Abstract
Dietary fats are not created equally, slight differences in structure lead to crucial differences in function. Muticellular organisms use polyunsaturated fatty acid as substrates to produce potent signaling molecules crucial for many physiological processes, including reproduction. Here we explored the mechanism responsible for germ cell loss induced by dietary supplementation of dihomo-gamma-linolenic acid (DGLA, 20:3n-6) in the roundworm Caenorhabditis elegans. In this study we found that C. elegans CYP-33E2 activity produces a range of epoxy and hydroxy metabolites from dietary DGLA. Knockdown of cyp-33E2 suppressed the DGLA-induced sterility phenotype. Additionally, direct exposure of two specific DGLA-derived epoxy products, 8,9- and 14,15-epoxyeicosadienoic acids, produced germ cell abnormalities in the C. elegans gonad. We propose that sterility is mediated by the production of toxic DGLA-derived epoxides that trigger germ cell destruction. These studies are the first to establish a biological activity for a CYP-produced metabolite of DGLA.
Similar content being viewed by others
Introduction
Liberation of polyunsaturated fatty acids (PUFAs) from phospholipids leads to the formation of powerful, short range hormones influencing diverse functions necessary for development and physiology1,2. Cyclooxygenase and lipoxygenase enzymes act on PUFAs to produce prostaglandins, leukotrienes and thromboxanes, collectively called eicosanoids3. The most well studied bioactive eicosanoids belong to series 2, indicating they are derived from arachidonic acid (AA, 20:4n-6). However, series 1 eicosanoids derived from dihomo-gamma-linolenic acid (DGLA, 20:3n-6) or series 3 eicosanoids derived from eicosapentaenoic acid (EPA, 20:5n-3) also exhibit biological activity. Eicosanoid mediators are best known for mediating pain and inflammation, but also regulate other physiological processes such as wound healing, bone metabolism, blood pressure, immune responses, ovulation, embryo implantation and the initiation of labor4,5,6. In Western diets, AA, an omega-6 fatty acid is often in excess, leading to an overproduction of pro-inflammatory eicosanoids. Dietary omega-3 fatty acids such as EPA, found in fish oil, are thought to be beneficial because eicosanoids derived from omega-3 fatty acids produce anti-inflammatory effects7.
A less understood class of eicosanoids are produced by cytochrome P450 monooxygenases (CYPs), which are a diverse collection of enzymes acting on various endogenous and xenobiotic molecules8. Fatty acid derived eicosanoids produced by the CYP pathway function in vascular dilation, angiogenesis, pain nociception and protection from ischemic/reperfusion damage1,9,10,11. In addition, epoxyeicosatrienoic acids (EETs) generated from AA by CYP epoxygenases were found to stimulate extensive multiorgan metastasis and escape from tumor dormancy in several tumor models12,13. Very few studies examining the direct effects of DGLA in mammals have been performed, although several cyclooxygenase- and lipoxygenase-derived eicosanoids appear to have anti-inflammatory and anti-proliferative properties14. Even though a great deal has been learned about eicosanoid formation and function, progress on elucidating beneficial and harmful functions of the thousands of diverse mediators has been limited due to the challenges imposed by low concentration, limited stability and restricted knowledge of receptors for many eicosanoid species. In particular, the biosynthesis and biological activity of DGLA-derived CYP-eicosanoids has never been described before.
Studies using C. elegans are beginning to shed light on the activities of eicosanoids in developmental processes of invertebrates2,15. Our previous work has identified the involvement of PUFAs in the maintenance of germ cells. Specifically, wild type C. elegans become sterile in a dose-dependent manner when grown on plates containing DGLA16. This sterility phenotype is remarkably specific, as high concentrations of dietary omega-3 fatty acids with similar physical properties did not affect fertility. The sterility, caused by destruction of germ cells, is highly amenable to genetic manipulation. For example, the fat-1 mutant strain, which produces excess endogenous DGLA17, or the nhr-49 strain, which displays multiple lipid homeostasis defects18,19 become sterile at lower doses of DGLA than wild type. Conversely, strains with activated stress response pathways, such as daf-2, do not become sterile upon exposure to DGLA20. This suggests that fatty acid metabolism is highly regulated to protect against potentially toxic dietary metabolites.
