Abstract
The inhibition of a prepotent response is an essential executive function which enables us to suppress inappropriate actions in a given context. Individuals with fencing expertise exhibit behavioral advantages on tasks with high demands on response inhibition. This study examines the electrophysiological basis for the superior response inhibition in experienced fencers. In the Go/Nogo task where frequent stimuli required a motor response while reaction had to be withheld to rare stimuli, the fencers, compared with the non-fencers, exhibited behavioral as well as electrophysiological advantages when suppressing prepotent responses. The superior response inhibition in the fencers was characterized by enhanced Nogo-N2 and reduced Nogo-P3. Single-trial analysis revealed that the amplitude difference of the Nogo-N2 between two groups was caused by lower single-trial latency variability in the fencers (may be due to low attentional fluctuation and/or stable neural processing speed) while the amplitude difference of the Nogo-P3 resulted from truly weaker neural activity in the fencers (may be because few cognitive sources are needed and few control efforts are made). The two inhibition-related components are distinct neurophysiological indexes that, on the one hand, provide effective guidance to titrate the level of executive function in fencers and on the other hand, facilitate to monitor fencers’ improvement in the training process.
Similar content being viewed by others
Introduction
Executive control refers to a set of top-down mental processes needed when you are engaged in situations requiring decision-making, conflict resolution, error correction and response inhibition1,2. Among these processes, the inhibition of a prepotent response is an essential executive function in everyday life3,4. Response inhibition is associated with the ability to suppress actions that are inappropriate/unwanted in a given context and that interfere with goal-driven behavior5,6,7. This is the ability that makes it possible for us to choose how we react rather than being unthinking creatures of habit and impulsion8.
The requirement to suppress a prepotent response is most frequently studied using the Go/Nogo task7,9,10. In this paradigm, the subject is required to respond with high speed to Go stimuli and to refrain from responding to Nogo stimuli11,12. By having many more Go than Nogo trials, responding rather than inhibiting is made prepotent7. Neuropsychology and cognitive neuroscience have revealed that Nogo stimuli elicit different response-related processes from those elicited by Go stimuli (i.e., response inhibition vs. response activation)11. According to functional magnetic resonance imaging studies, the inferior frontal gyrus and the pre-supplementary motor area show strong activations in the Nogo condition4,5,12,13,14. The event-related potential (ERP) studies have reported two major components in relation to response inhibition12. The first is Nogo-N2, which appears 200–400 ms with a frontal-midline maximum and displays larger negative amplitudes in the Nogo condition11,15,16,17,18,19. In general, the N2 enhancement to Nogo stimuli has been interpreted as a premotor inhibitory process that suppresses the incorrect response prior to reaction stage20,21,22,23. The second component is Nogo-P3, which appears 300–600 ms with a frontocentral maximum and displays larger and later positive amplitudes in the Nogo condition11,16,19,22,24. Unlike the Nogo-N2 (reflecting an early inhibition of response execution), the Nogo-P3 is usually considered as a later monitoring and evaluation of inhibition process12,15,25,26.
Recent years have witnessed an unprecedented interest in understanding the mechanisms of response inhibition, since it is thought to be a core deficit in attention-deficit/hyperactivity disorder (ADHD)6,7,27,28,29,30,31, drug abuse and addiction30,32,33,34,35,36, alcoholics36,37,38,39,40,41, Huntington’s disease42, obsessive-compulsive disorder5,43 and autism spectrum disorder44,45. However, while research on these diseases suggests that deficits in response inhibition are potentially inheritable characteristics and could service as candidate endophenotypes for genetic investigation7,12,46,47,48, very few studies have investigated the response inhibition in experts who have special executive talents or skills in a given area (except a handful of studies49,50,51,52). The importance of characterizing the response inhibition in experts is that, on the one hand, it may open new doors for a deeper understanding of the mechanisms behind inhibitory control deficits and thus facilitate the diagnosis, treatment and prevention of relevant diseases. On the other hand, because of the apparent demand to suppress inappropriate reactions in a large number of individual and social activities, distinct neurophysiological features in experts may serve as biomarkers with the capacity to measure the level of executive function and assess individuals’ potentials in special careers, such as open-skill sport, special troop, interpreter and pilot.
Contemporary fencing is an open-skill sport which requires fencers to quickly switch their states between offence and defense based on the judgment on whether the opponents offer an attack (a thrust with the purpose of landing a touch upon the opponent’s valid area) or a feint (a thrust with the purpose of provoking a reaction from the opponent). Individuals with fencing expertise exhibit behavioral advantages on tasks with high demands on executive functioning, particularly inhibitory control49,50,53, but the neural correlates of the enhanced inhibition of a prepotent response are unclear. In this study, ERP technique is employed together with the Go/Nogo task to elucidate the electrophysiological basis for the superior executive function of response inhibition in experienced fencers (compared with demographically matched non-fencers). It is expected that the Nogo-N2 and the Nogo-P3, as well as behavioral measures would show significant differences between fencers and non-fencers.
