Abstract
Phycodnaviruses are algae-infecting large dsDNA viruses that are widely distributed in aquatic environments. Here, partial genomic sequences of four novel algal viruses were assembled from a Yellowstone Lake metagenomic data set. Genomic analyses revealed that three Yellowstone Lake phycodnaviruses (YSLPVs) had genome lengths of 178,262 bp, 171,045 bp and 171,454 bp, respectively and were phylogenetically closely related to prasinoviruses (Phycodnaviridae). The fourth (YSLGV), with a genome length of 73,689 bp, was related to group III in the extended family Mimiviridae comprising Organic Lake phycodnaviruses and Phaeocystis globosa virus 16 T (OLPG). A pair of inverted terminal repeats was detected in YSLPV1, suggesting that its genome is nearly complete. Interestingly, these four putative YSL giant viruses also bear some genetic similarities to Yellowstone Lake virophages (YSLVs). For example, they share nine non-redundant homologous genes, including ribonucleotide reductase small subunit (a gene conserved in nucleo-cytoplasmic large DNA viruses) and Organic Lake virophage OLV2 (conserved in the majority of YSLVs). Additionally, putative multidrug resistance genes (emrE) were found in YSLPV1 and YSLPV2 but not in other viruses. Phylogenetic trees of emrE grouped YSLPVs with algae, suggesting that horizontal gene transfer occurred between giant viruses and their potential algal hosts.
Similar content being viewed by others
Introduction
Phytoplankton (microalgae), based on conservative estimates of more than 100,000 species1, is abundant in the sea. These algae form the base of the marine food web as their photosynthetic activities provide carbon sources and energy for life in the marine ecosystem and they regulate numerous aspects of the global environment1. Marine algae-infecting viruses, particularly phycodnaviruses, are important for controlling the composition of planktonic communities2.
The phycodnaviruses are a genetically diverse, morphologically similar group of double-stranded DNA viruses that infect eukaryotic algae and presently contain six genera3. Members of the Coccolithovirus, Phaeovirus, Prasinovirus, Prymnesiovirus and Raphidovirus genera infect marine algae, while chloroviruses (Chlorovirus) infect freshwater algae1,4. The genomes of phycodnaviruses are generally smaller (160 to 560 kb) than those of mimiviruses belonging to Lineage A of Group I, which typically have genomes larger than 1 Mb. Thus far, complete genomes of phycodnaviruses, such as Ostreococcus viruses (OtV1, OtV5)5,6, Micromonas sp. RCC1109 virus MpV17 and five chloroviruses8,9,10, have been well characterized.
The Phycodnaviridae, together with six other giant virus families, were defined as nucleo-cytoplasmic large DNA viruses (NCLDVs)11 and were proposed to be reclassified into a new order Megavirales due to the following shared features12: i) giant viral particles with capsid diameters >150 nm and genome sizes >100 kb; ii) the presence of nine class I core genes in all seven families or 47 NCLDV conserved genes in one or two families13; iii) potential for infection with virophages.
Interestingly, virophages were found to be associated with giant viruses since the first virophage Sputnik was isolated in association with a mamavirus, a relative of mimivirus, in a water-cooling tower in Paris14. Virophages have circular double-stranded DNA genomes of 18–30 kb which encode more than 20 genes. Recently, Zhou et al.15,16 observed extensive genetic diversity of virophages in Yellowstone Lake metagenomic datasets and have assembled seven complete virophage genomes (Yellowstone Lake virophages, YSLVs).
In this study, to provide insight into the diversity of giant viruses in Yellowstone Lake, we assembled giant viral genomes from the same metagenomic datasets in which YSLVs were discovered15,16. Based on comparative genomic and phylogenetic analyses, four novel giant viruses detected in YSL appeared to infect algae and horizontal gene transfer was observed among giant viruses, YSLVs and their potential algae hosts.