When C. elegans are grown on elevated DGLA for their full life, their gonads consist of somatic cells which lack germ cells of any stage16. However, the impact of DGLA supplementation on germ cells can also be observed by short term DGLA supplementation of young adult worms with mature gonads. The C. elegans gonad consists of two U-shaped arms attached at one end by the uterus and spermatheca organs. A primordial germ cell niche is formed in the distal gonad where germ cells proliferate mitotically and transition into meiosis as they are pushed toward the proximal gonad to mature into sperm or oocytes21. Short term feeding of DGLA to young adult C. elegans, followed by visualization of the germ cell membrane and nuclei, indicated that DGLA supplementation leads first to the formation of multi-nucleated germ cells, then subsequently to germ cell apoptosis and cell clearance20. The rapid formation of multinucleated germ cells appears to be due to cell fusion of meiotic cells in the germline syncytium. Alternatively, failed cytokinesis during mitosis in the distal gonad could also account for the multinucleated cells.
In this study, we examined the mechanism in which dietary DGLA induces germ cell demise in C. elegans. Reverse genetic, biochemical and morphological approaches were used to address the hypothesis that specific DGLA-derived eicosanoids have detrimental effects on germ cells survival. We found that CYP-33E2 activity produces hydroxy, epoxy and diol products from dietary DGLA. However, only two of these metabolites, 8,9- and 14,15-epoxyeicosadienoic acid (EED), trigger the formation of multi-nucleated germ cells when injected directly into the C. elegans gonad syncytium. Thus, these oxygenated metabolites are likely responsible for sterility induced by dietary DGLA.
Results
Reduction of CYP-33E2 activity suppresses DGLA-induced sterility
Physiological responses from PUFAs are often mediated by their conversion into oxygenated eicosanoid metabolites. To determine if DGLA-derived eicosanoids trigger the sterility induced by dietary DGLA, we used RNAi to inhibit the CYP and prostaglandin pathways that potentially metabolize DGLA (Fig. 1). In mammals, several CYP enzymes modify PUFAs, creating eicosanoids with epoxy (CYP2C and CYP2J isoforms) or hydroxy (CYP4A and CYP4F isoforms) modifications8.
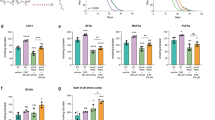
The C. elegans homolog CYP-33E2 produces a range of hydroxy and epoxy metabolites from linoleic acid (18:2n-6), AA (20:4n-6) and EPA (20:5n-3)22. We reasoned that similar metabolites might be formed from dietary DGLA and might trigger germ cell demise, leading to sterility in C. elegans. If a specific oxygenated DGLA metabolite is toxic to germ cells, then knocking down the enzyme producing the metabolite would be predicted to suppress the sterility phenotype. We used RNAi to knock-down cyp-33E2 and found, compared to empty vector controls, reduced populations of sterile worms when exposed to two concentrations of DGLA (Fig. 2A). GC/MS analysis revealed similar levels of DGLA uptake in the cyp-33E2 RNAi (11.5% DGLA for 0.3 mM) as in the empty vector control (10.5% DGLA for 0.3 mM), revealing that the cyp-33E2 is not simply blocking uptake or absorption of the dietary fatty acid. This result indicates that CYP-33E2 activity is involved in the process of germ cell destruction that leads to sterility in wild type worms supplemented with DGLA.
Epoxide hydrolase and prostaglandin synthesis do not contribute to DGLA-induced sterility
Based on our previous studies22,23, we predicted that CYP-33E2 activity generates epoxy and hydroxy metabolites from DGLA. Because epoxides derived from fatty acids may be toxic24,25, intracellular concentrations of fatty acid epoxides are regulated by soluble epoxide hydrolase enzymes, which convert epoxides into diols. While this modification often aids in the clearance of epoxide molecules, in some cases the resulting diol is also toxic24,25. To determine if diol metabolites of DGLA may also be toxic and contribute to germ cell demise, we obtained a ceeh-1 deletion and a ceeh-2 mutant carrying an opal stop codon in exon 1 from the C. elegans “million mutant” project26, predicted to be a homolog of human soluble epoxide hydrolase27. The ceeh-1 and ceeh-2 mutants resulted in wild type levels of sterility on DGLA, though a slight but not significant increase in the number of sterile nematodes was seen in ceeh-1 on 0.3 mM and in the ceeh-2 mutant on 0.1 mM DGLA. (Fig. 2B). These experiments suggest that soluble epoxide hydrolase activity is not involved in DGLA-mediated toxicity.