In addition to stimulus-locked averaging measures, single-trial analysis of ERP54,55,56,57 is used to investigate the trial-to-trial variations with regard to the amplitude and the latency of the Nogo-N2/P3 components. As illustrated in the study of Zhang et al.58, a larger amplitude in average N2 or P3 in one subject group may reflect truly stronger neural activity (more neural networks are allocated for inhibitory control) as compared with another group (Fig. 1A); or it may be due to the ERP responses in one group that show a more consistent latency from trial to trial (i.e. a smaller fluctuation in neural processing speed during the Go/Nogo task) (Fig. 1B). Therefore, ERP analysis on the single-trial level is essential to clarify the neural mechanisms underlying the different amplitudes of the average ERPs between fencers and non-fencers.
Method
Participants
Twenty-six healthy adults (14 females; age range = 18 to 22 years) and twenty-six fencers (13 females; age range = 19 to 23 years) were recruited from Shanghai University of Sport as paid participants. All the fencers had an experience in fencing (foil) for at least six years and had won at least one national/international championship. The subjects in control group were age- and education-matched non-athletes. All participants were right-handed and had normal or corrected-to-normal vision. They gave their written informed consent prior to the experiment. The experimental protocol was approved by the local ethics committee (Shanghai University of Sport) and this study was performed strictly in accordance with the approved guidelines.
Stimuli
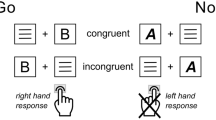
The fixation was a cross of 0.3° × 0.3° visual angle in the center of the computer monitor. The stimuli consisted of vertical and horizontal white bars on the black background (four pictures with a visual angle of 4° × 4°; Fig. 2). The four configurations were displayed randomly with equal probability within Go condition (p = 0.2, i.e., p = 0.1 for each Go picture) and within Nogo condition (p = 0.8, i.e., p = 0.4 for each Nogo picture). We used two stimuli in each condition to prevent the ceiling effect.
Procedure
Participants were seated in a dimly lit and sound-attenuated room. Stimuli were presented on a LCD monitor at a viewing distance of approximately 100 cm. Stimulus display and behavioral data acquisition were conducted using E-Prime 2.0 (Psychology Software Tools, Inc., Pittsburgh, PA).
Each trial started with a 200-to-300-ms fixation, followed by targets (Go stimuli) and non-targets (Nogo stimuli) that were presented for 200 ms. A black blank screen appeared after the stimulus and lasted for 1500 ms. Participants were required to press a button with their right index finger on the response box as quick as possible when the Go stimuli appeared, while they should withhold the behavioral responses when the Nogo stimuli appeared. The Go and Nogo trials were presented in random order with a probability of 4:1. The total experiment consisted of four blocks, with 100 trials in each block.
EEG recording and analysis
Brain electrical activity was recorded referentially against left mastoid and off-line re-referenced to the average of the left and right mastoids, by a 32-channel amplifier with a sampling frequency of 250 Hz (Brain Products, Gilching, Germany). All the electrooculogram and electroencephalogram (EEG) electrodes worked with electrode impedances kept below 5 kΩ. Ocular artifacts were removed from EEGs using a regression procedure implemented in NeuroScan software (Scan 4.3; NeuroScan Inc., Herndon, USA).
The data analysis and result display in this study were performed using Matlab R2011a (MathWorks, Natick, USA). The recorded EEG data were filtered with a 0.01–30 Hz finite impulse response filter with zero phase distortion. Filtered data were segmented beginning 200 ms prior to the onset of stimulus and lasting for 1200 ms. All epochs were baseline-corrected with respect to the mean voltage over the 200 ms preceding the onset of stimulus, followed by averaging in association with experimental conditions.
This study focused on the ERP components associated with inhibitory control. In particular, we analyzed the peak latency and peak amplitude of frontal N2, fronto-central P3 (Nogo) and parietal P3 (Go) between Go and Nogo conditions and between fencers and non-fencers. The ERP amplitudes were measured using different sets of electrodes in accordance with grand-mean ERP topographies and relevant literatures11,15,16,17,18,19,22,24. Accordingly, the mean of the peak amplitude of the N2 was calculated at the electrode sites of Fz, FC1 and FC2, with its peak detected within a time window of 200–400 ms after stimulus onset. The mean of the peak amplitude of the P3 was calculated at the electrode sites of Cz, FC1 and FC2 (Nogo condition) or at the electrode sites of Pz, CP1 and CP2 (Go condition), with its peak detected within a time window of 300–600 ms (Nogo condition) or 200–500 ms (Go condition) after stimulus onset.
Single-trial peak detection of N2 and P3 was performed on the average ERP waveforms computed based on the same electrode sets as in the average ERP analysis. The Maximum Likelihood Estimation (MLE) technique was employed to detect the occurrence of the component peak on the single-trial basis59. The MLE assumes that the ERP signal that hides in EEG background activity has an invariant shape but may vary both in its latency and amplitude. By maximizing the log likelihood function of the model in the frequency domain, the MLE estimates the unknown parameters of ERP signals such as latencies and amplitudes in single trials. Assuming that peak amplitude and peak latency across trials obey normal distributions60, the peak amplitude of the average ERP is primarily influenced by two variables, namely, the mean of the single-trial peak amplitude and the standard deviation of the single-trial peak latency, both of which are measured to characterized the peak amplitude of the average ERP in this study.
Statistics
Statistical analysis was performed using SPSS Statistics 20.0 (IBM, Somers, USA). Descriptive data were presented as mean ± standard error. The significance level was set at 0.05.
Two-way repeated-measures ANOVAs were performed on measurements of the behavioral and ERP data, with response assignment (Go and Nogo) as the within-subject factor and group (fencer and non-fencer) as the between-subject factor. Two-way repeated-measures ANOVA were also performed on the P3 peak amplitudes, with response assignment and electrode site (Fz, Cz and Pz) as the within-subject factors11. Greenhouse-Geisser correction for ANOVA tests was used whenever appropriate. Post-hoc testing of significant main effects was conducted using Bonferroni method. Significant interactions were analyzed using simple effects model. Partial eta-squared ( ) was reported to demonstrate the effect size in ANOVA tests.
) was reported to demonstrate the effect size in ANOVA tests.
To counterbalance the number of trials between Go and Nogo conditions, only 1/4 Nogo trials were randomly selected and used to statistically compare with Go trials and plot the ERP waveforms in the study.
Results
For the sake of brevity, this section only reports the most important results. Please refer to the supplementary material for the other significant findings.
Accuracy rate (ACC)
The interaction effect of response assignment by group was significant (F(1,50) = 4.80; p = 0.033;  = 0.088). Simple effect analysis indicated that the ACC in the Nogo trials was higher in the fencers (0.421 ± 0.033) compared with that in the non-fencers (0.310 ± 0.033) (F(1,50) = 5.51; p = 0.023). The ACC in the Go trials did not differ between groups (F(1,50) < 1; fencer = 0.989 ± 0.004; non-fencer = 0.983 ± 0.004).
= 0.088). Simple effect analysis indicated that the ACC in the Nogo trials was higher in the fencers (0.421 ± 0.033) compared with that in the non-fencers (0.310 ± 0.033) (F(1,50) = 5.51; p = 0.023). The ACC in the Go trials did not differ between groups (F(1,50) < 1; fencer = 0.989 ± 0.004; non-fencer = 0.983 ± 0.004).
Reaction time (RT)
The main effect of group was significant (F(1,50) = 6.30; p = 0.015;  = 0.112): the RT in the fencers (710 ± 20 ms) was shorter than that in the non-fencers (780 ± 20 ms).
= 0.112): the RT in the fencers (710 ± 20 ms) was shorter than that in the non-fencers (780 ± 20 ms).
Average measures of the N2
The interaction effect of response assignment by group was significant on the peak amplitude of the average N2 (F(1,50) = 4.18; p = 0.046;  = 0.077; Fig. 3). The N2 amplitude in the Nogo condition (F(1,50) = 8.75; p = 0.005) was larger in the fencers (−3.97 ± 0.45 μV) compared with the non-fencers (−2.07 ± 0.45 μV), whereas the group effect was not significant in the Go condition (F(1,50) < 1; fencer = 0.74 ± 0.45 μV; non-fencer = 0.82 ± 0.45 μV).
= 0.077; Fig. 3). The N2 amplitude in the Nogo condition (F(1,50) = 8.75; p = 0.005) was larger in the fencers (−3.97 ± 0.45 μV) compared with the non-fencers (−2.07 ± 0.45 μV), whereas the group effect was not significant in the Go condition (F(1,50) < 1; fencer = 0.74 ± 0.45 μV; non-fencer = 0.82 ± 0.45 μV).
The interaction effect of response assignment by group was significant on the peak latency of the average N2 (F(1,50) = 6.42; p = 0.014;  = 0.114; Fig. 3). The N2 latency in the Nogo condition (F(1,50) = 8.90; p = 0.004) was shorter in the fencers (280 ± 8.09 ms) compared with the non-fencers (314 ± 8.09 ms), while the group effect was not significant in the Go condition (F(1,50) < 1; fencer = 244 ± 7.80 ms; non-fencer = 236 ± 7.80 ms).
= 0.114; Fig. 3). The N2 latency in the Nogo condition (F(1,50) = 8.90; p = 0.004) was shorter in the fencers (280 ± 8.09 ms) compared with the non-fencers (314 ± 8.09 ms), while the group effect was not significant in the Go condition (F(1,50) < 1; fencer = 244 ± 7.80 ms; non-fencer = 236 ± 7.80 ms).