Material and Methods
Sequence assembly
Sequence assembly was performed as previously described by Zhou et al.15,16. Briefly, the Yellowstone Lake metagenomics dataset17 was downloaded from the CAMERA 2.0 Portal and assembled de novo using Newbler v2.6 (Roche). Contigs derived from the assembly were constructed as a local database in order to perform tBLASTx searches for viral major capsid protein (MCP)-related sequences. Since YSLVs are most closely related to Organic Lake virophage (OLV)15,16 and OLV is thought to be the viral parasite of Organic Lake phycodnaviruses (OLPVs)15,18, the potential giant viral hosts of YSLVs may be closely related to OLPVs. Therefore, MCPs of OLPVs were used as reference sequences to search (tblastx, E-value < 10−5) for homologous sequences in the constructed contig database described above. The OLPV MCP-related contigs over 10 kb in length with good quality (low E-value and high identity) were re-assembled using the Yellowstone Lake metagenomic dataset until the assembled sequences no longer extended. All sequence assemblies (with a minimum overlap length of 25 bp and minimum overlap identity of 95%) were performed using GeneiousPro15.
Assembly check
To further validate the assembled consensus sequences, duplicate reads were first removed from the data sets of scaffold reads with CD-HIT Suite19. Sequence identity cut-offs were set as: 0.97, 0.95 and 0.90. The obtained unique reads were then re-assembled and the consensus sequences were compared to the original sequences using the data sets without prior removal of duplicates in order to check the quality and accuracy of sequence assembly.
Genomic sequence analysis
The prediction and annotation of open reading frames (ORF) was performed as described by Zhou et al. in 201315. Each predicted ORF contained an ATG start codon and had a minimum size of 150 bp, standard genetic code and a stop codon. Translated amino acid sequences were used to search (E-value < 10−1) for homologs in NCBI nr database using the BLASTp program. One top hit to virus and/or non-virus was recorded. Functional annotation of ORFs was performed using the InterProScan program (http://www.ebi.ac.uk/Tools/pfa/iprscan/), Conserved Domain search20 on the NCBI server and HHpred (http://toolkit.tuebingen.mpg.de/hhpred).
In addition, 412 proteins of OLPV1, 326 of OLPV2 and 434 of Phaeocystis globosa virus 16 T (PgV-16 T) were analyzed for orthologous protein clusters shared with YSLGV proteins using the COG algorithm21.
All predicted ORFs (E-value < 10−1) were searched against a local database, which is comprised of all predicted proteins of seven YSLVs. Analysis of PgVV (a pro-virophage associated with PgV-16 T) proteins was performed by searching their homolog against an extensive database containing sequences of all the predicted proteins of known virophages.
Genomic sequences were aligned using the Mauve program on the Geneious Pro platform (default parameter)22. Repetitive sequences were checked on the softberry website (http://linux1.softberry.com/berry.phtml? topic = frep&subgroup = repeat&group = programs) with default parameters and long terminal repeats using LTR-Finder23.
Phylogenetic analysis
Homologs of MCP, DNA polymerase B family (PolB), poxvirus late transcription factor 3 (Pox-VLTF3), topoisomerase II (Topo II), vaccinia virus (VV) A32-like packaging ATPase, ribonucleotide reductase small subunit (RNR2), multidrug resistance protein (emrE) and OLV ORF2 (OLV2) were used to reconstruct phylogenetic trees. Reconstruction was initiated by aligning multiple amino acid sequences using the MUSCLE program24, followed by tree construction using the JTT model with a bootstrap value of 100. Phylogenetic analysis of emrE included sequences from three cellular life domains and was based on Bayesian Inference (parameter set: rate variation: gamma; rate matrix: poisson). All analyses were performed on the Geneious Pro platform.
Accession numbers
The genomic sequences of four YSL algal viruses have been deposited in DDBJ under accession numbers LC015646-LC015649 for YSLGV and YSLPV 1–3, respectively.
