Se1.56 device without and with red light illumination (λ = 610 nm) with various power intensity. With increasing power intensity, the photocurrent distinctly increases, which could be ascribed to the increasing number of photogenerated carriers. The photoresponsivity R, defined as the ratio between photocurrent increase (ΔI) and power intensity (P), ΔI/P, as a function of illumination power is shown in the inset of Fig. 6b. It is clear that R decreases with the increase of laser power, which may be attributed to the enhanced scattering or recombination rate of hot carriers at higher laser power intensity20. The relationship between photoresponsivity versus incident light power can be fitted by a power law relationship R ∝ Pα−1 20,46,47. The fitting parameter α = 0.80 was obtained in our measurement, which is comparable to that of layered SnS2 (α = 0.77)20 and few-layer MoS2 (α = 0.71)46, indicating that the recombination kinetics of photogenerated carriers is related to both trap states and interaction of carriers47.
Electrical and photodetector properties of SnS2−xSex alloys.
(a) IDS–VDS curves for the devices. The linearity indicates excellent Ohmic contacts in the SnS2−xSex devices. (b) IDS–VDS curves for SnS0.44Se1.56 device with various illumination power P (λ = 610 nm). The inset shows the logarithmic scale plot of photoresponsivity R as a function of light power (c) The photoresponsivity R of SnS0.44Se1.56 device at different illumination wavelengths (P = 16.36 μW) with a bias voltage of 2 V (red line) and solid phase UV-vis absorption spectrum of SnS0.44Se1.56 alloy (blue line). (d) The time trace of photocurrent response for SnS0.44Se1.56 device at a bias voltage of 2 V (λ = 610 nm, P = 16.36 μW).
The spectral responsivity of SnS0.44Se1.56 device as a function of illumination wavelength is presented in Fig. 6c and Supplementary Fig. S11. Large photocurrents could be observed at high light energy (34.9 mA W−1 for photon energy Eph = 4.88 eV and 20.6 mA W−1 for Eph = 3.40 eV). Interestingly, the photoresponsivity was not continuous enhanced with the increase of excitation energy. In the visible region, the largest photocurrent of 13.3 mA W−1 was obtained at Eph = 2.03 eV (λ = 610 nm), in accordance with the absorption spectrum of SnS0.44Se1.56, where it possesses the strongest photoabsorption abilities at λ = 632 nm. The results suggest that the photocurrent is derived from the absorption of light energy and the fast generation and separation process of electron-hole pairs in SnS0.44Se1.56 layers. It deserves to be noted that the photoresponsivity in our work is higher than the reported values in previous literatures (8.8 mA W−1 for layered SnS220, 92 μA W−1 for multilayer WS2 films48, 7.5 mA W−1 for MoS249). Furthermore, we calculate the external quantum efficiency (EQE) of SnS0.44Se1.56 photodetector, which represents the number ratio of photogenerated carriers to incident photons. EQE could be estimated according to the equation: EQE = hcR/(eλ), in which h means the Planck’s constant, c is the speed of light, λ is the incident light wavelength and e means the electron charge. The EQE of our device was estimated to be 2.69% at P = 16.36 μW and λ = 610 nm, which is a little higher than that of layered SnS2 obtained by CVD growth (EQE = 2.4%)20. In addition, time-resolved photoresponse behavior of the device was carried out and shown in Fig. 6d. The device exhibits an obvious current change (~180 nA) and good stability after a long-time operation with alternated turn-on and turn-off process. The long response time might be ascribed the combination of extrinsic traps, such as adsorbates at the SnS0.44Se1.56 surface and SiO2/Si substrate, or the contact barrier of SnS0.44Se1.56 film and, as well as intrinsic factors, including defect states in the SnS0.44Se1.56 itself prepared by solution synthesis process17.
In summary, ternary SnS2−xSex alloys with tuneable composition (0 ≤ x ≤ 2) have been prepared via a simple one-step solvothermal procedure. The crystal structures and morphologies of alloys presented large difference with the change of doped Se content. The lattice constant a and optical properties are found to be composition-dependent and could be tuned by altering S/Se ratio. Their band gaps would change from 2.23 to 1.29 eV, which is in line with the extended Vegard’s Law. The first-principles calculations theoretically proved the tunability of band structure in SnS2−xSex alloys and the calculation results are in consistent with experimental values. PDOS curves indicate that the VB and CB of ternary alloys are derived from different atom orbitals. SnS0.44Se1.56 NSs exhibit outstanding photoresponse behavior under the irradiation of green light. The PEC performances of materials not only just depend on their band structures, but also their morphologies. Furthermore, the high photoresponsivity (a maximum of 35 mA W−1) in a wide range of spectra, combined with their optical stability in SnS2−xSex devices can be attractive for a variety of optoelectronic applications.