In mammals, aldo-keto reductases (AKT) such as Prostaglandin F Synthase and isomerases such as Prostaglandin E Synthase act on prostaglandin H1 to produce series 1 F-class prostaglandins (PGF1)4. Although strong evidence exists for the synthesis of F-class prostaglandins in C. elegans28,29, the enzymes that catalyze the required cyclooxygenase activity have not been identified. We used RNAi knockdown of predicted homologs of human Prostaglandin E and F Synthases to determine if similar activities on DGLA were leading to the formation of a toxic metabolite. We tested three genes with ≥38% identity to human Prostaglandin F synthase: Y39G8B.1, T08H10.1 and C07D8.6 as well as R11A8.5 which has 45% identity to human Prostaglandin E Synthase 2. A significantly greater number of sterile nematodes were seen in the RNAi populations supplemented with DGLA than empty vector populations (Fig. 2C). This suggests instead that flux of DGLA through an alternative lipid metabolism pathway might prevent DGLA from conversion to more toxic eicosanoids produced by CYP-33E2.
C. elegans CYP-33E2 activity promotes successful reproduction
To test whether CYP-33E2 impacts successful reproduction in untreated C. elegans wild type, the brood size of individual worms was measured. Inhibition of cyp-33E2 resulted in a reduced brood size, approximately 30% lower than the empty vector control (Fig. 2D). Therefore, CYP-33E2 plays a positive role in reproduction under normal dietary conditions, possibly in the promotion of germ cell maintenance through eicosanoids synthesized from more abundant fatty acid substrates, such as eicosapentaenoic acid (EPA, 20:5n-3).
C. elegans synthesize epoxy and hydroxy DGLA metabolites via CYP-33E2 activity
Our genetic experiments infer that CYP-33E2 activity leads to toxic metabolite formation, while soluble epoxide hydrolases and conversion of DGLA into prostaglandins might protect worms from toxic DGLA-derived metabolites. To identify the DGLA metabolites produced by CYP-33E2 activity, microsomes from a baculovirus expression system co-expressing CYP-33E2 and human cytochrome P450 reductase (hCPR)22 were assayed with radiolabeled DGLA. Isolation and analysis of the resulting products by LC-MS/MS indicated that 8,9-, 11,12- and 14,15-EEDs as well as the hydroxy derivatives, 18- and 19-hydroxyeicosatrienoic acids (HETrE) were produced by this monooxygenase system (Fig. 3A, Table 1). CYP-33E2 was previously shown to produce similar epoxy and hydroxy metabolites from EPA or AA substrates22. This indicates that epoxide and/or hydroxide containing DGLA metabolites may induce germ cell death.
To confirm that the DGLA metabolites produced by the recombinant CYP-33E2 are synthesized endogenously by C. elegans, the CYP-eicosanoid pattern from whole nematodes fed 0 mM or 0.3 mM DGLA were assayed by using LC-MS/MS. In addition to the epoxy and hydroxy metabolites found in the CYP-33E2 assay (Fig. 3B,C), 8,9-, 11,12- and 14,15-di-hydroxyeicosadienoic acids (DHED), diols derived from the corresponding epoxides, were also produced (Fig. 3D). This suggests that endogenous epoxide hydrolase activity converts the corresponding epoxy metabolites to diols. Importantly, all three classes of eicosanoid metabolites were detected at a higher abundance in DGLA fed nematodes than in non-supplemented controls. This suggests that dietary supplementation of DGLA leads to the production of corresponding CYP-eicosanoids in the living worms.
Functional assays of DGLA-derived eicosanoids
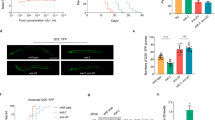
We sought evidence that specific DGLA metabolites induce the destruction of germ cells, leading to sterility. Of the DGLA metabolites found to be produced by C. elegans, only 14,15-EED was available commercially (Cayman Chemical). We produced the remaining metabolites by chemical oxidation (EEDs) following acidic hydrolysis (DHEDs) or by collection of CYP-33E2 produced 18-HETrE. All metabolites were purified by HPLC and quantified by MS. We developed a gonad injection assay in which the potency of specific metabolites could be directly assayed with smaller quantities of the purified eicosanoids. Because germ cells in the distal gonad are not completely enclosed, they share a common cytoplasm via a gonadal syncytium. Thus, multiple germ cells can be exposed to the metabolites from one injection. For proof of concept, DGLA was injected into the gonad of the C. elegans OD95 strain co-expressing fluorescent markers that target to the plasma membrane [GFP fusion that binds PI(4,5)P2] and chromosomes (mCherry-histone H2B)30. At a concentration of 10 μM, DGLA injections produced multinucleated, abnormal germ cells within several hours that were similar to those that developed when mature C. elegans were fed 0.3 mM DGLA (Fig. 4C). We then injected the purified DGLA-derived eicosanoids into gonads and observed gonad morphology 2–4 hours after injection. We found that injections of 8,9-EED or 14,15-EED at 1 and 2.5 μM produced multinucleated, abnormal germ cells in pre-meiotic and meiotic-transitioning germ cells (Fig. 4D–F). This suggests that these specific epoxide metabolites can trigger germ cell defects, because none of the other DGLA-derived eicosanoids produced the abnormal germ cell phenotype. Injections of DGLA at 1 and 2.5 μM did not produce abnormal germ cells compared to mock injections, suggesting that only a fraction of DGLA becomes modified into toxic metabolites and therefore DGLA must be present at higher concentrations to manifest the effect. Our previous feeding studies showed that the sterility phenotype is highly specific to supplementation of DGLA. Similarly, we found that injection of the EPA metabolites 8,9- and 14,15-epoxyeicosatetraenoic acids (EEQs) did not cause the formation of abnormal germ cells. Thus, the germ cell toxicity is highly specific to the dietary DGLA-derived 8,9- and 14,15-EED eicosanoids.