Results
Genomic features
A total of 677,637 contigs were obtained (100–199,335 bp in length) after de novo assembly and were used to search OLPV MCP-related contigs with tBLASTx. Six contigs (10 kb <length <50 kb, E value < e−80) were obtained, four of which were ultimately extended to 178,262, 171,454, 171,045 and 73,689 bp, after reference assembly (Table S1, Fig. S1). Duplicate reads were then removed from each scaffold data set. Re-assembled consensus sequences exhibited >99% nt identity to their corresponding sequences as described above, confirming the accuracy of the assembly.
Sequence analysis (see below) indicated that the 178,262, 171,045 and 171,454 bp-long contigs were closely related to phycodnaviruses, while the 73,689 bp-long contig was related to PgV-16 T and OLPVs, which is phylogenetically related with mimiviruses. Accordingly, they were named as Yellowstone Lake phycodnaviruses (YSLPVs 1–3) and Yellowstone Lake giant virus (YSLGV), respectively (Fig. 1). The numbers of predicted ORFs for each virus are shown in Fig. 1. The G/C content of the three YSLPVs (Fig. 1) was similar to that of prasinoviruses (48%), while the G/C content of YSLGV was similar to that of PgV-16 T (32%).
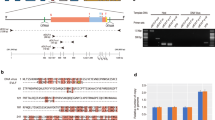
Physical maps of partial genomes of YSLPVs and YSLGV.
ORFs are indicated in box arrows and are labeled in different colors that represent different functional categories. Repeats are shown in diamond. Numbers outside the genomes indicate nucleotide positions. Viral names, genomic length and G+C content and the total number of predicted ORFs are shown in the center of the map. The blue line represents %G+C skew. The ends of each linear genome are indicated with black arrows.
Repeat sequences were found in three partial genomes. Inverted terminal repeats (382 bp) were detected only in YSLPV1 (Fig. 1). Other types of inverted terminal repeats were present on the complete genomic ends of phycodnaviruses, e.g., chloroviruses and phaeoviruses, suggesting that the assembled YSLPV1 genome was nearly complete. In addition, three different types of tandem repeats were also detected in YSLPV1, while one tandem repeat was detected in YSLPV3 and YSLGV (Fig. 1).
BLASTp analysis showed that 33% (YSLPV1), 36% (YSLPV2), 39% (YSLPV3) and 31% (YSLGV) of predicted ORFs were ORFans, which had no detectable homologs in GenBank (Fig. S2). With respect to virus hits, YSLPV1, −2 and −3 shared the most homologs with prasinoviruses (E-value ≤ 0.01, sequence identity 24.0–74.8%), particularly Ostreococcus and Micromonas viruses. In contrast, YSLGV shared the most homologs with OLPVs (10 hits) and PgV-16 T (40 hits) (Fig. 2). In YSLPV1, 91% of BLAST hits related to Phycodnaviridae were from Prasinovirus (42 hits to Micromonas virus, 38 to Ostreococcus virus and 18 to Bathycoccus virus). In YSLPV2 and −3, of the Phycodnaviridae-related hits, those from Prasinovirus accounted for 87% (34 to Micromonas virus, 38 to Ostreococcus virus and 13 to Bathycoccus virus) and 94% (38 to Micromonas virus, 48 to Ostreococcus virus and 17 to Bathycoccus virus), respectively. For non-virus hits, algal genes comprised more than 35% of eukaryotic hits of YSLPV and YSLGV ORFs (Fig. 3). In three YSLPVs, the majority of algae gene homologs were from the four algae classes Chlorophyceae, Trebouxiophyceae, Mamiellophyceae and Phaeophyceae, which were absent in YSLGV. For YSLGV, two algae hits related to Haptophyceae and Cryptophyceae were observed, one each to Bangiophyceae and Stramenopiles, respectively. In addition, several ORFs of YSLPV1, −2 and −3 showed similarity to bacterial genes, particularly to Cyanobacteria and Proteobacteria. YSLPV2 and −3 also had a significant number of hits to Verrucomicrobia bacterium: 20 hits of YSLPV2 and 34 of YSLPV3 (Table S2).