Methods
Synthesis of SnS2−xSex Alloys
All chemicals were of analytical grade and used as received without further purification. Tin (II) chloride dihydrate (SnCl2•2H2O), thioacetamide (TAA, C2H5NS), selenium dioxide (SeO2) and triethylene glycol (TEG, C6H14O4) were obtained from Sinopharm Chemical Reagent Co., Ltd. Polyvinylpyrrolidone (PVP, Mw = 55000) was purchased from Sigma-Aldrich.
SnS2−xSex nanosheets and nanosheets assemblies were prepared by solvothermal synthesis, similar to that for SnS2 nanosheets (NSs)32. SeO2 was used as Se source. For the synthesis of SnS2 NSs, 1 mmol (0.2257 g) SnCl2•2H2O, 2 mmol (0.1503 g) TAA and 0.5 g PVP were added into 30 mL of TEG. After complete dissolution through vigorous magnetic stirring at room temperature, the precursor solution was transferred into a 50 mL Teflon-lined stainless steel autoclave. The autoclave was heated at 220 °C for 12 h and then cooled down to room temperature naturally. The precipitate was centrifuged at 10, 000 rpm for 8 minutes and washed several times with deionized water and absolute ethanol, respectively. The final product was collected after dried at 60 °C overnight. SnSe2 plates were obtained by adding 2 mmol (0.2219 g) SeO2 as Se source instead of TAA. However, there would be little Se residue because of the low solubility of SeO2.
Ternary alloys were prepared by modulating the ratio of S and Se atoms with 2 mmol mixture of TAA and SeO2 powders. The initial S/Se molar ratio was set as 8:2, 6:4, 4:6 and 2:8. The obtained samples were denoted as SnS2−xSex, where x represented the molar ratio of doping Se.
Characterization
The crystal structure of samples was determined by Rigaku D/max-IIIB X-ray diffraction (XRD) (Cu Kα irradiation, λ = 1.54178 Å). Scanning electron microscope (SEM) and energy-dispersive spectroscopy (EDS) measurement were used to examine the morphology and chemical composition of as-obtained products on FEI Quanta 200F microscope. The thicknesses of SnS2−xSex nanosheets and nanosheets assemblies were measured by Bruker Dimension ICON-Pt atomic force microscopy (AFM). TEM and selected area electron diffraction (SAED) were also recorded on FEI Tecnai G2 F30 TEM. The absorption spectra were recored on PerkinElmer Lambda 950 UV/vis/NIR spectrometer. Raman spectra were measured on a LaBRAM HR800 (Jobin Yvon Horiba) Raman spectrometer with a He-Ne laser (λ = 532 nm). X-ray photoelectron spectroscopy (XPS) measurement was characterized on Thermo Fisher Scientific VG Kα Probe spectrometer.
Photoelectrochemical Measurements
Photoresponse behaviour of as-synthesized samples was carried out on a conventional three-electrode configuration with CHI 660E electrochemical workstation. A Pt wire and Ag/AgCl electrode were used as the counter and reference electrodes, respectively. 1 × 1 cm2 ITO conductive glass coated by dropping 1 mL 1.5 mg mL−1 of sample was used as work electrode. 0.5 M Na2SO4 aqueous solution (pH = 6) was used as electrolyte in all electrochemical tests. A 300W Xe lamp (CEL-HXF 300, Beijing Au-light, China, I = 20 A) with the monochromatic green light (λ = 550 nm) was employed as light source with distance of 10 cm to the photoelectrode placed in quartz cell. The current-time (I-t) curves were recorded with a bias potential of 0.5 V vs. Ag/AgCl electrode. Electrochemical impedance spectroscopy (EIS) was measured with a frequency of 100 kHz–100 mHz at a bias potential of 0.5 V vs. Ag/AgCl electrode.