Single-trial measures of the N2
The interaction effect of response assignment by group was significant on the standard deviation of the single-trial peak latency (F(1,50) = 5.39; p = 0.024;  = 0.097). The N2 latency jitter in the Nogo condition (F(1,50) = 11.3; p = 0.001) was smaller in the fencers (standard deviation of latency = 21.6 ± 0.46 ms) compared with the non-fencers (23.8 ± 0.46 ms), while the group effect was not significant in the Go condition (F(1,50) < 1; fencer = 25.7 ± 0.55 ms; non-fencer = 25.5 ± 0.55 ms).
= 0.097). The N2 latency jitter in the Nogo condition (F(1,50) = 11.3; p = 0.001) was smaller in the fencers (standard deviation of latency = 21.6 ± 0.46 ms) compared with the non-fencers (23.8 ± 0.46 ms), while the group effect was not significant in the Go condition (F(1,50) < 1; fencer = 25.7 ± 0.55 ms; non-fencer = 25.5 ± 0.55 ms).
No significant difference was found in the mean of the single-trial peak amplitude of the N2 across conditions or groups.
Average measures of the P3
The interaction effect of response assignment by group was significant on the peak amplitude of the average P3 (F(1,50) = 5.74; p = 0.020;  = 0.103; Figs 4 and 5). The P3 amplitude in the Nogo condition (F(1,50) = 5.95; p = 0.018) was larger in the non-fencers (10.4 ± 0.64 μV) compared with the fencers (8.24 ± 0.64 μV) while the group effect was not significant in the Go condition (F(1,50) < 1; fencer = 6.53 ± 0.37 μV; non-fencer = 6.95 ± 0.37 μV).
= 0.103; Figs 4 and 5). The P3 amplitude in the Nogo condition (F(1,50) = 5.95; p = 0.018) was larger in the non-fencers (10.4 ± 0.64 μV) compared with the fencers (8.24 ± 0.64 μV) while the group effect was not significant in the Go condition (F(1,50) < 1; fencer = 6.53 ± 0.37 μV; non-fencer = 6.95 ± 0.37 μV).
The interaction effect of response assignment by group was significant on the peak latency of the average P3 (F(1,50) = 4.47; p = 0.040;  = 0.082; Figs 4 and 5). The P3 latency in the Nogo condition (F(1,50) = 6.81; p = 0.012) was shorter in the fencers (482 ± 11.8 ms) compared with the non-fencers (526 ± 11.8 ms), while the group effect was not significant in the Go condition (F(1,50) < 1; fencer = 355 ± 9.93 ms; non-fencer = 356 ± 9.93 ms).
= 0.082; Figs 4 and 5). The P3 latency in the Nogo condition (F(1,50) = 6.81; p = 0.012) was shorter in the fencers (482 ± 11.8 ms) compared with the non-fencers (526 ± 11.8 ms), while the group effect was not significant in the Go condition (F(1,50) < 1; fencer = 355 ± 9.93 ms; non-fencer = 356 ± 9.93 ms).
Then the peak amplitude of the average P3 was tested for its topographic difference between Go and Nogo conditions. The interaction effect of response assignment by electrode site was significant (F(2,102) = 5.87; p = 0.006;  = 0.103; Figs 3, 4, 5). The P3 amplitude in the Go condition (F(2,102) = 9.46; p < 0.001) was largest at Pz (6.74 ± 0.26 μV) (Cz = 5.50 ± 0.55 μV; Fz = 4.34 ± 0.33 μV; Fz vs. Pz, p < 0.001). However, the P3 amplitude in the Nogo condition (F(2,102) = 18.2; p < 0.001) showed a different topographic pattern: it was largest at Cz (9.34 ± 0.48 μV) (Fz = 5.63 ± 0.46 μV; Pz = 7.95 ± 0.50 μV; Cz vs. Pz, p = 0.002; Fz vs. Cz, p < 0.001).
= 0.103; Figs 3, 4, 5). The P3 amplitude in the Go condition (F(2,102) = 9.46; p < 0.001) was largest at Pz (6.74 ± 0.26 μV) (Cz = 5.50 ± 0.55 μV; Fz = 4.34 ± 0.33 μV; Fz vs. Pz, p < 0.001). However, the P3 amplitude in the Nogo condition (F(2,102) = 18.2; p < 0.001) showed a different topographic pattern: it was largest at Cz (9.34 ± 0.48 μV) (Fz = 5.63 ± 0.46 μV; Pz = 7.95 ± 0.50 μV; Cz vs. Pz, p = 0.002; Fz vs. Cz, p < 0.001).
Single-trial measures of the P3
The interaction effect of response assignment by group was significant on the mean of the single-trial P3 peak amplitude (F(1,50) = 4.68; p = 0.035;  = 0.086). The P3 amplitude in the Nogo condition (F(1,50) = 4.74; p = 0.034) was larger in the non-fencers (13.9 ± 0.87 μV) compared with the fencers (11.2 ± 0.87 μV) while the group effect was not significant in the Go condition (F(1,50) < 1; fencer = 8.97 ± 0.50 μV; non-fencer = 9.36 ± 0.50 μV).
= 0.086). The P3 amplitude in the Nogo condition (F(1,50) = 4.74; p = 0.034) was larger in the non-fencers (13.9 ± 0.87 μV) compared with the fencers (11.2 ± 0.87 μV) while the group effect was not significant in the Go condition (F(1,50) < 1; fencer = 8.97 ± 0.50 μV; non-fencer = 9.36 ± 0.50 μV).
No significant difference was found in the standard deviation of the single-trial peak latency of the P3 across conditions or groups.
Discussion
Cancelling initiated responses timely is especially crucial and frequently happened during fencing practice (e.g., in the case when a feint is provided by the opponent). The present study employs ERPs to examine the neural correlates for fencers’ inhibition advantage according to the hypothesis that experienced fencers have enhanced executive function of response inhibition49,50,53.
Behavioral data
Previous behavioral studies in patients indicated a deterioration of Go/Nogo performance caused by an impaired ability to inhibit unwanted behavior. For example, individuals diagnosed with Huntington’s disease showed more false alarms in the Nogo condition42; patients with Tourette’s syndrome had longer RTs than healthy controls in Go trials and made more errors in total61; and obese children exhibited lower response accuracy relative to healthy weight children in the Nogo condition62. We found in the current study that the number of mistaken responses the fencers made on Nogo trials (false alarm) was significantly smaller than that made by the non-fencers. At the same time, the average RT to Go as well as to Nogo trials was significantly shorter in the fencers compared with the non-fencers. Similarly, using the Go/Nogo paradigm, Chan et al.49 observed that high-fit fencers committed significantly fewer errors compared to the non-fencers; and Kida et al.52 showed that the Go/Nogo RT for baseball players was significantly shorter than that of the non-athletes. The current study suggests that open-skill athletes are superior to non-athletes in both ACC and RT in the Go/Nogo task.
ERP data
It was observed in our data that the average Nogo-N2 elicited in the fencers was larger and with an earlier latency compared with that elicited in the non-fencers. The Nogo-N2 is known to reflect an inhibition of response execution20,21,23 and this inhibitory process is usually located at the pre-motor rather than at the motor level22. Previous Go/Nogo studies in healthy adults found that a smaller and later Nogo-N2 was evoked in subjects with a high false alarm rate compared with those with a low false alarm rate22. Similarly, studies in patients indicated that Nogo-N2 amplitudes were negatively correlated with the number of symptoms in ADHD20,27; and the N2 enhancement to Nogo trials was strongly reduced in the depressed individuals63. The current finding that Nogo stimuli elicited larger and earlier N2 in the fencers is consistent with previous studies, which suggested that the improvement of inhibitory control is accompanied by a larger and/or earlier Nogo-N250,51.
More importantly, this study examined the single-trial characteristics of the N2. Our result suggested that the enhanced Nogo-N2 in the fencers was due to smaller latency fluctuations on the trial-to-trial basis in this group (Fig. 1B). A potential explanation for the less variability in the Nogo-N2 latency is that fencers, compared with non-fencers, may have fewer attentional fluctuations and/or have a more stable neural processing speed when inhibition control is needed (see the study of Ford et al.57 for example), thus producing more consistent single-trial latencies at the early stage of response inhibition. This interpretation is in harmony with Rentrop et al.64, who observed in the Go/Nogo task that patients with schizophrenia displayed an increased variability in N2 latency, indicating a temporal instability of information processing associated with the disease.
Another interesting finding of this study was that the average Nogo-P3 differed significantly between groups: compared to the non-fencers, the fencers displayed an earlier and amplitude-reduced P3 in the Nogo condition. The Nogo-P3 is usually considered as the evaluation and monitoring of inhibition process12,15,25,26,65. Researchers have found that heavy drinkers showed significantly greater activity in the inhibitory control regions on the Nogo trials and displayed significantly larger NoGo-P3 amplitudes than controls; this result suggested that heavy drinkers may have impaired neural functions related to response inhibition so their brain needs to allocate more cognitive sources and make more control efforts to successfully withhold a response40,41. Similarly, Taddei et al.50 found that age-related compensatory neural responses occur during response inhibition, reflected by a delayed Nogo-P3 in older subjects compared with younger subjects. According to these results, we propose that the group difference in the Nogo-P3 of the current study indicates that fencing expertise improves the inhibitory function of individuals and makes their brain able to evaluate and monitor the inhibition of a prepotent response in a more efficient manner. Furthermore, single-trial peak estimates of the P3 indicated that the expertise-induced inhibitory improvement was reflected by true amplitude reductions in single trials (i.e. fewer neural resources were needed in response inhibition for experienced fencers) (Fig. 1A). It is known that P3 amplitude reflects demands on “perceptual-central” resources and is closely related to the intensity of processing66. The real P3 amplitude reduction observed in single trials provides strong evidence that long-term training in fencing significantly enhances the neural efficiency of inhibition processing, resulting in a compaction of associated cognitive sources and a minimization of control efforts in the inhibitory task.
