Abstract
Non-functioning pituitary macroadenomas (NFPAs) are the most prevalent pituitary macroadenomas. One common symptom of NFPA is hypogonadism, which may require long-term hormone replacement. This study was designed to clarify the association between the pre-operative tumor volume, pre-operative testosterone level, intraoperative resection status and the need of long-term post-operative testosterone replacement. Between 2004 and 2012, 45 male patients with NFPAs were enrolled in this prospective study. All patients underwent transsphenoidal surgery. Hypogonadism was defined as total serum testosterone levels of <2.4 ng/mL. The tumor volume was calculated based on the pre- and post-operative magnetic resonance images. We prescribed testosterone to patients with defined hypogonadism or clinical symptoms of hypogonadism. Hormone replacement for longer than 1 year was considered as long-term therapy. The need for long-term post-operative testosterone replacement was significantly associated with larger pre-operative tumor volume (p = 0.0067) and lower pre-operative testosterone level (p = 0.0101). There was no significant difference between the gross total tumor resection and subtotal resection groups (p = 0.1059). The pre-operative tumor volume and testosterone level impact post-operative hypogonadism. By measuring the tumor volume and the testosterone level and by performing adequate tumor resection, surgeons will be able to predict post-operative hypogonadism and the need for long-term hormone replacement.
Similar content being viewed by others
Introduction
Non-functioning pituitary macroadenomas (NFPAs) are the most prevalent pituitary macroadenomas. Although NFPAs are benign in origin, mass effects may lead to significant clinical symptoms, such as visual impairments and pituitary insufficiency. It is also well known that pituitary function may be affected by this primary mass effect. The pre-operative tumor volume, especially in cases of larger tumors, may significantly impact pre-operative hypogonadism and post-operative recovery from this deficiency. Dekkers et al. have reported that, in patients presenting with hypopituitarism, growth hormone (GH) deficiency and gonadal deficiency are observed in approximately 85% and 75% of all patients, respectively; alternatively, corticotroph (approximately 38%) and thyrotroph (approximately 32%) deficiencies are observed less frequently1,2,3,4,5,6,7,8. Moreover, Losa et al. have reported that diabetes insipidus occurred in 5.9% of their patients and true cases of permanent diabetes insipidus requiring life-long replacement therapy with desmopressin composed 1.6% of their sample9. However, because GH deficiency typically does not have a significant clinical impact, supplements for GH insufficiency are not prescribed unless the patient presents with a severe, clinically manifesting GH deficiency10,11,12. Gonadal function is the most sensitive endocrine axis of the pituitary gland; thus, it is the most vulnerable to external mass compression. Patients with hypogonadism may present with decreased libido, bone density, muscle mass/strength and fatigue that interfere with their daily activities13. Therefore, our study focused on hypogonadism and subsequent testosterone supplementation in male patients. Although permanent impairment of pituitary endocrine function has been reported to be caused by tumors, apoplexy, or surgical manipulations, surgery remains the treatment of choice for NFPAs. Additionally, relieving the pressure on the normal pituitary may promote post-operative recovery from hypopituitarism. Arafah has reported that improvement in pituitary function occurred more often in patients with tumors measuring ≤25 mm in diameter than in patients with larger tumors14. However, Webb et al. have stated that, in both functioning pituitary macroadenomas and NFPAs, the tumor size did not differ significantly between patients who did and who did not recover pituitary function after surgery15. Regardless of size, a one-dimensional measurement (diameter alone) is not as accurate as a three-dimensional measurement (volume) in predicting post-operative pituitary function. Moreover, Webb et al. have also noted that endocrine recovery tended to be less common in patients with NFPAs after subtotal tumor resection (STR, 26% post-operative recovery) than after gross total tumor resection (GTR, 44% recovery), although this difference did not reach statistical significance (p = 0.146)15. Therefore, whether the pre-operative tumor volume or intraoperative resection ratio significantly impacts post-operative hormone deficiency and whether long-term supplementation is needed warrant further investigation. To clarify the impact of the tumor volume and resection ratio on hypogonadism, we sought to quantify this correlation by calculating the most accurate pre- and post-operative NFPA volume by using a more accurate method. We also calculated the resection/residual tumor ratio accordingly. The aim of this study was to assess the relationship between the pre-operative tumor volume, the resection/residual ratio and hypogonadism and, if possible, to predict the necessity of long-term hormone replacement.
Results
Patient Subgroups
Between 2004 and 2012, 45 male patients with NFPAs underwent a total of 52 operations; 7 patients underwent a second operation because of residual tumor progression. As shown in Table 1, 29 cases (55.8%) were in the large tumor group (LTG), whereas 23 cases (44.2%) were in the small tumor group (STG). We performed STR in 24 cases (45.8%) and GTR in the remaining 28 cases (54.2%). Post-operative magnetic resonance imaging (MRI) revealed a residual tumor ratio of <0.22 in 40 cases (76.9%) and ≥0.22 in 12 cases (23.1%). The pre-operative testosterone level was <1.5 ng/mL in 28 cases (53.8%) and ≥1.5 ng/mL in the remaining 24 cases (46.2%).
Correlation Coefficient between the Pre-operative Tumor Volume and the Serum Testosterone Level
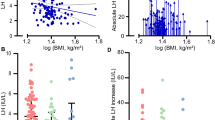
We found that a larger pre-operative tumor volume was negatively associated with the pre-operative serum testosterone level, as demonstrated by the scatter diagram shown in Fig. 1. We also calculated Pearson correlation coefficients, yielding a coefficient of −0.335 (p = 0.0151, adjusted R2 = 0.0946) and this result confirmed a negative relationship between the pre-operative tumor volume and the pre-operative testosterone level.
Subgroups Receiving Long-term Testosterone Replacement
The details about patients in different subgroups receiving long-term testosterone replacement are shown in Table 2. Most cases in the LTG (18/29, 62.1%) required long-term testosterone supplementation. Of the 12 cases in which the intraoperative residual ratio was ≥0.22, the majority (8/12, 66.7%) required testosterone supplementation. Of the 28 cases in which the pre-operative testosterone level was <1.5 ng/mL, 19 cases (67.9%) required testosterone supplementation. Moreover, of all 52 cases, 22 (42.3%) required long-term testosterone therapy and 48 of the 52 cases (92.3%) were diagnosed with GH deficiency. Furthermore, in all 22 cases that required long-term testosterone supplementation, 11 (50%) required concurrent long-term cortisone supplementation. Among these 11 cases, 2 (9.09%) required long-term thyroxine replacement, another case (4.55%) required desmopressin supplementation and a third case (4.55%) required simultaneous thyroxine and desmopressin treatment.
Univariate Logistic Regression and Categorical Analyses
To determine the cut-off value with the greatest sensitivity and most significant p value for predicting the need for long-term testosterone supplementation, we applied univariate logistic regression analysis. As shown in Table 3, when considering the tumor volume as a continuous variable, we found that the best sensitivity (0.8181) and an acceptable area under the receiver operating characteristic (ROC) curve (0.726) as well as the most significant p value (p = 0.0022) existed simultaneously at volumes of 7 and 8 cm3. As shown in Table 4, there was no obvious cut-off value of the residual ratio to predict the need for long-term testosterone replacement. As shown in Table 5, when considering the pre-operative testosterone level as a continuous variable, we found that at levels of 1.3, 1.4 and 1.5 ng/mL, the best sensitivity (0.8636), most acceptable area under the ROC curve (0.782) and most significant p value (p = 0.0003) existed simultaneously. As shown in Table 6, with regard to the categorical variables, also including the intraoperative resection status and patients who underwent second surgery or not, as our potential risk factors, chi-square and Fisher’s exact tests showed that there were significant differences in the need for long-term testosterone replacement based on the following cut-off values: tumor volume of 7 cm3 (p = 0.0012) and pre-operative testosterone level of 1.5 ng/mL (p < 0.0001). There were no statistically significant differences regarding the need for long-term testosterone replacement between the STR and GTR groups (p = 0.2986), in the residual ratio (cut-off value: 0.22, p = 0.0515) and in second surgery (p = 0.9999).
Evaluation of the Relationship between the Pre-operative Tumor Volume, the Serum Testosterone Level, the Residual Tumor Ratio, the Resection status, Second Surgery and the Need for Long-term Testosterone Therapy by Using Multivariate Logistic Regression Analysis
As shown in Table 7, when considering multiple variables together and the need for long-term testosterone therapy, we found that there were significant differences in the larger pre-operative tumor volume (p = 0.0067), the lower pre-operative testosterone level (p = 0.0101) and patients who underwent second surgery or not (p = 0.0250). There were no statistically significant differences in the resection status (p = 0.1059) or in the higher residual tumor ratio (p = 0.1040). As shown in Table 8, when considering the three significant factors shown in Table 7 together, we found that there were also significant differences in the larger pre-operative tumor volume (p = 0.0082), the lower pre-operative testosterone level (p = 0.0055) and patients who underwent second surgery or not (p = 0.0257). As shown in Table 9, we found that there were significant differences in the need for long-term testosterone therapy based on the following cut-off values: pre-operative tumor volume of 7 cm3 (p = 0.0144) and pre-operative testosterone level of 1.5 ng/mL (p = 0.0015). There was no statistically significant difference in patients who underwent second surgery or not (p = 0.1190). The choices and determination of the cut-off values will be discussed later.
Analysis of the Association between Risk Factors Associated with Long-Term Testosterone Therapy by Using Multivariate Logistic Regression Stratified by the Pre-Operative Tumor Volume
With regard to the residual tumor ratio, although it was determined to be an insignificant risk factor by the previous statistical results, the stratification analysis as shown in Table 10 revealed diverse statistically significant differences between the stratified pre-operative tumor volumes of <7 cm3 (p = 0.2723, insignificant) and ≥7 cm3 (p = 0.0367, significant) when regarding the higher residual tumor ratio as a risk factor of the need for long-term testosterone therapy. The same result was found when the stratified volume was at 8 cm3. Furthermore, the results of statistical analyses were not so diverse between volumes <9 cm3 (p = 0.3267, insignificant) and ≥9 cm3 (p = 0.0429, slightly significant). Moreover, the statistical analyses did not yield significant differences or diverse results between tumors with volumes of <10 cm3 (p = 0.3335) and ≥10 cm3 (p = 0.0608) and between tumors with volumes of <11 cm3 (p = 0.3617) and ≥11 cm3 (p = 0.1855). With regard to second surgery, there were no statistically significant differences between any of the subgroups stratified by the pre-operative tumor volume.
Discussion
Pituitary adenomas are benign tumors that arise from adenohypophyseal cells and represent 10–20% of all intracranial tumors16. NFPAs are often not diagnosed until they are sufficiently large to compress adjacent anatomical structures and cause visual disturbances, headaches and impaired pituitary function7,14,17,18. In patients with macroadenomas, hypopituitarism can be caused by three mechanisms: 1) compression of the pituitary stalk, which causes decreased availability of hypothalamic stimulatory hormones; 2) compression of functioning pituitary tissue; and 3) hypothalamic involvement of the pituitary tumor19.
Transsphenoidal surgery achieves the goal of alleviating endocrine, visual and other neurological defects by removing as much of the tumor as possible. The studies summarized in Table 11 included surgically treated pituitary macroadenomas, the majority of which were clinically non-functioning. After surgical decompression, recovery from hypopituitarism is possible if normal pituitary tissue is remaining15 and residual anterior pituitary function can be preserved and even improved after transsphenoidal surgery. If the pituitary tissue has been destroyed, the recovery of normal function is unlikely and lifelong hormone replacement therapy is required. Similar results have been obtained in recent studies20,21,22.
Pre-Operative Hypogonadism
In patients presenting with hypopituitarism, gonadal function was the most frequently observed impairment. Based on the mechanisms described above, the mass effect is clearly the dominating cause of pituitary insufficiency and gonadal function is the most vulnerable axis to external mass compression. Therefore, we focused on hypogonadism. Statistical analyses revealed that a larger tumor volume negatively impacted pre-operative hypogonadism (Fig. 1), which typically indicates a lower pre-operative testosterone level (Pearson correlation coefficient: −0.335, p = 0.0151, adjusted R2 = 0.0946). Therefore, a larger tumor volume would tend to cause more severe pre-operative hypogonadism. Although the pre-operative testosterone level is also a significant risk factor for post-operative testosterone replacement (p = 0.0101), we speculate that the tumor volume may be the primary factor causing long-term post-operative testosterone deficiency and that pre-operative hypogonadism may be only a secondary effect of the tumor volume. However, the pre-operative testosterone level could still be used as a predictor of the need for post-operative hormone supplementation.
Hypogonadism after Surgery
Dekkers et al. have reported that 90% of their patients were deficient in luteinizing hormone (LH) or follicle-stimulating hormone (FSH), 83% were GH-deficient, 60% were adrenocorticotropic hormone-deficient and 57% were thyroid stimulating hormone (TSH)-deficient post-operatively1. Our study revealed that, of the 52 cases, 22 (42.3%) required long-term testosterone therapy and 92.3% were also diagnosed as GH-deficient; however, we did not prescribe supplementation for GH deficiency as described above. We found that, of the 22 cases requiring testosterone replacement, 11 (50%) required concurrent long-term cortisone supplementation. Among these 11 cases, 2 cases (9.09%) required long-term thyroxine replacement, another case (4.55%) required desmopressin supplementation and a third case (4.55%) required simultaneous thyroxine and desmopressin treatment. In agreement with results from other studies, gonadal function was the most frequently affected hormonal axis that required replacement therapy both before and after surgery. Some studies have reported variable degrees of improvement in pituitary function after surgery4,6,8,15,23,24, whereas other studies have been unable to demonstrate a significant improvement in pituitary function2,7 or even have reported decreased pituitary function after transsphenoidal surgery2,16,25. Whether transsphenoidal surgery helps to improve pre-operative hypopituitarism remains a matter of debate. We propose two reasons for this uncertainty. First, although it is widely understood that larger tumors cause a lower pre-operative testosterone level, the exact cut-off value of the tumor volume for predicting recovery from hypogonadism is unknown. Therefore, even if adequate decompression is achieved in a patient whose tumor volume exceeds the cut-off value (e.g., 7 cm3 in our study), hypogonadism may not be ameliorated after the surgery. Second, excessively aggressive and blind surgical resection could result in unwanted pituitary gland damage.
Prediction of Post-Operative Pituitary Function
Before and during the operation, the prediction of post-operative pituitary function is important to enable the evaluation of whether the pituitary tissue will remain functionally viable after adequate surgical resection.
Determination of the Cut-Off Value
As we mentioned above, when considering tumor volume as the risk factor, the univariate logistic regression analysis produced the same results at volumes of 7 and 8 cm3. Although the same statistical results occurred for both volumes, a tumor volume larger than 7 cm3 may be associated with a risk of long-term hypogonadism at a lower threshold at an earlier time than a tumor volume ≥8 cm3. Thus, 7 cm3 may represent the optimal cut-off value of the tumor volume associated with the recovery of gonadal function and this threshold may predict the necessity of long-term hormone replacement. Conversely, when considering the pre-operative testosterone level as a risk factor, the univariate logistic regression analysis produced the same results at levels of 1.3, 1.4 and 1.5 ng/mL. Although the same statistical results were obtained in these three situations, a pre-operative testosterone level <1.5 ng/mL may predict a risk of long-term hypogonadism at a lower threshold at an earlier time than a level <1.3 ng/mL. Thus, 1.5 ng/mL may also represent the optimal cut-off value of the pre-operative testosterone level associated with the recovery of gonadal function and this threshold may also predict the necessity of long-term testosterone therapy. Regarding the residual tumor ratio, although a ratio of 0.22 was associated with a p value of 0.0595 and an odds ratio (OR) of 3.714 (0.949–14.541), the residual ratio was not such a strong risk factor in predicting the need for testosterone supplementation based on this statistical result. However, we still recognize the residual ratio as one of the potential risk factors influencing the need for post-operative long-term testosterone supplementation, as will be discussed in the following section.
Pre-operative Tumor Size and Volume
Arafah has observed more frequent improvement in pituitary function in patients with tumors measuring 2.5 cm or less than in patients with larger tumors14. However, Webb et al. have stated that in both functioning pituitary macroadenomas and NFPAs, the tumor size was not significantly associated with the improvement of pituitary function15; however, these authors divided the tumors into microadenoma and macroadenoma groups using a cut-off tumor diameter of 1 cm. Therefore, some tumors sized between 1 and 2.5 cm, which were categorized as macroadenomas in their study, may not exert a significant mass effect on the gland and may not require hormone replacement. Regardless of the aforementioned findings, the one-dimensional method of tumor measurement is not scientifically convincing. Therefore, to more accurately and scientifically assess these factors, we adopted a more quantitative volumetric method to calculate the tumor volume and determined the potential cut-off value of the tumor volume associated with the recovery of gonadal function to predict the necessity of long-term hormone replacement. From a statistical perspective, our study revealed that male patients with a larger pre-operative tumor volume have a greater risk of requiring post-operative long-term testosterone replacement (p = 0.0067, OR: 5.928, confidence interval [CI]: 1.637–21.465). Our study also revealed that the patients in the LTG (with a pre-operative tumor volume ≥7 cm3) exhibited an even greater risk for requiring post-operative long-term testosterone replacement than the patients in the STG, as determined by chi-square analysis (p = 0.0012, OR: 7.772, CI: 2.090–28.904) and multivariate logistic regression analysis (p = 0.0144, OR: 7.944, CI: 1.512–41.749). Therefore, a patient with a larger tumor, especially one ≥7 cm3, will have a greater risk of post-operative hypogonadism. Additionally, as mentioned above, a larger tumor volume would tend to cause more severe pre-operative hypogonadism. Based on our study, patients with more severe pre-operative hypogonadism, especially when the testosterone level is <1.5 ng/mL, will have a greater risk for the need for post-operative testosterone supplementation according to chi-square analysis (p < 0.0001, OR: 14.706, CI: 3.484–62.500) and multivariate logistic regression analysis (p = 0.0015, OR: 17.544, CI: 2.976–100.000). Therefore, the pre-operative testosterone level could also be used as a predictor of the need for testosterone replacement even though it may be only a secondary effect of the tumor volume.
Intraoperative Resection Status and Residual Tumor Ratio
We found that there was no significant difference between the GTR and STR groups in the need of post-operative long-term testosterone replacement by chi-square analysis (p = 0.2986) and multivariate logistic regression analysis (p = 0.1059). To explain the statistical results regarding GTR and STR, we assumed that we did our best to resect the tumor as much as possible during all operations to achieve optic nerve and pituitary gland decompression. In some cases, the operation was defined as STR because of the residual tumor tissue residing in the cavernous sinus rather than abutting the functional gland and these patients did not require long-term testosterone supplementation. The reason for this outcome was that, in these STR cases, the pituitary gland was also greatly decompressed, as demonstrated by intraoperative computed tomography (CT) and post-operative MRI, which rendered long-term testosterone supplementation unnecessary, as described in the GTR cases. Therefore, we suggest that even in probable STR cases, the tumor should be resected as much as possible during the operation to reduce the residual tumor ratio and, most importantly, to achieve decompression of the sella. In contrast, some other patients who received STR did require long-term testosterone supplementation. We found that these patients had a much larger tumor volume and greater paracavernous invasion (Fig. 2), which indicates that they were at a higher risk for requiring long-term testosterone supplementation because of the much larger mass effect, as discussed in the previous section despite the decompression of the sella (Fig. 3).
Regarding the residual ratio, although it was found to be insignificant based on chi-square analysis (cut-off value: 0.22, p = 0.0515), univariate logistic analysis (cut-off value: 0.22, p = 0.0595) and multivariate logistic regression analysis (p = 0.1040), the stratification analysis, as shown in Table 10, demonstrated statistically significant differences regarding the higher residual tumor ratio in the need of post-operative long-term testosterone supplementation, which may suggest that more residual tumor ratio is a possible risk factor for requiring long-term post-operative testosterone replacement. From a statistical perspective based on aforementioned results, we noted that there was a statistically significant difference between more residual tumor ratio and the need of long-term testosterone replacement once the tumor volume was larger than the stratified tumor volume (e.g., ≥7 cm3) compared with a smaller tumor volume (<7 cm3). Similar results were found when the stratified volumes were at 8 and 9 cm3, although the difference was not so significant when the volume was ≥9 cm3 (p = 0.0429). These results indicate that, although the pre-operative tumor volume is the primary cause of post-operative hypogonadism, a more residual tumor ratio may also carry a greater risk of the requirement of long-term testosterone replacement. Based on this result, we should make every effort to resect the tumor as much as possible, especially when it reaches a significant volume (i.e., 7 cm3), to reduce the residual ratio to achieve pituitary gland decompression. This result is in accordance with our previous conclusion. Additionally, we found that there were no significant differences in the need of long-term testosterone replacement when using a stratified volume of 10 and 11 cm3. This situation may be attributed to the fact that when the tumor exceeds our optimal cut-off value of 7 cm3 too much or in the case of extremely large tumors with paracavernous invasion even after a GTR attempt, the patient still has a greater risk of post-operative hypogonadism despite adequate decompression of the sella because of the much larger pre-operative tumor volume and its irreversible impact on post-operative hypogonadism. Therefore, these statistical outcomes may suggest that not only the pre-operative tumor volume but also the residual ratio may carry higher risks for the need for post-operative long-term testosterone supplementation.
As mentioned above, in GTR and probable STR cases, the goal of surgery was to achieve decompression of the sella and to reduce the residual tumor ratio; however, excessively aggressive resection should be avoided because it not only causes damage to the pituitary gland because of the blind surgical manipulation but also does not help to restore post-operative long-term pituitary function. Additionally, according to our previous report26, the extent of resection could be assessed as reliably with intraoperative CT as with post-operative MRI. As a result, the degree of sella clearance, tumor resection ratio and GTR/STR status could be determined via imaging as well as during the operation.
Second Surgery
Even though the multivariate logistic regression analysis showed a significant difference and a greater risk (OR: 183.160) in patients who underwent second operation (p = 0.0250) regarding the need of long-term post-operative testosterone replacement, the confidence interval ranged from 1.925 to 999.999, which may indicate that the result was not so convincingly significant. Two reasons for this result were hypothesized. First, the sample size may have been too small (only 7 cases). Second, we believe that the extreme significance of the pre-operative tumor volume and the testosterone level may have masked the importance of the second surgery. However, we still regard a second surgery as a potential risk factor for the need for long-term testosterone replacement not only because of the impact of recurrent/residual tumor itself but also because of the repeated surgical manipulations. However, further investigation should be performed by including more cases in the future.
In conclusion, regarding testosterone replacement in male patients, a larger pre-operative tumor volume (≥7 cm3), a lower pre-operative testosterone level (<1.5 ng/mL) and a higher residual tumor ratio are associated with a greater risk of the requirement of post-operative long-term testosterone replacement. Using intraoperative CT or MRI, after the sella is decompressed, we can terminate the surgical procedure, thus avoiding excessive, unnecessary and inappropriate surgical manipulation of the pituitary gland. We can predict the need for post-operative testosterone replacement pre-operatively based on the tumor volume, testosterone level and during the operation, we can eliminate unnecessary, harmful manipulation of the pituitary gland after the sella is decompressed.
Study Limitations
We believe that a second surgery is a potential risk factor for the need for long-term testosterone replacement; however, the sample size was small, which is a limitation of our study. By including more cases in the future, we could obtain more significant statistical results regarding the risk of a second surgery or other potential risk factors.
Methods and Materials
Patient Population
Between 2004 and 2012, 45 male patients with NFPAs were enrolled in this prospective long-term study. All patients underwent endonasal endoscopic transsphenoidal surgery for tumor removal and 7 patients required a second operation because of tumor recurrence. Pituitary function was assessed pre- and post-operatively. Routine post-operative MRI was performed within 3 months after surgery and annually thereafter. All experiments were performed in accordance with the relevant guidelines and regulations. The local ethics committee granted approval of the study and informed consent was obtained in each case. Approval was also obtained from the Institutional Review Board of Chang Gung Memorial Hospital.
Patient Subgroups
We divided the patients into different groups based on the pre-operative tumor volume, the pre-operative testosterone level, the resection status and the residual ratio (V2/V1 = post-operative volume/pre-operative volume). By applying univariate logistic regression, we found that at volumes of 7 and 8 cm3, which yielded the best sensitivity and p value and because we wanted to predict the need for long-term testosterone replacement, we had to choose the most sensitive value associated with the smallest tumor volume. Therefore, we could predict the need for long-term replacement at an earlier stage (smaller tumor volume). Conversely, when considering the pre-operative testosterone level, at levels of 1.3, 1.4 and 1.5 ng/mL at which the best sensitivity and p value existed, we had to choose the highest level to predict the need for long-term replacement at an earlier stage. Therefore, we determined a pre-operative tumor volume of 7 cm3 and a pre-operative testosterone level of 1.5 ng/mL as our cut-off values. Regarding the residual ratio, when the ratio was 0.22, although the p value did not reach a significant value, it approximately approached 0.05 (p value = 0.0595); therefore, we chose 0.22 as our cut-off value.
Assessment of Pituitary Function
A complete pituitary function test based on clinical signs and symptoms was performed to evaluate pituitary function and to reveal pre- and post-operative pituitary deficiencies. Basal hormonal measurements were obtained for all patients and the following criteria were used to define pituitary hormone deficiency1,27.
GH deficiency was defined as an IGF-I level below the reference range for age and sex28 and/or an insufficient increase in the GH level (absolute value <3 ng/mL) after stimulation during an insulin tolerance test. Hypocortisolism was diagnosed if the serum cortisol levels were low (<4.2 μg/dL) at 0800 h or below 1.7 μg/dL at 1600 h. Hypothyroidism was diagnosed if a subnormal serum-free T4 (FT4) level (<0.69 ng/dL) was associated with a low or normal TSH level (0.35–5.50 mIU/mL). In males, hypogonadism was diagnosed if the serum levels of testosterone were low (<2.4 ng/mL) in the presence of low or normal levels of gonadotropins (<10 IU/L). In postmenopausal women, hypogonadism was diagnosed if the serum LH and/or FSH levels were inappropriately low for the subject’s age (<1.5 mIU/mL). In premenopausal women, gonadotropin deficiency was diagnosed based on the presence of amenorrhea or oligomenorrhea and infertility, a low or low-normal basal level of gonadotropins (normal: LH, 2–16 mIU/mL; FSH, 2–10 mIU/mL) and persistently low estradiol levels (<30 pg/ml; <0.11 nmol/L). Diabetes insipidus was defined as polyuria not responding to fluid restriction but responding to vasopressin administration.
Imaging Interpretation
The surgeon interpreted the pre- and post-operative MRI findings. Subsequently, the neuroradiologist, who was blinded to the prior interpretation, provided an independent retrospective evaluation of the MRI in an attempt to decrease the reporting bias. The post-operative MRI findings were interpreted as GTR if enhancement included an exclusively normal pituitary gland and granulation tissue or as STR if residual enhancements in areas other than the normal pituitary gland and granulation tissue were evident.
Tumor Volume Calculation
To quantitatively define the tumor size and resection status, the volume of the tumor tissue was calculated based on the pre- and post-operative 1.5 T MRI using OsiriX software (www.osirix-viewer.com). We determined the pre-operative volume (V1), the post-operative volume (V2) and the residual tumor ratio (V2/V1), which was used to represent the completeness of the surgery. V1 and V2 were calculated as the averages of the tumor volume individually computed from the axial, sagittal and coronal images. According to Arafah, 2.5 cm is a critical cut-off tumor diameter for the improvement of pituitary function and the corresponding tumor volume is approximately (2.5)3/2 = 7.8125 cm3. Moreover, the univariate logistic regression showed that a volume of 7 cm3 was the best cut-off value, as mentioned above; thus, we used 7 cm3 as our cut-off value for the tumor volume. Tumors with a volume larger than 7 cm3 were categorized into the LTG; those measuring 7 cm3 or less were categorized into the STG. As previously noted, in cases defined as GTR, the volume obtained was associated with a normal pituitary gland and granulation tissue. To establish and standardize the average volume of the normal pituitary gland and the granulation tissue after GTR of a pituitary adenoma, we calculated the average volume of the residual pituitary gland and granulation tissue, which was regarded as the baseline value and ranged between 0.20 and 0.40 cm3 (mean ± 2SD). These data were obtained from 12 GTR patients in our previous report who were followed up for a long period after transsphenoidal pituitary surgery26.
Endocrine and Radiological Follow-Up
The endocrine tests that were performed pre-operatively were repeated at 7 days; at 1, 3, 6 and 12 months after surgery; and annually thereafter. After resection of a pituitary tumor, packing materials, post-operative debris, thickened mucosa and blood can interfere with imaging interpretation; however, these post-operative changes resolve within 3–4 months after surgery. Therefore, it is recommended to assess the effectiveness of surgery after approximately 3 months and annually thereafter for long-term follow-up after the initial surgery. The first post-operative MRI was performed within 3 months of surgery in most of our cases. Subsequent surveillance imaging studies were conducted at 1-year intervals for 2–3 years and then at longer intervals. However, patients with residual tumors received more frequent follow-ups.
Hormone Replacement
The hormone replacement regimen depended on the laboratory data and the clinical symptoms. Because clinical symptoms are always subtle, we initiated hormone replacement therapy primarily based on the laboratory data. If an endocrine deficiency was found, adequate substitution was initiated using hydrocortisone, thyroxine, gonadal steroids and desmopressin as appropriate. In our study, we focused on hypogonadism and prescribed testosterone supplementation for male patients with a serum testosterone level below 2.4 ng/mL or who exhibited clinical symptoms of hypogonadism. Hormone replacement for longer than 1 year was considered to indicate a “long-term” need for hormone supplementation.
Statistical Methodology
The chi-square test and Fisher’s exact test for independence were used to determine the statistical significance of the differences in the need for post-operative long-term testosterone replacement between different categorical variables. Univariate logistic regression analysis was performed to analyze the continuous variables and to determine the best cut-off values of the pre-operative tumor volume, testosterone level and residual tumor ratio. Multiple logistic regression analysis was performed to determine which variables and whether the chosen cut-off values independently predicted the need for long-term testosterone replacement. In all cases, a difference was considered significant if p < 0.05. SAS (Statistical Analysis System) software (version 9.3) was used (SAS Institute Inc., 100 SAS Campus Drive, Cary, NC 27513–2414, USA).
Conclusions
The pre-operative tumor volume and pre-operative testosterone level significantly impacted post-operative hypogonadism and the need for hormone replacement, especially when the pre-operative tumor volume was ≥7 cm3 or testosterone level <1.5 ng/mL. The residual tumor ratio and degree of sella decompression may also influence the long-term need for testosterone supplementation. We advocate using volumetric MRI techniques and direct volume measurements to evaluate pituitary tumors, their impact on the pituitary gland and the tumor resection ratio because these measurements can provide the most reliable results and can help surgeons to predict the degree of post-operative endocrine deficiency and the need for hormone replacement based on either the pre-operative tumor volume or to intraoperatively manipulate the degree of tumor resection.
Additional Information
How to cite this article: Lee, C.-C. et al. Prediction of Long-term Post-operative Testosterone Replacement Requirement Based on the Pre-operative Tumor Volume and Testosterone Level in Pituitary Macroadenoma. Sci. Rep. 5, 16194; doi: 10.1038/srep16194 (2015).
References
Dekkers, O. M. et al. Observation alone after transsphenoidal surgery for nonfunctioning pituitary macroadenoma. J Clin Endocrinol Metab. 91, 1796–1801 (2006).
Wichers, R. M., Hoven, S., Kristof, R. A., Bliesener, N. & Stoffel, W. B. Non-functioning pituitary adenomas: endocrinological and clinical outcome after transsphenoidal and transcranial surgery. Exp Clin Endocrinol Diabetes. 112, 323–327 (2004).
Alameda, C. et al. Experience in management of 51 non-functioning pituitary adenomas: indications for post-operative radiotherapy. J Endocrinol Invest. 28, 18 –22 (2005).
Marazuela, M. et al. Recovery of visual and endocrine function following transsphenoidal surgery of large nonfunctioning pituitary adenomas. J Endocrinol Invest. 17, 703–707 (1994).
Nomikos, P., Buchfelder, M. & Fahlbusch, R. The outcome of surgery in 668 patients with acromegaly using current criteria of biochemical ‘cure’. Eur J Endocrinol. 152, 379–387 (2005).
Arafah, B. M., Kailani, S. H., Nekl, K. E., Gold, R. S. & Selman, W. R. Immediate recovery of pituitary function after transsphenoidal resection of pituitary macroadenomas. J Clin Endocrinol Metab. 79, 348–354 (1994).
Comtois, R. et al. The clinical and endocrine outcome to trans-sphenoidal microsurgery of non-secreting pituitary adenomas. Cancer. 68, 860–866 (1991).
Nomikos, P., Ladar, C., Fahlbusch, R. & Buchfelder, M. Impact of primary surgery on pituitary function in patients with non-functioning pituitary adenomas—a study on 721 patients. Acta Neurochir (Wien). 146, 27–35 (2004).
Losa, M. et al. Early results of surgery in patients with nonfunctioning pituitary adenoma and analysis of the risk of tumor recurrence. J. Neurosurg. 108, 525–532 (2008).
Dekkers, O. M. et al. Quality of life is decreased after treatment for nonfunctioning pituitary macroadenoma. J Clin Endocrinol Metab. 91, 3364–3369 (2006).
Auernhammer, C. J. & Vlotides, G. Anterior pituitary hormone replacement therapy—a clinical review. Pituitary. 10, 1–15 (2007).
Vnitrni, L. Hypopituitarism–substitution therapy. Vnitr Lek. 53, 812–815 (2007).
Bassil, N., Alkaade, S. & Morley, J. E. The benefits and risks of testosterone replacement therapy: a review. Ther Clin Risk Manag. 5, 427–448 (2009).
Arafah, B. M. Reversible hypopituitarism in patients with large nonfunctioning pituitary adenomas. J Clin Endocrinol Metab. 62, 1173 (1986).
Webb, S. M., Rigla, M., Wagner, A., Oliver, B. & Bartumeus, F. Recovery of hypopituitarism after neurosurgical treatment of pituitary adenoma. J. Clin. Endocrinol. Metab. 84, 3696–3700 (1999).
Kovacs, K., Scheithauer, B. W., Horvath, E. & Lloyd, R. V. The World Health Organization classification of adenohypophysial neoplasms. A proposed five-tier scheme. Cancer. 78, 502–510 (1996).
Klibanski, A. Nonsecreting pituitary tumors. Endocrinol Metab Clin North Am. 16, 793–804 (1987).
Losa, M., Mortini, P., Barzaghi, R., Franzin, A. & Giovanelli, M. Endocrine inactive and gonadotroph adenomas: diagnosis and management. J Neurooncol. 54, 167–177 (2001).
Dekkers, O. M., Pereira, A. M. & Romijn, J. A. Treatment and follow-up of clinically nonfunctioning pituitary macroadenomas. J Clin Endocrinol Metab. 93, 3717–3726 (2008).
Arafah, B. M. et al. Recovery of pituitary function following surgical removal of large nonfunctioning pituitary adenomas. Clinical Endocrinology. 17, 213–222 (1982).
Arafah, B. M., Harrington, J. F., Madhoun, Z. T. & Selman, W. R. Improvement of pituitary function after surgical decompression for pituitary tumor apoplexy. J Clin Endocrinol Metab. 71, 323–328 (1990).
Nelson, A. T., Jr., Tucker, H. S., Jr. & Becker, D. P. Residual anterior pituitary function following transsphenoidal resection of pituitary macroadenomas. J. Neurosurg. 61, 577–580 (1984).
Aron, D. C. & Howlett, T. A. Pituitary incidentalomas. Endocrinol Metab Clin North Am. 29, 205–221 (2000).
Greenman, Y. et al. Relative sparing of anterior pituitary function in patients with growth hormone-secreting macroadenomas: comparison with nonfunctioning macroadenomas. J Clin Endocrinol Metab. 80, 1577–1583 (1995).
Greenman, Y. et al. Post-operative surveillance of clinically nonfunctioning pituitary macroadenomas: markers of tumour quiescence and regrowth. Clinical Endocrinology. 58, 763–769 (2003).
Lee, C. C. et al. Volumetric Measurement for Comparison of the Accuracy between Intraoperative Computed Tomography and Post-operative Magnetic Resonance Imaging in Pituitary Adenoma Surgery. Am J Neuroradiol. 32, 1539–1544 (2011).
Lindholm, J. et al. Hypopituitarism and mortality in pituitary adenoma. Clin Endocrinol (Oxf). 65(1), 51–58 (2006).
Hartman, M. L. et al. Which patients do not require a GH stimulation test for the diagnosis of adult GH deficiency? J Clin Endocrinol Metab. 87, 477–485 (2002).
Acknowledgements
This work was supported by the Institute of Biomedical Engineering of National Taiwan University, the Department of Neurosurgery of Chang Gung Memorial Hospital and Chang Gung University, Taoyuan, Taiwan. The English editing was performed by Nature Publishing Group (NPG) language editing and we sincerely appreciate their contributions and help.
Author information
Authors and Affiliations
Contributions
C.L. and C.C. wrote the main manuscript text; C.C. and S.L. collected the patient data; C.L. calculated the tumor volumes and prepared the figures and tables; P.P. and C.T. calculated and assessed the tumor volumes; C.C. helped to improve the quality of the figures for more precise measurement of the tumor volumes; and K.W. performed the statistical analyses. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Lee, CC., Chen, CM., Lee, ST. et al. Prediction of Long-term Post-operative Testosterone Replacement Requirement Based on the Pre-operative Tumor Volume and Testosterone Level in Pituitary Macroadenoma. Sci Rep 5, 16194 (2015). https://doi.org/10.1038/srep16194
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep16194
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.