Fabrication and Measurements of Photodetector Devices
The SnS2−xSex films were prepared onto the Si substrate with a 300-nm-thick SiO2 dielectric layer. Then, the Au electrodes with thickness of 120 nm were deposited by electron-beam evaporation with help of shadow mask and the channel length was about 2 mm. The electrical measurements were performed under ambient conditions using a Keithley semiconductor parameter analyzer, model 4200-SCS. The monochromatic light with different wavelengths was applied and the power intensity was calibrated by a power meter.
Additional Information
How to cite this article: Yu, J. et al. Ternary SnS2–xSex Alloys Nanosheets and Nanosheet Assemblies with Tunable Chemical Compositions and Band Gaps for Photodetector Applications. Sci. Rep. 5, 17109; doi: 10.1038/srep17109 (2015).
References
Yin, Z. et al. Preparation of MoS2–MoO3 Hybrid Nanomaterials for Light-Emitting Diodes. Angew. Chem., Int. Ed. 53, 12560–12565 (2014).
Sun, Y. et al. Freestanding Tin Disulfide Single-Layers Realizing Efficient Visible-Light Water Splitting. Angew. Chem., Int. Ed. 51, 8727–8731 (2012).
Voiry, D. et al. Enhanced Catalytic Activity in Strained Chemically Exfoliated WS2 Nanosheets for Hydrogen Evolution. Nat. Mater. 12, 850–855 (2013).
Lukowski, M. A. et al. Enhanced Hydrogen Evolution Catalysis from Chemically Exfoliated Metallic MoS2 Nanosheets. J. Am. Chem. Soc. 135, 10274–10277 (2013).
Cao, X. et al. Preparation of MoS2-Coated Three-Dimensional Graphene Networks for High-Performance Anode Material in Lithium-Ion Batteries. Small 9, 3433–3438 (2013).
Yang, S. et al. High-Performance Few-layer Mo-doped ReSe2 Nanosheet Photodetectors. Sci. Rep. 4, 5442 (2014).
Perea-López, N. et al. Photosensor Device Based on Few-Layered WS2 Films. Adv. Funct. Mater. 23, 5511–5517 (2013).
Zhang, Y. et al. Few-Layered SnS2 on Few-Layered Reduced Graphene Oxide as Na-Ion Battery Anode with Ultralong Cycle Life and Superior Rate Capability. Adv. Funct. Mater. 25, 481–489 (2015).
Li, H. et al. Growth of Alloy MoS2xSe2(1–x) Nanosheets with Fully Tunable Chemical Compositions and Optical Properties. J. Am. Chem. Soc. 136, 3756–3759 (2014).
Keene, J. D., McBride, J. R., Orfield, N. J. & Rosenthal, S. J. Elimination of Hole–Surface Overlap in Graded CdSxSe1–x Nanocrystals Revealed by Ultrafast Fluorescence Upconversion Spectroscopy. ACS Nano 8, 10665–10673 (2014).
Feng, Q. et al. Growth of Large-Area 2D MoS2(1-x)Se2x Semiconductor Alloys. Adv. Mater. 26, 2648–2653 (2014).
Kiran, V., Mukherjee, D., Jenjeti, R. N. & Sampath, S. Active Guests in the MoS2/MoSe2 Host Lattice: Efficient Hydrogen Evolution Using Few-Layer Alloys of MoS2(1− x)Se2x . Nanoscale 6, 12856–12863 (2014).
Xu, J., Yang, X., Yang, Q., Zhang, W. & Lee, C.-S. Phase Conversion from Hexagonal CuSySe1–y to Cubic Cu2–xSySe1–y: Composition Variation, Morphology Evolution, Optical Tuning and Solar Cell Applications. ACS Appl. Mater. Interfaces 6, 16352–16359 (2014).
Gong, Y. et al. Band Gap Engineering and Layer-by-Layer Mapping of Selenium-Doped Molybdenum Disulfide. Nano Lett. 14, 442–449 (2014).
Klee, V. et al. Superlinear Composition-Dependent Photocurrent in CVD-Grown Monolayer MoS2(1–x)Se2x Alloy Devices. Nano Lett. 15, 2612–2619 (2015).
Komsa, H.-P. & Krasheninnikov, A. V. Two-Dimensional Transition Metal Dichalcogenide Alloys: Stability and Electronic Properties. J. Phys. Chem. Lett. 3, 3652–3656 (2012).
