Abstract
The remarkable properties of graphene and transition metal dichalcogenides (TMDCs) have attracted increasing attention on two-dimensional materials, but the gate oxide, one of the key components of two-dimensional electronic devices, has rarely reported. We found the single-layer oxide can be used as the two dimensional gate oxide in 2D electronic structure, such as TiO2. However, the electrical performance is seriously influenced by the defects existing in the single-layer oxide. In this paper, a nondestructive and noncontact solution based on spectroscopic ellipsometry has been used to detect the defect states and energy level of single-layer TiO2 films. By fitting the Lorentz oscillator model, the results indicate the exact position of defect energy levels depends on the estimated band gap and the charge state of the point defects of TiO2.
Similar content being viewed by others
Introduction
Recently, many two-dimensional materials, such as graphene1,2 and TMDCs3,4, have received increasing attention due to their potential for applications in 2D nanoelectronic devices5,6. In fact, both the graphene and the TMDCs, used as channel materials in devices, are special materials of thin electrical and thermal conductor, with high carrier mobility properties. However, 2D nanoelectronic devices won’t be widely applied without the suitable 2D dielectric materials, which have rarely reported (except h-BN2,7). Due to the polar nature of the gate dielectrics used in MOSFETs, the carriers in the conducting channel couple electrostatically to the long-range polarization field created at the conductor/dielectric interface13. As a result, carrier mobility in two dimensional nanoelectronic devices is limited by surface optical phonons13,14, also limited by scattering from charged surface states and impurities8,9,14 and substrate surface roughness10,11,12. Even hexagonal boron nitride (h-BN) is an appealing substrate dielectric for use in improved 2D nanoelectronic devices, because it has an atomically smooth surface that is relatively free of dangling bonds and charge traps7, but h-BN is not suitable for being used in semiconductor manufacturing process, for it cannot be widely used in the CMOS manufacturing technology. As a potential 2D gate dielectric materials, single-layer TiO2 films can be act as the gate oxide in the 2D electronic devices.
With ~0.7 nm monolayer thickness15, single layer TiO2 is considered to be composed entirely of surface atoms, which endows its many special properties different from bulk titania, such as high crystallinity, wide bandgap of ~3.84 eV16, high chemical and thermal stability and large refractive index value, thus it involves certain useful applications, such as photocatalysts17, semiconductors18,19 and dielectric materials20. When the single-layer TiO2 films act as the gate dielectric layer, the electronic performance can be seriously affected by various defects and the leakage current will occur in the oxide gate dielectric layer due to the electron trapping effect. In order to understand the influence of defect disorder on the electronic properties, it is essential to clarify the energetic position of the various defects inside the band gap, which is not yet available.
In general, deep-level transient spectra (DLTS) and thermally stimulated current (TSC) methods are used to measure the electrical levels of film defects. However, these methods have some limitations and special requirements for samples, hence they are difficult for this application. In this article, a nondestructive and noncontact method21 based on spectroscopic ellipsometry was introduced to investigate the optical properties and electrical levels of point defects of single-layer TiO2. Samples were fabricated by immersing the substrates in a colloidal suspension of single-layer TiO2 films and investigated by atomic force microscopy (AFM), X-ray photoelectron spectroscopy (XPS) and spectroscopic ellipsometry (SE). Based on the detailed SE analysis and fitted by the Lorentz oscillator model, we got the electrical levels of various different charged defects in single-layer TiO2 films.
Results
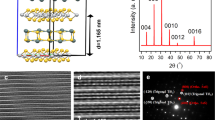
The AFM results are shown in Fig. 1. After being immersed in a colloidal suspension of single-layer TiO2 films for 20 minutes, a few packed single-layer TiO2 films with non-ignorable gaps have been deposited on SiO2/Si substrate. The surface morphology of these samples is depicted in Fig. 1(a–c), while based on results from Fig. 1(e–g), the edge thickness of these samples ranges from 1.3 to 1.75 nm. In addition, the crystal structure model of the single-layer TiO2 was shown in Fig. 1(i). Ti atom is coordinated with six oxygen atoms and resulting TiO6 octahedra are joined via edge-sharing to produce the 2D lattice22.
AFM analysis of single-layer TiO2 samples.