Discussion
Dietary DGLA causes C. elegans to become sterile due to destruction of germ cells during larval development. Through genetic, biochemical and morphological studies, we demonstrate here that CYP-33E2 acts on dietary DGLA to produce two toxic metabolites, 8,9- and 14,15-EED, that mediate the destruction of germ cells. This is the first example of a DGLA-derived epoxide with physiological activity.
We showed that RNAi knockdown of CYP-33E2 produced nematodes that were resistant to DGLA-induced sterility, however, we found that RNAi targeting genes that potentially direct DGLA towards other lipid metabolism pathways increased the sensitivity of C. elegans towards DGLA. Prostaglandins are well known fatty acid metabolites involved in multiple aspects of reproduction. In mammals, prostaglandins functions include the induction of labor and oocyte maturation31. In C. elegans, F-class prostaglandins produced by PUFAs in oocytes mediate nematode sperm guidance towards oocytes29. Under favorable conditions with abundant nutrients, pheromones sensed by ciliated neurons stimulate prostaglandin synthesis in the ovary, which in turn attracts sperm to oocytes and promotes optimal reproduction32.
Even though CYP-33E2 activity mediates the toxic effects of dietary DGLA, it is likely that this enzyme produces beneficial PUFA metabolites that promote reproduction, because cyp-33E2(RNAi) nematodes produce a smaller brood than control when grown on normal (non-supplemented) E. coli. Previously, epoxy fatty acids had been shown to mostly act in vascular regulation and angiogenesis1. In mammals AA and EPA derived epoxide metabolites regulate blood pressure and renal function during pregnancy33. Additionally, the abundance of EETs in pre-ovulatory follicular cells follows the estrus cycle and may influence estradiol formation34. In C. elegans, CYP-modified PUFA metabolites appear to regulate pharyngeal pumping, possibly through the activation of ion channels22,23. Recently, CYP-modified PUFAs, specifically 17,18- EEQ, was shown to impart protection against ischemic/reperfusion damage9,35. Thus, CYP-modified PUFA metabolites perform a range of important physiological functions in both vertebrates and invertebrates that are just beginning to be understood.
We previously showed that C. elegans mutant strains with constitutively active stress response pathways, including those resulting from inhibition of the insulin/IGF receptor DAF-2, were highly resistant to DGLA-induced sterility20. The daf-2 mutant strain shows increased expression of phase-II detoxification genes, such as gcs-1, which encodes an enzyme required for glutathione biosynthesis36. Phase II detoxification enzymes could act to clear the DGLA epoxides before they incur damage to germ cells. On the other hand, our studies did not support the hypothesis that soluble epoxide hydrolases aid the clearance of the toxic epoxides, because the ceeh-1 and ceeh-2 mutants did not significantly enhance the sterility phenotype.
Based on our finding that knockdown of cyp-33E2 leads to reduced brood size, we propose that under normal dietary conditions, polyunsaturated fatty acids are modified into epoxides by CYPs to promote successful reproduction. However, our studies demonstrate that an overabundance of DGLA in the diet leads to the synthesis of 8,9-EED and 14,15-EED, which may interfere with a physiological signal or act as toxins triggering germ defects and death. The specificity of these two isomers is critical to the germ cell phenotype, because exposure to 11,12-EED did not lead to germ cell abnormalities. Alternatively, our experiments do not rule out possibility that exposure to excess DGLA-derived epoxides lead to germ cell defects indirectly, for example, due to the disruption of signaling pathways that are regulated by a specific membrane lipid milieu37.