Huang, Y. et al. Tin Disulfide-An Emerging Layered Metal Dichalcogenide Semiconductor: Materials Properties and Device Characteristics. ACS Nano 8, 10743–10755 (2014).
Song, H. S. et al. High-Performance Top-Gated Monolayer SnS2 Field-Effect Transistors and Their Integrated Logic Circuits. Nanoscale 5, 9666–9670 (2013).
Fang, Z. et al. The Enhanced Photoelectrochemical Response of SnSe2 Nanosheets. Cryst. Eng. Comm. 16, 2404–2410 (2014).
Su, G. et al. Chemical Vapor Deposition of Thin Crystals of Layered Semiconductor SnS2 for Fast Photodetection Application. Nano Lett. 15, 506–513 (2015).
Pan, T. et al. Field Effect Transistors with Layered Two-Dimensional SnS2−xSex Conduction Channels: Effects of Selenium Substitution. Appl. Phys. Lett. 103, 093108 (2013).
Williams, R. H., Murray, R. B., Govan, D. W., Thomas, J. M. & Evans, E. L. Band Structure and Photoemission Studies of SnS2 and SnSe2. I. Experimental. J. Phys. C 6, 3631 (1973).
Hadjiev, V., De, D., Peng, H., Manongdo, J. & Guloy, A. Phonon Probe of Local Strains in SnSxSe2−x Mixed Crystals. Phys. Rev. B 87, 104302 (2013).
Chuo, H., Wang, T. & Zhang, W. Optical Properties of ZnSxSe(1-x) Alloy Nanostructures and Their Photodetectors. J. Alloy.Compd. 606, 231–235 (2014).
Wang, Z. et al. ZnO/ZnSxSe1−x Core/Shell Nanowire Arrays as Photoelectrodes with Efficient Visible Light Absorption. Appl. Phys. Lett. 101, 073105 (2012).
Ma, Q. et al. Postgrowth Tuning of the Bandgap of Single-Layer Molybdenum Disulfide Films by Sulfur/Selenium Exchange. ACS Nano 8, 4672–4677 (2014).
Garaje, S. N. et al. Template-Free Synthesis of Nanostructured CdxZn1–xS with Tunable Band Structure for H2 Production and Organic Dye Degradation Using Solar Light. Environ. Sci. Technol. 47, 6664–6672 (2013).
Xu, J. et al. Synthesis of Homogeneously Alloyed Cu2−x(SySe1−y) Nanowire Bundles with Tunable Compositions and Bandgaps. Adv. Funct. Mater. 20, 4190–4195 (2010).
Zhong, X., Feng, Y., Knoll, W. & Han, M. Alloyed ZnxCd1-xS Nanocrystals with Highly Narrow Luminescence Spectral Width. J. Am. Chem. Soc. 125, 13559–13563 (2003).
Wang, M., Fei, G. T., Zhang, Y. G., Kong, M. G. & Zhang, L. Tunable and Predetermined Bandgap Emissions in Alloyed ZnSxSe1–x Nanowires. Adv. Mater. 19, 4491–4494 (2007).
Vegard, L. & Schjelderup, H. Constitution of Mixed Crystals. Physik. Z 18, 93–96 (1917).
Yu, J. et al. Monodisperse SnS2 Nanosheets for High-Performance Photocatalytic Hydrogen Generation. ACS Appl. Mater. Interfaces 6, 22370–22377 (2014).
Ma, J. et al. Controlled Synthesis of One-Dimensional Sb2Se3 Nanostructures and Their Electrochemical Properties. J. Phys. Chem. C 113, 13588–13592 (2009).
Lokhande, C. A Chemical Method for Tin Disulphide Thin Film Deposition. J. Phys. D: Appl. Phys 23, 1703–1705 (1990).
Zhong, J. et al. Supercritical Solvothermal Preparation of A ZnxCd1−xS Visible Photocatalyst with Enhanced Activity. J. Mater. Chem. A 2, 19641–19647 (2014).
Swafford, L. A. et al. Homogeneously Alloyed CdSxSe1-x Nanocrystals: Synthesis, Characterization and Composition/Size-Dependent Band Gap. J. Am. Chem. Soc. 128, 12299–12306 (2006).
Wei, H. et al. Novel SnSxSe1-x Nanocrystals with Tunable Band Gap: Experimental and First-Principles Calculations. J. Mater. Chem. 21, 12605–12608 (2011).
Yoon, Y.-J. et al. Synthesis of ZnxCd1-xSe (0 ≤ x ≤ 1) Alloyed Nanowires for Variable-Wavelength Photodetectors. J. Mater. Chem. 20, 2386–2390 (2010).
Wei, S.-H., Zhang, S. B. & Zunger, A. First-Principles Calculation of Band Offsets, Optical Bowings and Defects in CdS, CdSe, CdTe and Their Alloys. J. Appl. Phys. 87, 1304–1311 (2000).
Sun, Y. et al. Highly Depressed Temperature-Induced Metal-Insulator Transition in Synthetic Monodisperse 10-nm V2O3 Pseudocubes Enclosed by {012} Facets. Nanoscale 3, 2609–2614 (2011).
Tang, J., Zou, Z. & Ye, J. Effects of Substituting Sr2+ and Ba2+ for Ca2+ on the Structural Properties and Photocatalytic Behaviors of CaIn2O4 . Chem. Mater. 16, 1644–1649 (2004).
Bickley, R. I., González-Carreño, T., Palmisano, L., Tilley, R. J. D. & Williams, J. M. Relative Proportions of Rutile and Pseudo-Brookite Phases in the Fe(III)-TiO2 System at Elevated Temperature. Mater. Chem. Phys. 51, 47–53 (1997).
De, D. et al. High on/off ratio field effect transistors based on exfoliated crystalline SnS2 nano-membranes. Nanotechnology 24, 025202 (2013).
Mitzi, D. B., Kosbar, L. L., Murray, C. E., Copel, M. & Afzali, A. High-mobility ultrathin semiconducting films prepared by spin coating. Nature 428, 299–303 (2004).
Huang, L., Yu, Y., Li, C. & Cao, L. Substrate Mediation in Vapor Deposition Growth of Layered Chalcogenide Nanoplates: A Case Study of SnSe2 . J. Phys. Chem. C 117, 6469–6475 (2013).
Tsai, D. S. et al. Few-Layer MoS2 with High Broadband Photogain and Fast Optical Switching for Use in Harsh Environments. ACS Nano 7, 3905–3911 (2013).
Buscema, M. et al. Fast and Broadband Photoresponse of Few-Layer Black Phosphorus Field-Effect Transistors. Nano Lett. 14, 3347–3352 (2014).
Perea-López, N. et al. Photosensor Device Based on Few-Layered WS2 Films. Adv. Funct. Mater. 23, 5511–5517 (2013).
Yin, Z. et al. Single-layer MoS2 phototransistors. ACS Nano 6, 74–80 (2011).
Acknowledgements
This work was supported by National Basic Research Program of China (2012CB934102) and Fundamental Research Funds for the Central Universities (Grant No. HIT.BRETIII.201203).
Author information
Authors and Affiliations
Contributions
J.Y., C.X., P.H and L.Z. conceived and designed the experiments. J.Y. and Y.L. performed the experiments. F.Z. contributed to calculations and X. C. measured the photodetector properties. All authors discussed the results and J.Y., C.X. and Y.L. contributed to the manuscript preparation. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Yu, J., Xu, CY., Li, Y. et al. Ternary SnS2–xSex Alloys Nanosheets and Nanosheet Assemblies with Tunable Chemical Compositions and Band Gaps for Photodetector Applications. Sci Rep 5, 17109 (2015). https://doi.org/10.1038/srep17109
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep17109
This article is cited by
-
Large-area epitaxial growth of 2D ZrS2(1−x)Se2x semiconductor alloys with fully tunable compositions and bandgaps for optoelectronics
Science China Materials (2023)
-
Optothermal and electrical properties of ultrathin alloys of mixed dichalcogenides
Journal of Materials Science (2022)
-
Stacked 2D nanoflake-structured thin films of chalcogenide SnSxSe(y−x) grown by spray pyrolysis: structural, optical and electrical properties
Journal of Materials Science: Materials in Electronics (2020)
-
Locally Gated SnS2/hBN Thin Film Transistors with a Broadband Photoresponse
Scientific Reports (2018)
-
Selective oxidation mediated synthesis of unique SexTey nanotubes, their assembled thin films and photoconductivity
Nano Research (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.