The relative percentages of ORFs, homologous to distinct viral families and to the genera of Phycodnaviridae (three pie graphs on the right), in (A) YSLPV1, (B) YSLPV2, (C) YSLPV3 and (D) YSLGV. The same viral family or genus is indicated by a single color. Numbers represent the corresponding percentage of ORFs homologous to that of different viral families or genera.
The relative percentage of ORFs, homologous to three domains of life (center pie chart), bacterial phyla (left pie chart) and eukaryotes (right pie chart), in (A) YSLPV1, (B) YSLPV2, (C) YSLPV3 and (D) YSLGV. The same group of organism is indicated by a single color. Numbers represent the corresponding percentages of ORFs homologous to that of different groups of organisms.
Whole genome alignment of YSLPV1, −2 and −3 showed that three highly conserved regions were shared among the three genomes (Fig. 4), confirming the close phylogenetic relationship as described below. Genome inversion occurred between YSLPV1 and −2, while YSLPV3 experienced genome rearrangement compared to YSLPV1 and −2 (Fig. 4).
Conserved genes of NCLDVs present in YSLPVs and YSLGV
Based on the results of COG analysis, 39 YSLGV proteins were clustered with OLPG comprising OLPVs and PgV-16 T. Based on conserved domain and functional analyses, 23 NCVOGs were found in YSLPV1, 18 in YSLPV2, 17 in YSLPV3 and 6 in YSLGV (Table 1). Among these, four genes (DNA polymerase sliding clamp (PCNA), VV A32-like packaging ATPase, MCP and VLTF3) were present in all four sequences. Nine genes, present only in YSLPV1, −2 and −3, were identified as YqaJ viral recombinase family protein, ribonucleotide reductase large subunit (RNR1), ribonucleotide reductase small subunit (RNR2), mRNA-capping enzyme, transcription initiation factor IIB, TATA-box binding protein, DNA helicase of superfamily II, thioredoxin fold protein and serine/threonine protein kinase. In addition, YSLPV1 and −2 shared 18 NCVOGs (Table 1). However, a number of core genes in prasinoviruses were absent in YSLPVs and YSLGV. For example, PolB was not detected in YSLGV and YSLPV3 and Rnase H was absent in YSLPVs 1–3. These omissions were likely a result of incomplete genomic sequences or due to high divergence of the corresponding proteins.
Gene duplication, which is commonly present in giant dsDNA viruses, was also found in YSLPVs and YSLGV. For example, YSLPV2 encodes two PolB paralogs (ORF143 and ORF195), which shared 69% amino acid identity and were most similar to their homologs in prasinoviruses (Table S2); YSLGV contained two copies of topoisomerase gene similar to PgV-16 T25. In addition, a conserved gene cluster, comprising three core genes of PolB, MCP and Topo II, was observed in YSLPV1 and −2 as well as in other prasinovirus genomes (Fig. 5). Interestingly, this gene cluster was duplicated in YSLPV2, which has not been identified in other NCLDV viruses. This duplication is unlikely to be due to the presence of sequence assembly artifacts in the duplicated gene cluster, since their corresponding scaffold sequences were reliable with good quality and high coverage.
Other key functional genes
Genes involved in DNA replication, nucleotide metabolism, transcription and virion packaging were frequently found in YSLPVs and YSLGV (Table 1). Genes involved in sugar metabolism, such as glycosyltransferase, were detected in YSLPVs and YSLGV (Table S2) and these genes are also common in NCLDVs. Other functional genes that are involved in lipid metabolism, such as patatin-like phospholipase, were also identified in YSLPVs and YSLGV. The detailed information is shown in Table 1 and Table S2. It is worth noting that YSLPVs encoded several genes, including deoxynucleoside kinase, HNH nuclease, SKP1 protein and ornithine: arginine decarboxylase, were uniquely found in chloroviruses7.