How the DGLA-derived epoxides induce germ cell abnormalities and germ cell death remains to be investigated. Similarly, the mechanisms of action for oxygenated fatty acids in mammalian processes such as vascular dilation are undefined. Though strong evidence indicates that epoxide fatty acid metabolites act in signal transduction, no receptors for these molecules have been identified38,39. Recently discovered functions for specific eicosanoids in C. elegans, such as the requirement for F-series prostaglandins in sperm guidance28,29,32, CYP involvement in response to hypoxia and re-oxygenation9,35 and the sensitivity of germ cells to oxygenated metabolites of dietary DGLA, demonstrate the utility of the genetically amenable C. elegans system for the discovery and elucidation of cellular mechanisms of eicosanoid signaling in physiological processes.
Methods
C. elegans maintenance and strains
Strains used in this study include: Wild type (N2 Bristol), RB2321 [ceeh-1(ok3153)], VC20710 [ceeh-2(gk379719)] and OD95 [Itls37, Itls38]30. The VC20710 strain was outcrossed 5x to N2. Nematode growth media (NGM) was used to maintain C. elegans with E. coli strain OP50 at 20 °C. Feeding RNAi was performed on NGM plates supplemented with 100 μg/ml ampicillin and 2 mM isopropyl-β-D-thiogalactopyranoside using the Ahringer lab RNAi library clones in E. coli strain HT11540,41. All RNAi constructs were sequence verified using Sanger sequencing.
Sterility assay
Wild-type strains were supplemented with DGLA on either standard NGM or RNAi medium as previously described16,20,42. Light microscopy was used to identify sterile worms as a lack of embryos in the adult uterus. For each concentration of DGLA, the percentage of sterile worms in the population was determined by scoring at least 30 worms per plate as fertile or sterile and averaging the sterility of populations on 4–8 plates. Comparisons between sterility levels of control populations and experimental populations were made using two-tailed student’s t tests. Exogenous DGLA uptake was confirmed by gas chromatography/mass spectrometry (GC/MS) of nematodes16,20,42.
CYP-33E2 activity on DGLA
C. elegans CYP-33E2 and hCPR were co-expressed in Sf9 cells as previously described22. For the enzymatic activity assay, microsomes with 9 pmol of CYP-33E2 were incubated with 10 μM [1-14C]-DGLA (ARC, St. Louis, MO, USA) at 25 °C for 10 minutes. The analysis of the obtained metabolites were performed by RP-HPLC and LC-MS/MS were prepared as previously described22.
Endogenous CYP-produced DGLA metabolites
Endogenous eicosanoids of C. elegans were identified exactly as previously described22. The synthesized DGLA metabolites were identified by their molecular weight and their characteristic fragmentation pattern under collision induced dissociation. The mass of negative molecular ions are m/z = 321 for epoxides and hydroxides and m/z = 339 for diols. The characteristic cleavage beside the oxygen functional group allows the differentiation of all regioisomeres (Table 1). The retention time was in an expected range compared to a wide spectrum of well-known oxylipins.
Production of DGLA derived eicosanoids
Whereas only 14,15-EED was available commercially (Cayman Chemical), the both other regioisomeric monoepoxides were synthesized by reacting the parental DGLA, supplemented with trace amounts of the corresponding [1-14C]-labeled compound, with m-chloroperoxybenzoic acid as described previously for the chemical oxidation of AA to EETs43. To produce EED derived diols (DHEDs) we hydrolyzed each of the three epoxides with 1 mL of a 1:1 acetic acid/water mixture over night at 45 °C while shaking with 70 rpm. All reaction products were resolved and purified by RP- and subsequent NP-HPLC as described in detail previously44. 18- and 19-HETrE were produced enzymatically by recombinant CYP-33E2/hCPR activity as described above and isolated after RP-HPLC purification. The identity and quantity of all regioisomeric metabolites was confirmed by LC-MS/MS.
Fatty acid and eicosanoid injection assay
For proof of concept, sodium dihomo-gamma-linolenic acid was dissolved in water at a concentration of 10 μM. All other fatty acids and metabolites arrived in ethanol and were diluted to 1 or 2.5 μM with water, mock injections used 1% ethanol. A glass capillary tube (World Precision Instruments Inc., TW100F-4) was drawn into a needle, loaded and used to inject into the gonad syncytium of the OD95 strain using an inverted light microscope. For each concentration of fatty acid metabolite, 8–12 individual nematodes were injected. Nematodes were recovered on NGM seeded with E. coli for 2 hours at 20˚C. Nematodes were then transferred to a drop of 0.1 mg/mL levamisole on a 2% agarose pad and a coverslip was applied. Germ cell membrane and nuclei were viewed with laser excitation at 488 and 561 nm (filtered at 495/535 nm and 600/670 nm for GFP and mCherry excitation/emission) and imaged on a Leica TCS SP5 confocal microscope with a 63X oil immersion lens. Images were processed identically using Adobe Photoshop CS4. The mean number of defective germ cells per injected worm was determined and two-tailed student’s t test was used to compare the number of defective germ cells in injected worms compared to mock injections.
Additional Information
How to cite this article: Deline, M. et al. Epoxides Derived from Dietary Dihomo-Gamma-Linolenic Acid Induce Germ Cell Death in C. elegans. Sci. Rep. 5, 15417; doi: 10.1038/srep15417 (2015).
References
Spector, A. A. & Kim, H. Y. Cytochrome P epoxygenase pathway of polyunsaturated fatty acid metabolism. Biochim Biophys Acta 1851, 356–365 (2015).
Vrablik, T. L. & Watts, J. L. Polyunsaturated fatty acid derived signaling in reproduction and development: insights from Caenorhabditis elegans and Drosophila melanogaster. Mol Reprod Dev 80, 244–259 (2013).
Astarita, G., Kendall, A. C., Dennis, E. A. & Nicolaou, A. Targeted lipidomic strategies for oxygenated metabolites of polyunsaturated fatty acids. Biochim Biophys Acta 1851, 456–468 (2015).
Funk, C. D. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science 294, 1871–1875 (2001).
von Moltke, J. et al. Rapid induction of inflammatory lipid mediators by the inflammasome in vivo. Nature 490, 107–111 (2012).
Brock, T. G. Regulating leukotriene synthesis: the role of nuclear 5-lipoxygenase. J Cell Biochem 96, 1203–1211 (2005).
Smyth, E. M., Grosser, T., Wang, M., Yu, Y. & FitzGerald, G. A. Prostanoids in health and disease. J Lipid Res 50 Suppl, S423–428 (2009).
Nebert, D. W., Wikvall, K. & Miller, W. L. Human cytochromes P450 in health and disease. Philos Trans R Soc Lond B Biol Sci 368, 20120431 (2013).
Ma, D. K. et al. Cytochrome P450 drives a HIF-regulated behavioral response to reoxygenation by C. elegans. Science 341, 554–558 (2013).
Schuck, R. N. et al. The cytochrome P450 epoxygenase pathway regulates the hepatic inflammatory response in fatty liver disease. PLoS One 9, e110162 (2014).
Wagner, K., Vito, S., Inceoglu, B. & Hammock, B. D. The role of long chain fatty acids and their epoxide metabolites in nociceptive signaling. Prostaglandins Other Lipid Mediat 113–115, 2–12 (2014).
Wang, D. & Dubois, R. N. Epoxyeicosatrienoic acids: a double-edged sword in cardiovascular diseases and cancer. J Clin Invest 122, 19–22 (2012).
Panigrahy, D. et al. Epoxyeicosanoids stimulate multiorgan metastasis and tumor dormancy escape in mice. J Clin Invest 122, 178–191 (2012).
Wang, X., Lin, H. & Gu, Y. Multiple roles of dihomo-gamma-linolenic acid against proliferation diseases. Lipids Health Dis 11, 25 (2012).
Zhu, H. & Han, M. Exploring developmental and physiological functions of fatty acid and lipid variants through worm and fly genetics. Annu Rev Genet 48, 119–148 (2014).
Watts, J. L. & Browse, J. Dietary manipulation implicates lipid signaling in the regulation of germ cell maintenance in C. elegans. Dev Biol 292, 381–392 (2006).
Watts, J. L. & Browse, J. Genetic dissection of polyunsaturated fatty acid synthesis in Caenorhabditis elegans. Proc Natl Acad Sci USA 99, 5854–5859 (2002).
Van Gilst, M. R., Hadjivassiliou, H., Jolly, A. & Yamamoto, K. R. Nuclear hormone receptor NHR-49 controls fat consumption and fatty acid composition in C. elegans. PLoS Biol 3, e53 (2005).
Van Gilst, M. R., Hadjivassiliou, H. & Yamamoto, K. R. A Caenorhabditis elegans nutrient response system partially dependent on nuclear receptor NHR-49. Proc Natl Acad Sci USA 102, 13496–13501 (2005).
Webster, C. M., Deline, M. L. & Watts, J. L. Stress response pathways protect germ cells from omega-6 polyunsaturated fatty acid-mediated toxicity in Caenorhabditis elegans. Dev Biol 373, 14–25 (2013).
Hubbard, E. J. & Greenstein, D. Introduction to the germ line. (September 1, 2005), WormBook, ed. The C. elegans Research Community, WormBook, doi/10.1895/wormbook. 1. 18. 1 http://www.wormbook.org.
Kosel, M. et al. Eicosanoid formation by a cytochrome P450 isoform expressed in the pharynx of Caenorhabditis elegans. Biochem J 435, 689–700 (2011).
Kulas, J., Schmidt, C., Rothe, M., Schunck, W. H. & Menzel, R. Cytochrome P450-dependent metabolism of eicosapentaenoic acid in the nematode Caenorhabditis elegans. Arch Biochem Biophys 472, 65–75 (2008).
Kosaka, K., Suzuki, K., Hayakawa, M., Sugiyama, S. & Ozawa, T. Leukotoxin, a linoleate epoxide: its implication in the late death of patients with extensive burns. Mol Cell Biochem 139, 141–148 (1994).
Zheng, J., Plopper, C. G., Lakritz, J., Storms, D. H. & Hammock, B. D. Leukotoxin-diol: a putative toxic mediator involved in acute respiratory distress syndrome. Am J Respir Cell Mol Biol 25, 434–438 (2001).
Thompson, O. et al. The million mutation project: a new approach to genetics in Caenorhabditis elegans. Genome Res 23, 1749–1762 (2013).
Harris, T. R. et al. Identification of two epoxide hydrolases in Caenorhabditis elegans that metabolize mammalian lipid signaling molecules. Arch Biochem Biophys 472, 139–149 (2008).
Edmonds, J. W. et al. Insulin/FOXO signaling regulates ovarian prostaglandins critical for reproduction. Dev Cell 19, 858–871 (2010).
Hoang, H. D., Prasain, J. K., Dorand, D. & Miller, M. A. A heterogeneous mixture of F-series prostaglandins promotes sperm guidance in the Caenorhabditis elegans reproductive tract. PLoS Genet 9, e1003271 (2013).
McNally, K., Audhya, A., Oegema, K. & McNally, F. J. Katanin controls mitotic and meiotic spindle length. J Cell Biol 175, 881–891 (2006).
Nunes, C., Silva, J. V., Silva, V., Torgal, I. & Fardilha, M. Signalling pathways involved in oocyte growth, acquisition of competence and activation. Hum Fertil (Camb) 18, 149–55 (2015).
McKnight, K. et al. Neurosensory perception of environmental cues modulates sperm motility critical for fertilization. Science 344, 754–757 (2014).
Huang, H. et al. Epoxyeicosatrienoic Acid inhibition alters renal hemodynamics during pregnancy. Exp Biol Med (Maywood) 231, 1744–1752 (2006).
Newman, J. W. et al. Cytochrome p450-dependent lipid metabolism in preovulatory follicles. Endocrinology 145, 5097–5105 (2004).
Keller, J. et al. CYP-13A12 of the nematode Caenorhabditis elegans is a PUFA-epoxygenase involved in behavioural response to reoxygenation. Biochem J 464, 61–71 (2014).
An, J. H. & Blackwell, T. K. SKN-1 links C. elegans mesendodermal specification to a conserved oxidative stress response. Genes & development 17, 1882–1893 (2003).
Kniazeva, M., Shen, H., Euler, T., Wang, C. & Han, M. Regulation of maternal phospholipid composition and IP(3)-dependent embryonic membrane dynamics by a specific fatty acid metabolic event in C. elegans. Genes & development 26, 554–566 (2012).
Ding, Y. et al. The biological actions of 11,12-epoxyeicosatrienoic acid in endothelial cells are specific to the R/S-enantiomer and require the G(s) protein. J Pharmacol Exp Ther 350, 14–21 (2014).
Akbulut, T. et al. 20-HETE activates the Raf/MEK/ERK pathway in renal epithelial cells through an EGFR- and c-Src-dependent mechanism. Am J Physiol Renal Physiol 297, F662–670 (2009).
Kamath, R. S. & Ahringer, J. Genome-wide RNAi screening in Caenorhabditis elegans. Methods 30, 313–321 (2003).
Kamath, R. S., Martinez-Campos, M., Zipperlen, P., Fraser, A. G. & Ahringer, J. Effectiveness of specific RNA-mediated interference through ingested double-stranded RNA in Caenorhabditis elegans. Genome Biol 2, RESEARCH0002 (2001).
Deline, M. L., Vrablik, T. L. & Watts, J. L. Dietary supplementation of polyunsaturated fatty acids in Caenorhabditis elegans. J Vis Exp 81, 10.3791/50879 (2013).
Falck, J. R., Yadagiri, P. & Capdevila, J. Synthesis of epoxyeicosatrienoic acids and heteroatom analogs. Methods Enzymol 187, 357–364 (1990).
Arnold, C. et al. Arachidonic acid-metabolizing cytochrome P450 enzymes are targets of {omega}-3 fatty acids. J Biol Chem 285, 32720–32733 (2010).
Acknowledgements
We thank Shirin Bahmanyar and Karen Oegema for the OD95 strain. Some C. elegans strains were provided by the CGC, which is funded by National Institutes of Health Office of Research Infrastructure Programs (P40 OD010440). Funding for this study was provided by grants from the National Institutes of Health (USA), R01 DK74114 (JLW) and T32 GM083864 (MD) as well as the German Research Foundation (Germany), SCHU 822/5-2 (WHS) and ME 2056/3-1 (RM).
Author information
Authors and Affiliations
Contributions
M.D. and J.L.W. conceived and coordinated the study and wrote the paper. M.D. and J.L.W. designed, performed and analyzed the experiments shown in Figure 2. J.K., M.R., W.H.S. and R.M. designed, performed and analyzed the experiments shown in Figure 3. J.K., M.R., W.H.S. and R.M. provided reagents for use in the experiments shown in Figure 4. M.D. and J.L.W. designed, performed and analyzed the experiments shown in Figure 4. All authors reviewed the results and approved the final version of the manuscript.
Genetic manipulation of DGLA cascade alters sterility from DGLA supplementation.
Potential DGLA metabolism pathways were inhibited with RNAi or mutant alleles and assayed for sterility induced by DGLA supplementation. (A) RNAi knock-down of cyp-33E2 suppresses DGLA-induced sterility. (B) ceeh-1 and ceeh-2 mutations do not significantly alter sterility. (C) Knockdown of alpha-keto reductase and PGE synthase homologs increases sensitivity to DGLA supplementation. (D) Knock-down of cyp-33E2 decreases C. elegans reproduction success. For all panels, EV = empty vector control; ND = no detectable sterility on 0 mM DGLA; error bars, S.E.M, **P < 0.0005 compared to empty vector control.
C. elegans DGLA metabolism is mediated by CYP activity.
(A) Microsomes from a CYP-33E2/hCPR expressing baculovirus system were assayed with radiolabeled DGLA and separated by HPLC. The resulting products were identified and quantified by LC-MS/MS. (B–D) Endogenous DGLA metabolites of C. elegans fed 0 mM or 0.3 mM DGLA, assayed by LC-MS/MS. (B) Monohydroxy-metabolites (HETrE) increase in the presence of 0.3 mM DGLA. (C) Epoxy-metabolites (EED) and (D) Dihydroxy-metabolites (DHED) also increase in the presence of 0.3 mM DGLA. For all panels, error bars are S.E.M., *P < 0.05, **P < 0.001 compared to 0 mM DGLA.
Specific epoxides derived from DGLA trigger germ cell abnormalities.
Confocal microscopy of injected gonads indicates the defects produced by direct injection of specific DGLA metabolites. (A) Full gonad image of mock injection. White box indicates area of gonad enlarged for B–E. (B) Mock injected, (C) 10 μM DGLA injection, (D) 2.5 μM 14,15-EED, (E) 2.5 μM 8,9-EED. (F) The resulting defects are summarized as the mean number of defective germ cells per injection. White bars at the top right of A and B indicate 50 μm length. White arrows indicate multinucleated germ cells. Error bars, S.E.M, *P < 0.01 compared to mock injection. Injection of other DGLA metabolites did not result in increased germ cell defects compared to mock injections.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Deline, M., Keller, J., Rothe, M. et al. Epoxides Derived from Dietary Dihomo-Gamma-Linolenic Acid Induce Germ Cell Death in C. elegans. Sci Rep 5, 15417 (2015). https://doi.org/10.1038/srep15417
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep15417
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.