The top column depicts the surface morphology of different samples: immersed in a colloidal suspension of single-layer TiO2 for 20 minutes for (a) sample 1, (b) sample 2 and (c) sample 3 and (d) immersed in colloidal suspension having been diluted 100 times for 30 seconds. The bottom is the height profile of marked line in (a–d), respectively and structure model of single-layer Ti1-δ O2 in (I).
Quantitative XPS analysis was measured on single-layer TiO2 samples to characterize the chemical state of the samples. As shown in Fig. 2(a,b), the spectra of Ti 2p and O 1s are observed at binding energies of 456–467 and 528–535 eV, respectively. From Fig. 2(c), the Ti 2p spectra of sample 1 is fitted with the Ti 2p1/2 and Ti 2p3/2 spin-orbital splitting photoelectrons peaks (area ratio is 2), located at binding energies of 464.03 and 458.35 eV, respectively. The FWHM of the Ti 2p3/2 signal was 1.206 eV for sample 1. Also the O 1s signal in Fig. 2(d) is fitted with two peaks: O 1s peak of Si-O species at 532.75 eV and O 1s peak of Ti-O species at 530.08 eV, closely resembling the reported values23,24. The values of the FWHM of the peaks were 1.409 eV and 1.391 eV, respectively.
According to XPS results, even the binding energy of Ti 2p peaks closely resemble the reported literature spectra25,26,27 and the peak separation of 5.68 eV between the Ti 2p1/2 and Ti 2p3/2 signals agree well with the reported values28, the binding energy of Ti 2p peaks still has a slight chemical shift to lower binding energy28. Furthermore, the ratio of titanium to oxygen in single-layer TiO2 samples, determined by integrating the areas under the Ti 2p and O 1s peaks and correcting the areas by the respective Scofield photoionization cross sections of the core level photoelectrons29, was about 0.748: 1. Therefore, we can deduce that the three single-layer samples exist oxygen vacancies.
Figure 3 shows the spectroscopic ellipsometry results of the single-layer TiO2. To investigate the defect states and energy levels of single-layer TiO2 films, three Lorentz oscillators are used in the data analysis with key parameters listed in Table 1. Besides one oscillator used to describe the band-gap energy of single-layer TiO2 samples, two other oscillators in LOM were adopted to characterize two different charged defects.
From Fig. 3, the solid curves generated from the LOM dispersion obtained by fitting described above exhibited good agreement with experimental data. Also the thickness of three samples measured from atomic force microscopy were about 1.3, 1.4 and 1.75 nm, respectively, closely resembling the value of SE fitting results. Therefore, the three-oscillator model is suitable to characterize the single layer of TiO2 with different defects. However, the calculated results presented the value of A3 is the largest of A1–A3, indicating C3 is dominant oscillator of C1–C3 for three samples and the oscillator center energies Ci for samples converge to three average values, 1.85 eV, 2.22 eV and 4.05 eV.
According to the definition of the LOM, these oscillators are fundamental characteristics of single-layer TiO2 with defects. While the probability of the electronic transitions from the conduction band to valance band or defect traps was expressed by the parameter Ai, whose value represents the percentage contribution of oscillator i in the whole system. According to Table 1, the value of A3 is the largest of A1–A3 and the oscillator 3 center energy of 4.02 eV is very close to the bandgap energy of single-layer TiO2 (3.8 eV30), much larger than that of anatase TiO2 (3.2 eV), resulting in size quantization effects. Hence, the calculated band-gap energy for three single-layer TiO2 samples should be about 4.02 eV. Therefore, the remaining two oscillators 1 and 3 are used to characterize the two different charged defects appearance in the single-layer TiO2 samples. Combined with the XPS results, the center energies of C1 (1.89 eV) and C3 (2.22 eV) can be explained as two different oxygen vacancy defects. As shown in Fig. 4, the band structure of titania ultrathin films assembled by single-layer TiO2 films is obtained. In addition, the value of A1 is notably larger than A2, indicating the center energy located at 1.89 eV is the dominant defect configuration.
Discussion
Figure 1(a–c) show the surface morphology of those samples which were immersed in colloidal suspension of single-layer TiO2 films for 20 minutes. From Fig. 1(e–g), the edge thickness of these samples ranges from 1.3 to 1.75 nm. However, the crystallographic height of single-layer TiO2 (0.7 nm) consist of the vertical distance between the levels of upper and bottom oxygen atoms of the host layer (0.42 nm) and the ionic radius of these two oxygen atoms (0.28 nm)31. There is big difference between the experimental thickness and crystallographic height. In order to ensure the ultrathin films assembled by single-layer TiO2, one substrate had been immersed in colloidal suspension having been diluted 100 times for 30 seconds. Then this sample was investigated by AFM, as shown in Fig. 1(d). While Fig. 1(h) shows that the edge thickness of the single-layer TiO2 films was ~1.5 nm. As a result, those samples shown in Fig. 1(a–c) were assembled by single-layer TiO2 films. However, the difference between the experimental thickness and crystallographic height is mainly caused by adsorbed charge-compensating protons, oxonium ions, or water molecules, as is the case for other nanosheets19,32,33,34,35.
In summary, we successfully deposited ~1.5 nm single-layer TiO2 on substrate and the properties of single layer TiO2 films have been investigated by AFM, XPS and SE. The results of XPS demonstrate that some oxygen vacancy defects were formed in single-layer TiO2 samples. Also the parameters extracted from SE data by Lorentz oscillator model fitting illustrate that the probabilities and transition energies for different charged oxygen vacancy defects. Furthermore, the thickness of samples measured by atomic force microscopy exhibits excellent agreement with spectroscopic ellipsometry fitting values. Therefore, the method based on spectroscopic ellipsometry investigating the optical properties and electrical levels of point defects of single-layer TiO2 is appropriate. By fitting the Lorentz oscillator model, we get the electrical levels of different charged defects in single-layer of TiO2.
Methods
Fabrication of colloidal suspension of single-layer TiO2
According to the previous reported method37, reagents such as TiO2, K2CO3, Li2CO3 and MoO3 were mixed with a molar ratio of 1.73: 1.67: 0.13: 1.27. Then, the mixture was placed in a Pt crucible and reacted at 1473 K. After keeping this temperature for 10 h, the mixture was cooled spontaneously when the temperature reached 1223 K. By dissolving a K2MoO4 flux melt with water, the titanate crystals of K0.8[Ti1.73Li0.27]O4 were recovered and converted into a protonic form. After stirred in a 0.5 mol dm−3 HCl solution (2 dm3) at room temperature for 5 days, the acid-exchanged crystals, H1.07Ti1.73O4·H2O, were collected. Then, the protonic titanate crystals, H1.07Ti1.73O4·H2O, was attempted by reaction with a tetrabutylammonium hydroxide solution ((C4H9)4NOH; hereafter TBAOH) and little bit of protonic titanate was immersed in the TBAOH solution. After 10 days of vigorous shaking, colloidal suspension of single-layer TiO2 films was obtained.
Fabrication and Measurement
SiO2/Si wafers (2 × 2 cm2) were cleaned by ultrasonic treatment in acetone for 15 minutes, followed by ultrasonic treatment in absolute ethyl alcohol for 5 minutes. Before being immersed in a colloidal suspension of single-layer TiO2 films for 20 min, the SiO2/Si wafers were washed with copious water, as seen in Fig. 5. It should be noticed that the samples should be washed with water and dried before characterization. The surface topography and the thickness of three samples were measured under the ambient conditions by using a Veeco MultiMode VIII instrument equipped with a Nanoscope V controller. The XPS spectra of the samples were measured using ultra high resolution XPS analyzer PHOIBOS of SPECS customized UHV surface analysis system, while the SE spectra of the samples were measured using SOPRA GES5E Spectroscopic Ellipsometer range from 260 nm to 800 nm with a fixed incidence angle of 74°.
Ellipsometry Modelling and Fitting
Ellipsometry is an optical technique used to investigate the dielectric properties of thin films that exploits phase information and the polarization state of light and the physical thickness measurement can reach to angstrom resolution36. Also the Lorentz oscillator model (LOM) has been demonstrated that it is an effective way to fit the ellipsometric parameters tan Ψ and cos Δ, which are defined as

where rp and rs are the complex reflection coefficients of polarized light parallel and perpendicular to the incidence plane, respectively.
The SE spectra of the samples were analyzed by building a four-phase simple model structure consisting of substrate Si/SiO2/film (TiO2)/ambient. In this model, fitting variables include the unknown parameters of film thickness (d) and dielectric constant (ε). However, the LOM is suitable to characterize the dielectric function of the single-layer TiO2 films as follows:

where ε∞ is the light-frequency dielectric constant; Ai, Ci and vi are the amplitude, center energy and damping coefficient of each oscillator in eV, respectively. The Ai value also represents the percentage contribution of oscillator i in the whole system.
Additional Information
How to cite this article: Song, X. F. et al. Electrical level of defects in single-layer two-dimensional TiO2. Sci. Rep. 5, 15989; doi: 10.1038/srep15989 (2015).
References
Li, X. et al. Large-area synthesis of high-quality and uniform graphene films on copper foils. Science 324, 1312–1314 (2009).
Liu, Z. et al. Direct growth of graphene/hexagonal boron nitride stacked layers. Nano Lett. 11, 2032–2037 (2011).
Mattheis, L. F. Band structures of transition-metal-dichalcogenide layer compounds. Phys. Rev. B 8, 3719–3740 (1973).
Wilson, J. A. & Yoff, A. D. The transition metal dichalcogenides discussion and interpretation of the observed optical, electrical and structural properties. Adv. Phys. 18, 193–335 (1969).
Radisavljevic, B., Radenovic, A., Brivio, J., Giacometti, V. & Kis, A. Single-layer MoS2 transistors. Nature Nanotech. 6, 147–150 (2011).
Berger, C. et al. Electronic confinement and coherence in patterned epitaxial graphene. Science 312, 1191–1196 (2006).
Dean, C. R. et al. Boron nitride substrates for high-quality graphene electronics. Nature Nanotech. 5, 722–726 (2010).
Ando, T. Screening effect and impurity scattering in monolayer graphene. J. Phys. Soc. Jpn. 75, 074716 (2006).
Hwang, E. H., Adam, S. & Sarma, S. D. Carrier transport in two-dimensional graphene layers. Phys. Rev. Lett. 98, 186806 (2007).
Ishigami, M., Chen, J. H., Cullen, W. G., Fuhrer, M. S. & Williams, E. D. Atomic structure of graphene on SiO2 . Nano Lett. 7, 1643–1648 (2007).
Katsnelson, M. I. & Geim, A. K. Electron scattering on microscopic corrugations in graphene. Philos. T. Roy. Soc. A 366, 195–204 (2007).
Morozov, S. V. et al. Giant intrinsic carrier mobilities in graphene and its bilayer. Phys. Rev. Lett. 100, 145–150 (2008).
Fratini, S. & Guinea, F. Substrate-limited electron dynamics in graphene. Phys. Rev. B 77, 195415 (2008).
Chen, J. H., Jang, C., Xiao, S., Ishigami, M. & Fuhrer, M. S. Intrinsic and extrinsic performance limits of graphene devices on SiO2 . Nature Nanotech. 3, 206–209 (2008).
Sasaki, T., Ebina, Y., Kitami, Y. & Watanabe, M. Two-dimensional diffraction of molecular nanosheet crystallites of titanium oxide. J. Phys. Chem. B. 105, 6116–6121 (2001).
Sakai, N., Ebina, Y., Takada, K. & Sasaki, T. Electronic band structure of titania semiconductor nanosheets revealed by electrochemical and photoelectrochemical studies. J. Am. Chem. Soc. 126, 5851–5858 (2004).
Shibata, T., Sakai, N., Fukuda, K., Ebina, Y. & Sasaki, T. Photocatalytic properties of titania nanostructured films fabricated from titania nanosheets. Phys. Chem. Chem. Phys. 9, 2413–2420 (2007).
Sasaki, T. & Watanabe, M. Semiconductor nanosheet crystallites of quasi-TiO2 and their optical properties. J. Phys. Chem. B. 101, 10159–10161 (1997).
Xiang, Q., Yu, J. & Jaroniec, M. Graphene-based semiconductor photocatalysts. Chem. Soc. Rev. 41, 782–796 (2012).
Osada, M. et al. High-κ dielectric nanofilms fabricated from titania nanosheets. Adv. Mater. 18, 1023–1027 (2006).
Chen, Y. R., Zhou, P. & Chen, L. Y. Electrical levels of defect investigation of ZrO2 thin film by spectroscopic ellipsometry. J. Vac. Sci. Technol. B. 27, 1030–1034 (2009).
Osada, M. & Sasaki, T. Two-dimensional dielectric nanosheets: novel nanoelectronics from nanocrystal building blocks. Adv. Mater. 24, 210–228 (2012).
McCafferty, E. & Wightman, J. P. Determination of the concentration of surface hydroxyl groups on metal oxide films by a quantitative XPS method. Surf. Interface Anal. 26, 549–564 (1998).
Nefedov, V. I., Gati, D. & Dzhurinskii, B. F. X-ray electron studies of oxides of some element. Zh. Neorg. Khim. 20, 2307–2314 (1975).
Sanjinés, R. et al. Electronic structure of anatase TiO2 oxide. J. Appl. Phys. 75, 2945–2951 (1994).
Netterfield, R. P. et al. Ion-assisted deposition of mixed TiO2-SiO2 films. J. Appl. Phys. 66, 1805–1809 (1989).
Ong, J. L., Lucas, L. C., Raikar, G. N. & Gregory, J. C. Electrochemical corrosion analyses and characterization of surface-modified titanium. Appl. Surf. Sci. 72, 7–13 (1993).
Hofmann, S. & Sanz, J. M. Quantitative XPS analysis of the surface layer of anodic oxides obtained during depth profiling by sputtering with 3 keV Ar+ ions. J. Trace Micropobe Tech. 1, 213–264 (1982).
Scofield, J. H. Hartree-Slater subshell photoionization cross-sections at 1254 and 1487 eV. J. Electron. Spectrosc. Relat. Phenom. 8, 129–137 (1976).
Wang, L. & Sasaki, T. Titanium oxide nanosheets: graphene analogues with versatile functionalities. Chem. Rev. 114, 9455–9486 (2014).
Dong, X. et al. Synthesis of mn-substituted titania nanosheets and ferromagnetic thin films with controlled doping. Chem. Mater. 21, 4366–4373 (2009).
Ozawa, T. C., Fukuda, K., Akatsuka, K., Ebina, Y. & Sasaki, T. Preparation and characterization of the Eu3+ doped perovskite nanosheet phosphor: La0.90Eu0.05Nb2O7 . Chem. Mater. 19, 6575–6580 (2007).
Li, L., Ma, R., Ebina, Y., Iyi, N. & Sasaki, T. Positively charged nanosheets derived via total delamination of layered double hydroxides. Chem. Mater. 17, 4386–4391 (2005).
Ma, R., Liu, Z., Li, L., Iyi, N. & Sasaki, T. Exfoliating layered double hydroxides in formamide: a method to obtain positively charged nanosheets. J. Mater. Chem. 16, 3809–3813 (2006).
Ma, R. et al. Synthesis and exfoliation of Co2+-Fe3+ layered double hydroxides: an innovative topochemical approach. J. Am. Chem. Soc. 129, 5257–5263 (2007).
Shen, Y. et al. Evolution of the band-gap and optical properties of graphene oxide with controllable reduction level. Carbon 62, 157–164 (2013).
Tanaka, T., Ebina, Y., Takada, K., Kurashima, K. & Sasaki, T. Oversized titania nanosheet crystallites derived from flux-grown layered titanate single crystals. Chem. Mater. 15, 3564–3568 (2003).
Acknowledgements
Project supported by the National Natural Science Foundation of China (61376093), Shanghai Municipal Science and Technology Commission (13QA1400400), National Science and Technology Major Project (2011ZX02707) and Innovation Program of Shanghai Municipal Education Commission (12ZZ010).
Author information
Authors and Affiliations
Contributions
X.F.S. and D.H.L. contribute to performing the experiment and drafting the manuscript. L.F.H. and P.Z. contribute to designing this research and analyzing the results. L.C., Q.S. and D.W.Z. analyzed the data and discussed the results. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Song, X., Hu, L., Li, D. et al. Electrical level of defects in single-layer two-dimensional TiO2. Sci Rep 5, 15989 (2015). https://doi.org/10.1038/srep15989
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep15989
This article is cited by
-
Understanding and prediction of metastable single-layer metallene oxides
Science China Materials (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.