Conclusion remarks
The current study investigated the effect of fencing expertise on the executive function of response inhibition. In the Go/Nogo task where frequent (80%) stimuli required a motor response while reaction had to be withheld to rare (20%) stimuli, the fencers, compared with the non-fencers, exhibited behavioral as well as electrophysiological advantages when suppressing prepotent responses. The superior response inhibition in the fencers was characterized by enhanced Nogo-N2 (may be due to low attentional fluctuation and stable neural processing speed in response inhibition process) and reduced Nogo-P3 (may be because few cognitive sources are allocated and few control efforts are made). These two inhibition-related ERP components are distinct neurophysiological indexes that may, on the one hand, provide effective guidance to titrate the level of executive function in fencers and on the other hand, facilitate to monitor fencers’ improvement in the training process. Furthermore, in line with the single-trial findings, we infer that diseases relevant to inhibitory control deficits, such as ADHD and drug abuse, are likely accompanied by instability of information processing and excessive demand for neural networks during response inhibition.
Additional Information
How to cite this article: Zhang, D. et al. Enhanced response inhibition in experienced fencers. Sci. Rep. 5, 16282; doi: 10.1038/srep16282 (2015).
References
Baddeley, A. & Della Sala, S. Working memory and executive control. Philos Trans R Soc Lond B Biol Sci 351, 1397–1404 (1996).
Miller, E. K. & Cohen, J. D. An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24, 167–202 (2001).
Aron, A. R. The neural basis of inhibition in cognitive control. Neuroscientist 13, 214–228 (2007).
Chikazoe, J. Localizing performance of go/no-go tasks to prefrontal cortical subregions. Curr Opin Psychiatry 23, 267–272 (2010).
Chambers, C. D, Garavan, H. & Bellgrove, M. A. Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neurosci Biobehav Rev 33, 631–646 (2009).
Mostofsky, S. H. & Simmonds, D. J. Response inhibition and response selection: two sides of the same coin. J Cogn Neurosci 20, 751–761 (2008).
Aron, A. R. & Poldrack, R. A. The cognitive neuroscience of response inhibition: relevance for genetic research in attention-deficit/hyperactivity disorder. Biol Psychiatry 57, 1285–1292 (2005).
Diamond, A. Executive functions. Annu Rev Psychol 64, 135–168 (2013).
Aron, A. R. Introducing a special issue on stopping action and cognition. Neurosci Biobehav Rev 33, 611–612 (2009).
Bari, A. & Robbins, T. W. Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol 108, 44–79 (2013).
Eimer, M. Effects of attention and stimulus probability on ERPs in a Go/Nogo task. Biol Psychol 35, 123–138 (1993).
Huster, R. J., Enriquez-Geppert, S., Lavallee, C. F., Falkenstein, M. & Herrmann, C. S. Electroencephalography of response inhibition tasks: functional networks and cognitive contributions. Int J Psychophysiol 87, 217–233 (2013).
Aron, A. R. From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biol Psychiatry 69, e55–e68 (2010).
Sharp, D. J. et al. Distinct frontal systems for response inhibition, attentional capture and error processing. Proc Natl Acad Sci USA 107, 6106–6111 (2010).
Pfefferbaum, A., Ford, J. M., Weller, B. J. & Kopell, B. S. ERPs to response production and inhibition. Electroencephalogr Clin Neurophysiol 60, 423–434 (1985).
Kopp, B., Mattler, U., Goertz, R. & Rist, F. N2, P3 and the lateralized readiness potential in a nogo task involving selective response priming. Electroencephalogr Clin Neurophysiol 99, 19–27 (1996).
Jodo, E. & Kayama, Y. Relation of a negative ERP component to response inhibition in a Go/Nogo task. Electroencephalogr Clin Neurophysiol 82, 477–482 (1992).
Thorpe, S., Fize, D. & Marlot, C. Speed of processing in the human visual system. Nature 381, 520–522 (1996).
Kok, A. Effects of degradation of visual stimulation on components of the event-related potential (ERP) in go/nogo reaction tasks. Biol Psychol 23, 21–38 (1986).
Pliszka, S. R, Liotti, M. & Woldorff, M. G. Inhibitory control in children with attention-deficit/hyperactivity disorder: event-related potentials identify the processing component and timing of an impaired right-frontal response-inhibition mechanism. Biol Psychiatry 48, 238–246 (2000).
Geczy, I., Czigler, I. & Balazs, L. Effects of cue information on response production and inhibition measured by event-related potentials. Acta Physiol Hung 86, 37–44 (1999).
Falkenstein, M., Hoormann, J. & Hohnsbein, J. ERP components in Go/Nogo tasks and their relation to inhibition. Acta Psychol 101, 267–291 (1999).
Roche, R. A., Garavan, H., Foxe, J. J. & O’Mara, S. M. Individual differences discriminate event-related potentials but not performance during response inhibition. Exp Brain Res 160, 60–70 (2005).
Simson, R., Vaughan, H. G. & Ritter, W. The scalp topography of potentials in auditory and visual Go/NoGo tasks. Electroenceph Clin Neurophysiol 43, 864–875 (1977).
Schmajuk, M., Liotti, M., Busse, L. & Woldorff, M. G. Electrophysiological activity underlying inhibitory control processes in normal adults. Neuropsychologia 44, 384–395 (2006).
Bokura, H., Yamaguchi, S. & Kobayashi, S. Electrophysiological correlates for response inhibition in a Go/NoGo task. Clin Neurophysiol 112, 2224–2232 (2001).
Woltering, S., Liu, Z., Rokeach, A. & Tannock, R. Neurophysiological differences in inhibitory control between adults with ADHD and their peers. Neuropsychologia 51, 1888–1895 (2013).
Chamberlain, S. R. & Sahakian, B. J. The neuropsychiatry of impulsivity. Curr Opin Psychiatr 20, 255–261 (2007).
Slaats-Willemse, D., Swaab-Barneveld, H., de Sonneville, L., van der Meulen, E. & Buitelaar, J. Deficient response inhibition as a cognitive endophenotype of ADHD. J Am Acad Child Adolesc Psychiatry 42, 1242–1248 (2003).
Pattij, T. & Vanderschuren, L.J. The neuropharmacology of impulsive behaviour. Trends Pharmacol Sci 29, 192–199 (2008).
Groman, S. M., James, A. S. & Jentsch, J. D. Poor response inhibition: at the nexus between substance abuse and attention deficit/hyperactivity disorder. Neurosci Biobehav Rev 33, 690–698 (2009).
Belin, D., Mar, A. C., Dalley, J. W., Robbins, T. W. & Everitt, B. J. High impulsivity predicts the switch to compulsive cocaine-taking. Science 320, 1352–1355 (2008).
Dalley, J. W. et al. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science 315, 1267–1270 (2007).
Volkow, N. D., Fowler, J. S., Wang, G. J. & Swanson, J. M. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol Psychiatry 9, 557–569 (2004).
Roberts, G. M. & Garavan, H. Evidence of increased activation underlying cognitive control in ecstasy and cannabis users. Neuroimage 52, 429–435 (2010).
Nigg, J. T. et al. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. J Am Acad Child Adolesc Psychiatry 45, 468–475 (2006).
Rubio, G. et al. The role of behavioral impulsivity in the development of alcohol dependence: a 4-year follow-up study. Alcohol Clin Exp Res 32, 1681–1687 (2008).
Pandey, A. K. et al. Neurocognitive deficits in male alcoholics: an ERP/sLORETA analysis of the N2 component in an equal probability Go/NoGo task. Biol Psychol 89, 170–182 (2012).
Kamarajan, C. et al. Alcoholism is a disinhibitory disorder: neurophysiological evidence from a Go/No-Go task. Biol Psychol 69, 353–373 (2005).
Ames, S. L. et al. Neural correlates of a Go/NoGo task with alcohol stimuli in light and heavy young drinkers. Behav Brain Res 274, 382–389 (2014).
López-Caneda, E., Rodríguez-Holguín, S., Corral, M., Doallo, S. & Cadaveira, F. Evolution of the binge drinking pattern in college students: neurophysiological correlates. Alcohol 48, 407–418 (2014).
Beste, C., Saft, C., Andrich, J., Gold, R. & Falkenstein, M. Response inhibition in Huntington’s disease—A study using ERPs and sLoreta. Neuropsychologia 46, 1290–1297 (2008).
van Velzen, L. S., Vriend, C., de Wit, S.J. & van den Heuvel, O. A. Response inhibition and interference control in obsessive-compulsive spectrum disorders. Front Hum Neurosci 8, 419 (2014).
Langen, M. et al. Fronto-striatal circuitry and inhibitory control in autism: findings from diffusion tensor imaging tractography. Cortex 48, 183–193 (2012).
Duerden, E.G. et al. Neural correlates of inhibition of socially relevant stimuli in adults with autism spectrum disorder. Brain Res 1533, 80–90 (2013).
Chamberlain, S. R. et al. Impaired cognitive flexibility and motor inhibition in unaffected first-degree relatives of patients with obsessive-compulsive disorder. Am J Psychiatry 164, 335–338 (2007).
Ersche, K. D. et al. Abnormal brain structure implicated in stimulant drug addiction. Science 335, 601–604 (2012).
Vink, M., Ramsey, N. F., Raemaekers, M. & Kahn, R. S. Striatal dysfunction in schizophrenia and unaffected relatives. Biol Psychiatry 60, 32–39 (2006).
Chan, J., Wong, A., Liu, Y., Yu, J. & Yan, J. Fencing expertise and physical fitness enhance action inhibition. Psychol Sport Exerc 12, 509–514 (2011).
Taddei, F., Bultrini, A., Spinelli, D. & Di Russo, F. Neural correlates of attentional and executive processing in middle-age fencers. Med Sci Sports Exerc 44, 1057–1066 (2012).
Moreno, S., Wodniecka, Z., Tays, W., Alain, C. & Bialystok, E. Inhibitory control in bilinguals and musicians: event related potential (ERP) evidence for experience-specific effects. PLoS One 9, e94169 (2014).
Kida, N., Oda, S. & Matsumura, M. Intensive baseball practice improves the Go/Nogo reaction time, but not the simple reaction time. Brain Res Cogn Brain Res 22, 257–264 (2005).
Vertopoulos, E., Tsolakis, C. & Remoundou, M. A preliminary study of visual memory and rule detection in fencing: a comparative study. Biology of Exercise 6, 37–45 (2010).
Unsal, A. & Segalowitz, S. J. Sources of P300 attenuation after head injury: single-trial amplitude, latency jitter and EEG power. Psychophysiology 32, 249–256 (1995).
Walhovd, K. B., Rosquist, H. & Fjell, A. M. P300 amplitude age reductions are not caused by latency jitter. Psychophysiology 45, 545–553 (2008).
Rousselet, G. A., Husk, J. S., Bennett, P. J. & Sekuler, A. B. Single-trial EEG dynamics of object and face visual processing. Neuroimage 36, 843–862 (2007).
Ford, J. M., White, P., Lim, K. O. & Pfefferbaum, A. Schizophrenics have fewer and smaller P300s: a single-trial analysis. Biol Psychiatry 35, 96–103 (1994).
Zhang, D., Luo, W. & Luo, Y. Single-trial ERP evidence for the three-stage scheme of facial expression processing. Sci China Life Sci 56, 835–847 (2013).
Jaśkowski, P. & Verleger, R. Amplitudes and latencies of single-trial ERP’s estimated by a maximum-likelihood method. IEEE Trans Biomed Eng 46, 987–993 (1999).
Spencer, K. M. Averaging, detection and classification of single-trial ERPs. In Event-Related Potentials: a Method Handbook (ed. Handy, T. C. ) (The MIT Press, London, 2005).
Thomalla, G. et al. Costs of control: decreased motor cortex engagement during a Go/NoGo task in Tourette’s syndrome. Brain 137, 122–136 (2014).
Kamijo, K. et al. The association of childhood obesity to neuroelectric indices of inhibition. Psychophysiology 49, 1361–1371 (2012).
Katz, R. et al. Cognitive control in late-life depression: response inhibition deficits and dysfunction of the anterior cingulate cortex. Am J Geriatr Psychiatry 18, 1017–1025 (2010).
Rentrop, M. et al. Temporal variability and spatial diffusion of the N2 event-related potential in high-functioning patients with schizophrenia. Schizophr Res 131, 206–213 (2011).
Beste, C., Willemssen, R., Saft, C. & Falkenstein, M. Response inhibition subprocesses and dopaminergic pathways: basal ganglia disease effects. Neuropsychologia 48, 366–373 (2010).
Kok, A. On the utility of P3 amplitude as a measure of processing capacity. Psychophysiology 38, 557–577 (2001).
Acknowledgements
This study was funded by the National Natural Science Foundation of China (31571120; 31300867) and the National Key Basic Research Program of China (973 Program, 2014CB744600).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: X.W. and C.Q.; performed the experiments: H.D. and X.W.; analyzed the data: D.Z. and X.W.; wrote the manuscript: D.Z. and Y.L.; contributed materials and analysis tools: C.Q.; provided lab equipment for running the study: X.W. and Y.L.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Zhang, D., Ding, H., Wang, X. et al. Enhanced response inhibition in experienced fencers. Sci Rep 5, 16282 (2015). https://doi.org/10.1038/srep16282
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep16282
This article is cited by
-
Does transcranial direct current stimulation enhance cognitive performance in Parkinson’s disease mild cognitive impairment? An event-related potentials and neuropsychological assessment study
Neurological Sciences (2022)
-
Modality-specific improvements in sensory processing among baseball players
Scientific Reports (2021)
-
Characterizing the brain’s dynamical response from scalp-level neural electrical signals: a review of methodology development
Cognitive Neurodynamics (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.