Phylogenetic analysis
Five conserved proteins of NCLDVs, DNA polymerase B, MCP, VLTF3, topoisomerase II and VV A32-like packaging ATPase, were used to construct phylogenetic trees. According to the tree topology (Fig. 6; Fig. S3), YSLPV1, −2 and −3 formed a distinct monophyletic group, which represented a sister lineage to and shared a common ancestor with prasinoviruses. YSLGV clustered with the OLPG group, including PgV-16 T and OLPVs. Taken together, both sequence and phylogenetic analyses indicated that YSLPV1, −2 and −3 appeared to be a novel algal virus lineage most closely related to Prasinovirus and that YSLGV seemed to be a novel member of OLPG.
Unrooted phylogenetic trees reconstructed using amino acid sequences of NCLDV conserved genes of PolB and MCP that are present in YSLPVs, YSLGV and selected other giant virus families.
Viral families, genera of Phycodnaviridae and three groups of Mimiviridae are shaded in different colors. The Mimiviridae family has currently only two ICTV-approved members, Acanthamoeba polyphaga mimivirus and Cafeteria roenbergensis virus. The subgroupings into Group I-III are arbitrary, non-formal classifications. Bootstrap values (100 iterations) are indicated on the branching of the tree.
Relevance to virophages
A total of nine non-redundant homologous counterparts shared between YSLPVs/YSLGV and YSLVs were determined based on BLAST searches of a local database containing all YSLV ORFs (n = 193), using YSLPVs/YSLGV ORFs as query sequences (Table 2). YSLV4 ORF04 shared the highest similarity with YSLPV1, −2 and −3 ORFs (>50%, E-value < e-90), which were homologous to RNR2, a conserved gene in NCLDVs. Phylogenetic analysis of viral RNR2 protein sequences (Fig. 7) showed that YSLV4 RNR2 was clustered with that of OLPG and the RNR2 homologs of YSLPVs were grouped into a single clade that is closely related to prasinovirues. Although RNR2 was not detected in YSLGV, it does not exclude the possibility of its presence since the YSLGV genomic sequence was incomplete and YSLGV appeared to be a new member of OLPG. OLV ORF2 is a gene with unknown function that is conserved in YSLV 1–4 and −6 and is present in both YSLPV1–3 and YSLGV (Table 2).
Phylogenetic tree reconstructed using amino acid sequences of RNR2 of YSLPVs, YSLV4-ORF04 and selected other giant viral families.
Mimiviridae, OLPG group and Phycodnaviridae are labeled with lines. YSLPVs and YSLV4-ORF04 are indicated in blue. African swine fever virus (ASFV) was used to root the tree. Bootstrap values (100 iterations) are shown on the branching of the tree. The accession numbers of the sequences used are as follows: ASFV (NP_042738), ATCV1 (YP_001426516), BpV1 (YP_004061574), Cowpox virus 2 (ADZ29378), EhV 86 (YP_293780), Grouper iridovirus (AAV91049), Insectomime virus (AHA46032), Mamavirus (AEQ60501), Marseillevirus (YP_003407004), Megavirus chiliensis (YP_004894645), Mimivirus (YP_003986815 ), Moumouvirus goulette (AGF85216), MpV PL1 (AET43587), MpV SP1 (AET84875), OLPV1 (ADX05815), OLPV2 (ADX06189), OsV5 (YP_001648251), Pandoravirus dulcis (YP_008319229), PBCV NY2B (AGE54331), PBCV NYs1 (AGE55017), PgV-16 T (YP_008052426), Rana grylioirido virus 9506 (AAS67856), Singapore grouper iridovirus (YP_164142), Tiger frog virus (AAL77807) and Vaccinia virus (AEY74987).
Host defense gene in YSLPVs
YSLPV2 ORF158 and -ORF210 contain a multidrug resistance domain. YSLPV1 ORF29 showed sequence similarity with the quaternary ammonium compound resistant protein of Natrinema gari (29.8%, E value 0.035), while YSLPV1 ORF218 shared 33.3% sequence similarity with the multidrug resistant protein MdtJ of Yersinia pseudotuberculosis IP 31758, an enterobacteria species, with an E value of 5.88e-04 (Table S2). Based on HHpred analysis, YSLPV1 ORF29, -ORF218, YSLPV2 ORF158 and -ORF210 were homologous to a multidrug transporter emrE protein (Table S3) that is approximately 110 amino acids in size and exports positively charged hydrophobic drugs in exchange for protons, thereby conferring bacterial resistance to toxic compounds26. Phylogenetic analysis revealed that YSLPV emrE shares a common ancestor with eukaryotic algae emrE that possibly originated from bacteria (Fig. 8).
Rooted Bayesian phylogenetic tree reconstructed using amino acid sequences of emrE in YSLPV 1–2 and selected bacterial, eukaryotic and archaeal species. YSLPVs, Algae, Bacteria and Archaea groups are marked with lines.
YSLPVs are shown in blue. Bayesian posterior probabilities are indicated on the branching of the tree. The tree is rooted using Archaea emrE. The accession numbers of the sequences used are as follows: Haloarcula hispanica (WP_014039315), Haloferax (WP_004045282), Sphingobacterium spiritivorum (WP_002995628), Escherichia coli (WP_032285312), Escherichia coli O104:H4 str. C227-11 (WP_001304280), Wigglesworthia glossinidia endosymbiont (WP_011070390), Elizabethkingia anophelis (CDN79378), Prevotella bergensis (WP_007174771), Bacteroides sp.3_1_33FAA (WP_008654963), Ostreococcus tauri (XP_003082847), Coccomyxa subellipsoidea c-169 (XP_005651632), Bathycoccus prasinos (XP_007513508) and Micromonas pusilla CCMP1545 (XP_003063730).
Discussion
In this study, partial genomic sequences of four novel giant viruses were obtained from YSL metagenomic data sets, from which seven virophages had previously been discovered15,16. The YSLPV1 genome appears nearly complete as it contains a pair of inverted repeats flanking both ends of the genome and a more complete set of NCVOGs than the other three genomes. YSLGV is far from complete as it is the shortest assembled sequence, approximately 73 kb in length, which does not meet the criteria for genome length of giant viruses (>100 kb). Despite the lack of several core genes in the partial genome, including PolB, RNR1, RNR2 and DNA ligase, which are fundamental to DNA replication and nucleotide metabolism, YSLGV contains capsid protein, Topoisomerase II, Packaging ATPase, PCNA and late transcription factor VLTF3 that all clustered with those of OLPG. Moreover, the presence of the RNA polymerase gene distinguishes YSLGV from YSLPVs and prasinoviruses, since most phycodnaviruses do not contain RNA polymerase genes27,28.
Based on homolog and phylogenetic analyses, YSLPV 1–3 belong to the phycodnaviruses and YSLGV is grouped to the OLPG clade affiliated with mimiviruses. YSLPVs represent a novel viral lineage in Phycodnaviridae and are more closely related to prasinoviruses that infect marine algae than to chloroviruses that infect freshwater algae. Since Yellowstone Lake is a freshwater ecosystem containing hundreds of hydrothermal vents29, YSLPVs, unlike their marine algae-infecting relatives of Prasinovirus, have the potential to infect freshwater algae similarly to chloroviruses. In addition, YSLGV appears to be a novel member of Group III, the extended family of Mimiviridae, whose eukaryotic hosts are thought to be algae, not protozoa. The Yellowstone Lake therefore contains a diverse set of giant algal viruses, which await further study.
Virophages were found to be associated with mimiviruses whose genome sizes are typically larger than 1 megabase and replicate in cytoplasmic viral factory14. Sputnik30 and Mavirus31 are parasites of mimivirus group I and group II (Cafeteria roenbergensis virus, CroV), respectively. OLV18 was thought to be associated with tentative group III mimiviruses (OLPVs). The recently isolated virophage Zamilon is capable of infecting lineage C (Group I) members of Mimiviridae32. A virophage-like sequence, termed PgVV, was obtained during assembly of the third largest genome of marine virus of PgV-16 T (459,984 bp in length) using metagenomic data25, although no virophage particles were observed. The PgVV genomic features are unique: 1-kb telomeric-like repeats flanking the genome, 16 coding regions that all transcribe from the same strand and a putative jelly-roll capsid protein (PgVV ORF12) that bears little similarity to that of other virophages33. The discovery of pro-virophage PgVV is the first report of a virophage associated with a mimivirus-like giant algal virus and it also implies that virophages are capable of infecting giant viruses of comparatively smaller genome sizes34. Thus far, however, no evidence has been provided to indicate that virophages are able to infect giant algal viruses of phycodnaviruses.
It has been shown that virophages and their associated giant viral hosts share homologous genes (Table S4). In this study, comparative genomic analysis revealed that YSLV 1–4 and −6 exhibit genetic links with YSLPVs and YSLGV. For example, nine non-redundant homologous genes are shared between YSLVs and YSLPVs/YSLGV (Table 2) and this number may increase when the complete genomes of YSLPVs and YSLGV are available. The homolog counterparts of function-unknown OLV ORF2, which is conserved in YSLV 1–4 and −6, OLV and PgVV (ORF01) (Table S5), were present in YSLGV and YSLPV 1–3 and phylogenetic analysis of the OLV ORF2 homolog grouped YSLV2 with YSLPVs, suggesting that gene transfer may have occurred between them (Fig. 9). Taken together, although it is too preliminary to conclude a potential association between virophages and phycodnaviruses, the study of giant algal viruses may be informative in the search for novel virophages.
Surprisingly, homologs of multidrug transport protein emrE were identified in YSLPV1 and −2. To our knowledge, emrE has not previously been reported in phycodnaviruses or in any other known virus. It is likely that YSLPVs derived emrE genes from their potential algal hosts that contained emrE genes obtained from their symbiotic bacteria through horizontal gene transfer (Fig. 8). The function of emrE and its ecological and evolutionary fitness to YSLPVs remain to be explored.
In conclusion, four algal viral partial genomic sequences were discovered from the Yellowstone Lake metagenomics dataset. The corresponding novel viruses of YSLPV 1–3 and YSLGV are related to members of the Phycodnaviridae and the OLPG clade related to Mimiviridae, respectively, indicating the diversity of algal virus species in YSL ecosystem. Genetic links between YSLPVs/YSLGV and YSLVs were observed, while their potential associations await future study. Yellowstone Lake is a hotspot for studying the diversity of algal giant viruses and virophages.
Additional Information
How to cite this article: Zhang, W. et al. Four novel algal virus genomes discovered from Yellowstone Lake metagenomes. Sci. Rep. 5, 15131; doi: 10.1038/srep15131 (2015).
References
Wilson, W. H., Van Etten, J. L. & Allen, M. J. The Phycodnaviridae: the story of how tiny giants rule the world. Curr Top Microbiol Immunol 328, 1–42 (2009).
Suttle, C. A. Viruses in the sea. Nature 437, 356–361 (2005).
International Committee on Taxonomy of Viruses. In Virus Taxonomy: Classification and Nomenclature of Viruses:NINTH REPORT OF THE INTERNATIONAL COMMITTEE ON TAXONOMY OF VIRUSES (eds King, A. et al. ), 249–262 (2012).
Dunigan, D. D., Fitzgerald, L. A. & Van Etten, J. L. Phycodnaviruses: a peek at genetic diversity. Virus Res 117, 119–132 (2006).
Derelle, E. et al. Life-cycle and genome of OtV5, a large DNA virus of the pelagic marine unicellular green alga Ostreococcus tauri. PLoS One 3, e2250 (2008).
Weynberg, K. D., Allen, M. J., Ashelford, K., Scanlan, D. J. & Wilson, W. H. From small hosts come big viruses: the complete genome of a second Ostreococcus tauri virus, OtV-1. Environ Microbiol 11, 2821–2839 (2009).
Moreau, H. et al. Marine prasinovirus genomes show low evolutionary divergence and acquisition of protein metabolism genes by horizontal gene transfer. J Virol 84, 12555–12563 (2010).
Fitzgerald, L. A. et al. Sequence and annotation of the 314-kb MT325 and the 321-kb FR483 viruses that infect Chlorella Pbi. Virology 358, 459–471 (2007).
Fitzgerald, L. A. et al. Sequence and annotation of the 369-kb NY-2 A and the 345-kb AR158 viruses that infect Chlorella NC64A. Virology 358, 472–484 (2007).
Fitzgerald, L. A. et al. Sequence and annotation of the 288-kb ATCV-1 virus that infects an endosymbiotic chlorella strain of the heliozoon Acanthocystis turfacea. Virology 362, 350–361 (2007).
Iyer, L. M., Balaji, S., Koonin, E. V. & Aravind, L. Evolutionary genomics of nucleo-cytoplasmic large DNA viruses. Virus Res 117, 156–184 (2006).
Colson, P., de Lamballerie, X., Fournous, G. & Raoult, D. Reclassification of giant viruses composing a fourth domain of life in the new order Megavirales. Intervirology 55, 321–332 (2012).
Yutin, N., Wolf, Y. I., Raoult, D. & Koonin, E. V. Eukaryotic large nucleo-cytoplasmic DNA viruses: clusters of orthologous genes and reconstruction of viral genome evolution. Virol J 6, 223 (2009).
La Scola, B. et al. The virophage as a unique parasite of the giant mimivirus. Nature 455, 100–104 (2008).
Zhou, J. et al. Diversity of virophages in metagenomic data sets. J Virol 87, 4225–4236 (2013).
Zhou, J. et al. Three novel virophage genomes discovered from Yellowstone Lake metagenomes. J Virol 89, 1278–1285 (2015).
Clingenpeel, S. et al. Yellowstone Lake: high—energy geochemistry and rich bacterial diversity. Environ Microbiol 13, 2172–2185 (2011).
Yau, S. et al. Virophage control of antarctic algal host-virus dynamics. PNAS 108, 6163–6168 (2011).
Huang, Y., Niu, B., Gao, Y., Fu, L. & Li, W. CD-HIT Suite: a web server for clustering and comparing biological sequences. Bioinformatics 26, 680–682 (2010).
Marchler-Bauer, A. et al. CDD: conserved domains and protein three-dimensional structure. Nucleic Acids Res 41, D348–352 (2013).
Tatusov, R. L. et al. The COG database: new developments in phylogenetic classification of proteins from complete genomes. Nucleic Acids Res 29, 22–28 (2001).
Darling, A. C., Mau, B., Blattner, F. R. & Perna, N. T. Mauve: multiple alignment of conserved genomic sequence with rearrangements. Genome Res 14, 1394–1403 (2004).
Xu, Z. & Wang, H. LTR_FINDER: an efficient tool for the prediction of full-length LTR retrotransposons. Nucleic Acids Res 35, W265–268 (2007).
Edgar, R. C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32, 1792–1797 (2004).
Santini, S. et al. Genome of Phaeocystis globosa virus PgV-16 T highlights the common ancestry of the largest known DNA viruses infecting eukaryotes. PNAS 110, 10800–10805 (2013).
Ninio, S., Elbaz, Y. & Schuldiner, S. The membrane topology of EmrE—a small multidrug transporter from Escherichia coli. FEBS Lett 562, 193–196 (2004).
Yutin, N., Colson, P., Raoult, D. & Koonin, E. V. