Abstract
Heart failure with preserved ejection fraction (HFPEF) is characterized by myocardial interstitial fibrosis. A total of 146 patients with HFPEF, were recruited. HFPEF severity was determined using Doppler imaging (E/Em) and also cardiac magnetic resonance imaging (CMRI). Canine modeling of HFPEF was induced by aortic banding. Hemodynamic and echocardiographic data were obtained before and after pressure loading and myocardial Galectin-3 was determined. Mechanical stretch of cultured cardiomyocytes served as the cellular model of HFPEF. Patients with severe HFPEF had significantly higher plasma Galectin-3 levels. Significant correlation between plasma Galectin-3 and E/Em in advanced HFPEF patients was noted. After 2 weeks of pressure overload in canine models, the protein expression of Galectin-3 from LV myocardial tissue was significantly increased (p < 0.01) compared with controls. Galectin-3 expression paralleled the severity of LV diastolic dysfunction by evaluation of CMRI (r = −0.58, p = 0.003) and tissue fibrosis (r = 0.59, p = 0.002). After adjusting for confounders for diastolic dysfunction, Galectin-3 levels were still associated with diastolic parameters both in humans (p < 0.001) and canine model (p = 0.041). Mechanical stretch increased Galectin-3 secretion in cultured cardiomyocytes. Both plasma and myocardial Galectin-3 levels correlated with severity of cardiac diastolic dysfunction.
Similar content being viewed by others
Introduction
Heart failure with preserved ejection fraction (HFPEF) has become an increasing concern in recent years. Studies suggest that at least one-third of patients with congestive heart failure actually have HFPEF1.
The symptoms and morbidity associated with HFPEF are similar to systolic heart failure2. In order to differentiate HFPEF from systolic heart failure, a new consensus was proposed3. Although N-terminal of the prohormone brain natriuretic peptide (NT-proBNP) was incorporated in the consensus as a diagnostic cytokine, its level fluctuated in HFPEF patients and often fell below the cut-off value when applied to large cohorts4. Brouwers et al. evaluated 8592 patients and concluded that risk factors such as age, history of atrial fibrillation or even cystatin C level were even better associated with the risk of HEPEF than NT-proBNP5 Another study also found that NT-proBNP was much lower in HFPEF group than in the systolic HF group6. Recently, Edelmann, et al. also showed that plasma Galectin-3 level are elevated in patients with stable HFpEF and relate to functional performance and quality of life but not NT-proBNP7.
Several new biomarkers, including Galectin-3, have been used for the diagnosis of HFPEF in recent years8. Galectin-3 is secreted by activated macrophages and modulates several physiological and pathological processes that are associated with the development of HFPEF, including inflammation and fibrosis9. Up-regulation of myocardial Galectin-3 has been demonstrated in animal models of pressure overload which are prone to HFPEF and a recent study also suggested that plasma Galectin-3 levels correlated with echocardiographic diastolic function parameters10. De Boer et al. followed a cohort of heart failure (HF) patients and compared the value of Galectin-3 in predicting the different types of HF. The authors concluded that Galectin-3 was an independent marker for outcome in HF patients and appeared to be particularly useful in HFPEF patients11.
Although the regulation of Galectin-3 has been extensively studied, the expression of Galectin-3, as it relates to hemodynamic changes, has not yet been described. In addition, the role of Galectin-3 in the diagnosis and differentiation of HFPEF has not been determined. We developed a canine model of HFPEF induced by aortic banding to compare hemodynamic changes with cardiac Galectin-3 expression. We also tested whether cardiomyocytes secrete Galectin-3 after stretch stimulation using an in vitro cellular model. In order to further elucidate the role of Galectin-3 in HFPEF patients, patients from the Taiwan Diastolic Heart Failure Registry (TDHFR) were enrolled and their plasma Galectin-3 levels were correlated with echocardiographic DHF severity. We hypothesized that both plasma and myocardial Galectin-3 levels would correlate with severity of DHF. Because CMRI (cardiac magnetic resonance imaging) T1 mapping along with ECV (extracellular volume fraction) quantification are promising modalities for noninvasive evaluation of diffuse myocardial fibrosis, we assessed the correlation between ECV and plasma Galectin-3 levels in current study.
Materials and Methods
Human model of diastolic heart failure
Taiwan Diastolic Heart Failure Registry
This study was performed in accordance with the Declaration of Helsinki and was approved by the institutional review board of the National Taiwan University Hospital (NTUH-REC No. 20070313R) and all subjects provided their written informed consent prior to participation in the study. The study group consisted of heart failure patients admitted to the cardiovascular ward of National Taiwan University Hospital from July 2007 to March 2011. Patients with the diagnosis of DHF (as defined in previous reports as well as by the recent consensus statement of the European Society of Cardiology) were enrolled in TDHFR12,13,14. In brief, diastolic heart failure (DHF) was defined as: (1) heart failure on the basis of Framingham criteria and normal systolic function (ejection fraction ≥50%); and (2) echocardiographic evidence of left ventricular diastolic dysfunction.
Finally, 146 patients with DHF (56 men and 90 women) were included in the current study. All participants received echocardiographic examinations as well as blood sampling for the estimation of plasma NT-proBNP and Galectin-3 levels. Using tissue Doppler imaging results and the recommendations of American society of echocardiography15, patients were divided into a severe DHF group (E/Em ≥ 15) vs. mild DHF group (15 > E/Em ≥ 8) (Table 1). We also describe the distribution of mild and severe DHF of our current population by other criteria in supplemental Table 1. The detailed inclusion and exclusion criteria are listed in the online data supplement in Supplemental Text 1.
Measurements of plasma Galectin-3 levels
All blood samples were collected with each patient after 12 h of fasting. Plasma Galectin-3 was measured with high-sensitivity enzyme-linked immunosorbent assay (ELISA)(catalog no. DGAL30, R&D Systems, Inc 614 McKinley Place NE Minneapolis, MN 55413, USA). Further detailed methods are provided in Supplemental Text 1.
Myocardial fibrosis assessed by cardiac magnetic resonance contrast-enhanced T1 mapping
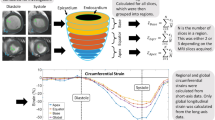
We performed CMRI on 35 randomly selected subjects (25 patients with DHF, 10 control patients), using a clinical 3.0-T CMRI scanner (Trio, Siemens, Erlangen, Germany), as described previously16. The ECV was calculated and each ECV value was averaged over five short-axis slices for each subject (Figure 1). Further detailed methods are provided in Supplemental Text 1. Figure 1 shows the ECV quantification of a DHF patient with diffuse myocardial fibrosis.
Image Analysis for Left Ventricular Systolic Function and Diastolic Function
LV diastolic and systolic function were calculated according to previous study17. Briefly, endocardial and epicardial contours of the LV were determined at each slice level on cine MRI and the area enclosed by each contour was computed. From the interpolated curve of dV/dt, systolic and diastolic functional indices were determined at the minimal and maximal values as peak ejection rate (PER) and peak filling rate (PFR), respectively. A representative description for the PER and PFR is shown in supplemental Figure 3. Image analysis was performed using software developed in-house provided by Matlab 7.9 (Mathworks, Inc., Natick, MA, USA). Further detailed methods are provided in Supplemental Text 1.
In vivo animal model of diastolic dysfunction
Canine model of LV diastolic dysfunction
Use of animals adhered to the NIH guidelines for the care and use of laboratory animals; protocols were approved by the Institutional Animal Care and Use Committee. Twelve dogs between 1 and 2 years of age, of either sex, were used in the experiments. Nine dogs were assigned to the aortic banding group, while the others comprised the sham-operated control group. The baseline body weight of each dog was measured and the dogs were anesthetized with 0.15 mL/kg fentanyl-droperidol, intubated and ventilated with nitrous oxide and oxygen (1:3 ratio) before surgery. Anesthesia was maintained by sufentanyl (0.15 mg/kg·min) and 1% isoflurane.
Thoracic aortic banding was performed, as previously described, to induce LV diastolic dysfunction due to chronic pressure overload18. Further detailed methods are provided in Supplemental Text 1.
Galectin-3 protein expression in the canine model
Cardiac tissue samples were homogenized in 50 mmol/L HEPES (pH 7.5), 150 mmol/L NaCl, 5 mmol/L EDTA and protease inhibitors. Cell debris was removed by centrifugation for 2 min at 12,000 g and protein concentration was determined with the Bradford reagent (Bio-Rad Laboratories, Hercules, CA, USA). Extracts were normalized to equal protein amounts and separated by SDS-PAGE. Galectin-3 protein concentrations were determined by western blot analysis with a GADPH control.At the beginning and end of the protocol, a complete echocardiographic study, including transthoracic echocardiography, was performed under anesthesia (Sonos 7500; Hewlett-Packard, Andover, MA, USA). Further detailed methods are provided in Supplemental Text 1.
In vitro cellular model of pressure overload
Cell culture and in vitro stretch
HL-1 myocytes were cultured in Claycomb medium (JRH Bioscience, Lenexa, KS, USA) supplemented with 10% fetal bovine serum and maintained in a humid 10% CO2 incubator at 37 °C, as previously described12. In vitro mechanical stretch of cultured HL-1 myocytes was performed, as previously described18. HL-1 cardiomyocytes were then seeded (3 × 106 cells/well) onto 6-well collagen I-coated Bioflex plates (Flexcell International Corp, Hillsborough, NC) and cyclically strained via vacuum to 20% of elongation at a frequency of 1 Hz for 2, 6, or 24 hours. Further detailed methods are provided in Supplemental Text 1.
Galectin-3 concentration detection
Conditioned medium samples obtained from 12 strained samples were collected at the indicated times and frozen at −80 °C until assayed. Galectin-3 assessment was performed using ELISA (USCN Life Science & Technology Company).
Statistical analysis
Data were analyzed using SPSS 15.0 software (SPSS Inc., Chicago, IL, USA). Continuous variables were represented as mean values ± standard deviation, while categorical variables were represented as frequencies. The association between categorical variables was tested by Pearson’s χ2 test. To test for normal distribution, the Kolmogorov-Smirnov test was applied. Comparisons between data showing normal distribution were performed using the Student’s t-test, or otherwise, by the Mann-Whitney U-test. The associations between cytokines and Doppler parameters or cytokines and CMRI diffuse fibrosis value were studied using Pearson’s correlation coefficient if the data met the criteria for normal distribution, or otherwise, by Spearman’s correlation test. Multiple linear regression modeling was applied followed by a forward stepwise analysis method to determine the factors associated with echocardiographic E/Em levels, including baseline, echocardiographic and laboratory parameters. Receiver operating characteristic (ROC) curves were used to assess the discriminative power of Galectin-3, NT-proBNP and LV mass/fibrosis for severe DHF. The area under the curve (AUC) was calculated. The optimal cutoff point for Galectin-3, defined as that with the minimum value of (1-sensitivity)2 + (1-specificity)2, or the shortest distance from the left upper corner to the ROC curve, was reported. Integrated discrimination improvement (IDI) of Galectin-3 for severe DHF was calculated by PredictABEL, R version 3.2.2 package (Netherlands) as previously reported19. A value of p < 0.05 was considered statistically significant for all tests.
Results
Human model of diastolic heart failure
Baseline characteristics of DHF patients
The baseline characteristics of mild vs. severe DHF patients are shown in Table 1. The baseline characteristics of both groups were comparable except for the type of medication administered. There were no significant differences in LV systolic function or left ventricular diameters between the two groups. Patients with severe DHF had larger sized atriums, longer mitral flow deceleration times and larger E/Em ratios.
Plasma levels of Galectin-3 in DHF patients
Plasma Galectin-3 levels were significantly higher in severe DHF patients compared with mild DHF patients (severe DHF, 19.4 ± 12.4 ng/mL; mild DHF, 6.8 ± 5.3 ng/mL; p < .001) (Table 1). Galectin-3 levels were significantly associated with DT and E/Em levels in the whole DHF group (r = 0.30, p = 0.001 and r = 0.65, P < 0.001, respectively) (Table 2) (Fig. 2A).
Human model of diastolic heart failure (DHF).
(A) Correlations between plasma Galectin-3 levels with echocardiographic diastolic parameter (E/e’) in all DHF patients (B) Plasma Galectin-3 levels progressively increase concurrently with the ECV in the DHF groups, indicating good correlation between plasma Galectin-3 and severity of myocardial fibrosis. (C) Correlations between plasma Galectin-3 levels with peak ejection rate (PER) and (D) peak filling rate (PFR) in DHF patients.
Linear regression analysis was then performed and all the factors that might influence echocardiographic E/Em levels were incorporated. After correcting for LV mass index, diabetes, age, gender, plasma NT-proBNP and prescribed drugs (ACE inhibitor or diuretics), plasma Galectin-3 levels still significantly correlated with E/Em levels (B = 0.195, p < 0.001; Table 3). We also did the ROC analyses for the development of mild or severe DHF. The area under curve value is 0.87, 0.64 and 0.82 for Galectin-3, NT-proBNP and LV mass/fibrosis, respectively (supplemental Fig. 2A–C). The addition of Galectin-3 to traditional risk factors resulted a significant IDI (10.8% [CI, 3.4% to 18.1%]; P = 0.003). The best cut point of Galectin-3 for mild/severe DHF was 10.68 ng/ml with high sensitivity (0.80) and moderate specificity (0.74). To focus on HFPEF patients with overload-derived diastolic dysfunction¸ we excluded DHF patients with additional diagnosis of coronary heart disease (n = 28) and repeated correlation and linear regression analysis. The results were similar to the entire cohort which revealed that Galectin-3 levels were significantly associated with E/Em (r = 0.549, p < 0.001). Plasma Galectin-3 levels still significantly correlated with E/Em levels after adjustment for confounding factors. (B = 0.239, p < 0.001).
Relationship of myocardium fibrosis to plasma Galectin-3 level
Plasma Galectin-3 levels progressively increased concurrently with the ECV in the DHF groups (Fig. 2B) (r = 0.59, p = 0.002), indicating good correlation between plasma Galectin-3 levels and the severity of myocardial fibrosis. We performed correlation analysis between plasma Galectin-3 and LV functional index. Galectin-3 level was significantly correlated with PER (r = 0.59, p = 0.003) and PFR (r = −0.58, p = 0.003) (Fig. 2C,D).
Animal model of LV diastolic dysfunction
Myocardial Galectin-3 expression was elevated in aortic banding group
After 2 weeks of aortic banding, the aortic blood pressure increased significantly after the aortic banding (Fig. 3A). The echocardiographic parameter for diastolic function (E/e′) was approximately 1.5-fold higher compared with controls, indicating that the aortic banding induced LV diastolic dysfunction (Fig. 3B). To test whether local Galectin-3 expression was an early and reliable marker for the severity of LV diastolic dysfunction (as observed in the clinical study), the myocardial expression of Galectin-3 was measured. After aortic banding, the tissue Galectin-3 increased significantly after 2 weeks (Fig. 3D). As in the severe DHF patients, tissue Galectin-3 correlated with the echocardiographic diastolic parameter, E/Em. After adjusting for LV mass, aortic pressure and LV ejection fraction by multiple linear regression method, tissue Galectin-3 still significantly correlated with echocardiographic E/Em ratios (B = 7.6, p = 0.041; Table 4).
In vivo animal model of diastolic dysfunction.
(A) After 2 weeks of aortic banding, the aortic blood pressure increases significantly. (B) The echocardiographic parameter for diastolic function (E/e′) is approximately 1.5-fold higher compared with controls, indicating that aortic banding induced LV diastolic dysfunction. (C) Calculated LV mass also increases significantly. (D) After 2 weeks of aortic banding, the tissue Galectin-3 also increases significantly. Cropped western blots were compared between controls and aortic banding animals for Galectin-3 protein concentrations. All the gels have been run under the same experimental conditions. Full-length blots are included in Supplemental Figure 1.
Cell model of LV pressure overload
Cellular Galectin-3 expression after mechanical stretch
In vitro mechanical stretch of cardiomyocytes was used to mimic the in vivo myocardial pressure overload involved in the pathogenesis of diastolic dysfunction. Compared with control cardiomyocytes, stretched cardiomyocytes exhibited a significant increase in Galectin-3 secretion into the culture medium. Further analysis showed a 32% increase in Galectin-3 secretion after 6 h of stretch when compared with control cardiomyocytes (p = 0.02; Fig. 4).
Discussion
Based on CMRI results, the level of plasma Galectin-3 correlated with the severity of LV diastolic dysfunction as well as the severity of cardiac fibrosis. In addition, plasma Galectin-3 was significantly associated with echocardiographic parameters for diastolic dysfunction, especially in advanced HFPEF patients. Accordingly, in the animal model, we also showed that cardiac Galectin-3 increased significantly as early as 2 weeks after pressure overload. Moreover, the expression of Galectin-3 paralleled the severity of LV diastolic dysfunction. In the cellular model, cardiomyocytes produced and secreted more Galectin-3 after mechanical stretch (mimicking pressure overload stimulation). These results implied that Galectin-3 level may directly reflect changes in LV diastolic function or cardiac fibrosis and may serve as a sensitive marker to monitor the effect of treatment.
In the present study, the level of Galectin-3 increased after myocardial stretch. In our previous study, myocardial stretch was also associated with upregulation of connective tissue growth factor (CTGF) which may be the downstream messenger of Galecin-320. CTGF could be an important intermediary for sensing stimulation and promoting further fibrosis cascades which in turn lead to the development of HFPEF. In addition, Galectin-3 plays an important role in the inflammatory response, which is important in cardiac remodeling21. Galectin-3 has also been convincingly linked to cardiac fibrosis and damage22. Systemic inflammation may cause cardiac diastolic dysfunction by decreasing diastolic calcium re-uptake through downregulation of Sarcoplasmic reticulum Ca2+-ATPase (SERCA) gene expression12. SERCA is one of the most extensively studied calcium channels with respect to diastolic dysfunction. Decreased activity of the SERCA pump slows the removal of calcium from the cytosol, which impairs the diastolic relaxation of contractile proteins17. It is logical to speculate that through augmentation of the inflammatory process, Galectin-3 may lead to down-regulation of SERCA and cardiac diastolic dysfunction.
Previous studies have shown that the change of myocardial expression of both Galectin-3 mRNA and protein paralleled the change of plasma Galectin-3 level23,24. However, The source of increasing cardiac Galectin-3 in various cardiovascular diseases remains unclear. In the present study, we measured Galectin-3 concentration in pure cardiomyocytes (cell model), intact heart tissue (animal model) and plasma (human study). We found that Galectin-3 increased significantly even after only a slight increase in blood pressure (animal model) or after short-term stretching (cell model). These results strengthen the hypothesis that cardiomyocytes could respond to the stimulation of elevated blood pressure or mechanical load change by secreting Galetin-3, which in turn could lead to paracrine responses, e.g., up-regulation of fibrosis or inflammation. In addition, the results of our human study were also consistent with our animal and cellular models in that the concentration of Galectin-3 was significantly higher in severe HFPEF patients compared with mild HFPEF patients. After adjusting for the influence of plasma pro-BNP level, Galectin-3 remained an independent factor for cardiac diastolic dysfunction. The correlation between cardiac diastolic function indicators was stronger for Galectin-3, compared with pro-BNP, in our population. Therefore, Galectin-3 may be useful for early detection, phenotyping, risk stratification and therapeutic targeting of individuals with early or established HFPEF11.
Several studies have addressed the prognostic value of Galectin-3 in patients with either systolic heart failure or HFPEF. One recent study measured plasma Galecin-3 levels repeatedly at baseline and after serial follow-up in two large cohorts of patients with either chronic or acute decompensated heart failure. The authors concluded that Galectin-3 level provided important and significant prognostic value in patients with chronic or acute decompensated heart failure25. We found that Galectin-3 levels not only reflect the severity of cardiac diastolic dysfunction and fibrosis (in our human study) but are also sensitive to shearing force or pressure changes. Therefore, modern therapies for HFPEF that target only downstream factors (e.g., fibrosis and renin-angiotensin aldosterone systems) may not be able to decrease plasma Galectin-3 levels. Targeting Galectin-3 may be an upstream therapeutic option for the treatment of all types of heart failure. There is still much uncertainty regarding the development of a therapy which can target Galectin-3 directly. First, we do not know how Galectin-3 is regulated at the transcriptional and translational levels in the heart. Previous mechanistic studies performed on cardiac fibroblasts and macrophages have shown that the TGF-β/Smad pathway was involved26. Although inflammatory signals also contribute to the regulation of Galectin-3, the signals or cytokines which govern the production and secretion of Galectin-3 remain enigmatic, warranting future explorative pharmacological studies. The etiology of HFPEF are multiple and complex. In our current studies, we tried to prove one of the possible mechanisms (our central hypothesis) that myocardium could secret Galectin-3 under the stimulation of myocardial stretch (or pressure overload) and the secreted Galectin-3 (in plasma or within myocardium) may in turn trigger myocardial fibrosis, which results in diastolic dysfunction or HFPEF (see Fig. 5 for the schematic presentation of our central hypothesis). To prove this hypothesis, single human studies might not be adequate and supplementary animal model is mandatory. We therefore adopted an aortic-banding canine model with aortic banding that has been a well-documented myocardial pressure-overload model. Finally, although we had proven that myocardial Galectin-3 was increased after pressure overload, we did not know the source of increased Galectin-3 (fibroblasts or cardiomyocytes). Furthermore, the response to aortic band may be diverse in different animals. Therefore, we further used a pure cellular model to prove that single stretch per se could stimulate the cultured cardiomycytes to secret Gaelectin-3.
Schematic representations for the etiology of diastolic heart failure (DHF) and the multi-face influences of Galectin-3 towards the disease.
HFPEF, heart failure with preserved ejection fraction; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease; CAD, coronary artery disease; DM, diabetes mellitus; MRI, magnetic resonance imaging.
Our study had several limitations. We did not directly measure the tissue Galectin-3 expression in patients with HFPEF. Theoretically, this is not feasible in human studies. In addition, our animal model only demonstrated pressure overload-induced LV diastolic dysfunction. There are other mechanisms of LV diastolic dysfunction, such as myocardial ischemia. The role of Galecin-3 in ischemia-induced or other causes of LV diastolic dysfunction is less studied. We did not measure plasma Galecin-3 levels in our canine model and we did not perform CMRI in all recruited patients. In addition, there are more structural, functional and molecular biological evidence supports that DHF is a distinct syndrome. The relative wall thickness and LV mass index seemed showed a trend of increase in patients with severe DHF. We recognized from numerous previous studies that DHF is a distinct form of HF other than systolic HF. Structurally, most patients with systolic HF have eccentric LV hypertrophy, whereas those with DHF have concentric LV hypertrophy27. In our current study, the relative wall thickness and the severity of concentric hypertrophy increased as the disease progressed. The concentric LV hypertrophy or concentric remodeling associated with DHF is frequently caused by chronic pressure overload (e.g. hypertension). In addition to abnormal relaxation due to increased LV stiffness, concentric LV remodeling may activate the calcineurin/nuclear factor pathways of activated T cells (NFAT) pathway and result in the increased expression of fetal β-myosin heavy chain and downregulation of sarcoplasmic reticulum28. Therefore, the severity of LV remodeling is also a factor to determine the severity of DHF in our patients. In patients with diabetes mellitus, an increasing rate of diastolic dysfunction was noted which could be explained by the infiltration of advanced glycation end products29. Higher BMI, or visceral adipose tissue amount could also lead to subclinical LV diastolic dysfunction by low-grade inflammation or even accumulation of epicardial fat30,31. We did not control renal function in our current study. However, chronic kidney disease is associated with fluid overload, higher prevalence of hypertension and LV hypertrophy which contribute to formation of LV diastolic dysfunction32. Finally, pulmonary hypertension or right ventricular dysfunction is highly prevalent and often severe in HFPEF33. We did not obtain the data of pulmonary artery systolic pressure (PASP) and were not able to analysis the role between PASP and Galectin-3.
In conclusion, we found a significant correlation between plasma and tissue Galectin-3 levels and the degree of diastolic dysfunction and severity of myocardial fibrosis. We also demonstrated that both cellular and tissue Galecin-3 responded well to changes in stretching and pressure overload, respectively, indicating that Galecin-3 could be an important intermediary in promoting further fibrosis and/or inflammatory cascades. While there is little information regarding the medical management of HFPEF, novel therapies that downregulate the overexpression of Galectin-3 may indicate a new direction for HFPEF therapy. Targeting Galectin-3 may be an upstream therapeutic option for the treatment of all types of heart failure.
Additional Information
How to cite this article: Wu, C.-K. et al. Galectin-3 level and the severity of cardiac diastolic dysfunction using cellular and animal models and clinical indices. Sci. Rep. 5, 17007; doi: 10.1038/srep17007 (2015).
References
Owan, T. E. et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 355, 251–259 (2006).
Vasan, R. S. et al. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population-based cohort. J Am Coll Cardiol 33, 1948–1955 (1999).
Paulus, W. J. et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J 28, 2539–2550 (2007).
Massie, B. M. et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 359, 2456–2467 (2008).
Brouwers, F. P. et al. Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community-based cohort: 11-year follow-up of PREVEND. Eur Heart J 34, 1424–1431 (2013).
Bishu, K. et al. Biomarkers in acutely decompensated heart failure with preserved or reduced ejection fraction. Am Heart J 164, 763–770 e763 (2012).
Edelmann, F. et al. Galectin-3 in patients with heart failure with preserved ejection fraction: results from the Aldo-DHF trial. Eur J Heart Fail 17, 214–223 (2015).
Santhanakrishnan, R. et al. Growth differentiation factor 15, ST2, high-sensitivity troponin T and N-terminal pro brain natriuretic peptide in heart failure with preserved vs. reduced ejection fraction. Eur J Heart Fail 14, 1338–1347 (2012).
Yang, R. Y., Rabinovich, G. A. & Liu, F. T. Galectins: structure, function and therapeutic potential. Expert Rev Mol Med 10, e17 (2008).
Sharma, U. C. et al. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation 110, 3121–3128 (2004).
de Boer, R. A. et al. Predictive value of plasma galectin-3 levels in heart failure with reduced and preserved ejection fraction. Ann Med 43, 60–68 (2011).
Wu, C. K. et al. Plasma levels of tumor necrosis factor-alpha and interleukin-6 are associated with diastolic heart failure through downregulation of sarcoplasmic reticulum Ca2+ ATPase. Crit Care Med 39, 984–992 (2011).
Wu, C. K. et al. Genetic polymorphisms of the angiotensin II type 1 receptor gene and diastolic heart failure. J Hypertens 27, 502–507 (2009).
Wu, C. K. et al. Demonstrating the pharmacogenetic effects of angiotensin-converting enzyme inhibitors on long-term prognosis of diastolic heart failure. Pharmacogenomics J 10, 46–53 (2010).
Nagueh, S. F. et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 22, 107–33 (2009).
Tseng, W. Y., Liao, T. Y. & Wang, J. L. Normal systolic and diastolic functions of the left ventricle and left atrium by cine magnetic resonance imaging. J Cardiovasc Magn Reson 4, 443–457 (2002).
Ver Heyen, M. et al. Replacement of the muscle-specific sarcoplasmic reticulum Ca(2+)-ATPase isoform SERCA2a by the nonmuscle SERCA2b homologue causes mild concentric hypertrophy and impairs contraction-relaxation of the heart. Circ Res 89, 838–846 (2001).
Cheng, W. P., Wang, B. W., Chen, S. C., Chang, H. & Shyu, K. G. Mechanical stretch induces the apoptosis regulator PUMA in vascular smooth muscle cells. Cardiovasc Res 93, 181–189 (2012).
Kundu, S., Aulchenko, Y. S., van Duijn, C. M. & Janssens, A. C. PredictABEL: an R package for the assessment of risk prediction models. Eur J Epidemiol 26, 261–264 (2011).
Wu, C. K. et al. Connective tissue growth factor and cardiac diastolic dysfunction: human data from the Taiwan Diastolic Heart Failure Registry and molecular basis by cellular and animal models. Eur J Heart Fail 16, 163–172 (2014).
Frangogiannis, N. G. The immune system and cardiac repair. Pharmacol Res 58, 88–111 (2008).
Sasaki, S., Bao, Q. & Hughes, R. C. Galectin-3 modulates rat mesangial cell proliferation and matrix synthesis during experimental glomerulonephritis induced by anti-Thy1.1 antibodies. J Pathol 187, 481–489 (1999).
Beiras-Fernandez, A. et al. Local expression of myocardial galectin-3 does not correlate with its serum levels in patients undergoing heart transplantation. Ann Transplant 18, 643–650 (2013).
Lopez, B. et al. Galectin-3 and histological, molecular and biochemical aspects of myocardial fibrosis in heart failure of hypertensive origin. Eur J Heart Fail 17, 385–392 (2015).
van der Velde, A. R. et al. Prognostic value of changes in galectin-3 levels over time in patients with heart failure: data from CORONA and COACH. Circ Heart Fail 6, 219–226 (2013).
de Boer, R. A., Yu, L. & van Veldhuisen, D. J. Galectin-3 in cardiac remodeling and heart failure. Curr Heart Fail Rep 7, 1–8 (2010).
Katz, A. M. & Zile, M. R. New molecular mechanism in diastolic heart failure. Circulation 113, 1922–1925 (2006).
Wilkins, B. J. et al. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ Res 94, 110–118 (2004).
Berg, T. J. et al. Serum levels of advanced glycation end products are associated with left ventricular diastolic function in patients with type 1 diabetes. Diabetes Care 22, 1186–1190 (1999).
Wu, C. K. et al. The Relationship Among Central Obesity, Systemic Inflammation and Left Ventricular Diastolic Dysfunction as Determined by Structural Equation Modeling. Obesity (Silver Spring) 20, 730–737 (2012).
Lin, H. H. et al. Accumulation of epicardial fat rather than visceral fat is an independent risk factor for left ventricular diastolic dysfunction in patients undergoing peritoneal dialysis. Cardiovasc Diabetol 12, 127 (2013).
London, G. M. Cardiovascular disease in chronic renal failure: pathophysiologic aspects. Semin Dial 16, 85–94 (2003).
Lam, C. S. et al. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol 53, 1119–1126 (2009).
Acknowledgements
This work was supported, in part, by grants from the IBMS CRC Research Program of the Institute of Biomedical Sciences, Academia Sinica (IBMS-CRC102-P01), the National Science Council of the Republic of China (102-2314-B-002-058-, 104-2314-B-002-194-MY3, 102-2628-B-002 -035 -MY3) and the TVGH-NTUH Joint Research Program (VN104-01).
Author information
Authors and Affiliations
Contributions
C.W., C.T., F.L. and J.C. designed the whole study, analyzed and interpreted the data. M.S. performed the laboratory work. C.W. wrote the manuscript. C.W. and Jen-Kuang L. collected and analyzed the clinical data. F.C., J.H., Jiunn-Lee L. and C.T. recruited the patients and critically reviewed the manuscript for important intellectual content. C.T. was also in charge of the whole program. All authors discussed the implications of the results and critical reviewed the manuscript at all stages.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Wu, CK., Su, MY., Lee, JK. et al. Galectin-3 level and the severity of cardiac diastolic dysfunction using cellular and animal models and clinical indices. Sci Rep 5, 17007 (2015). https://doi.org/10.1038/srep17007
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep17007
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.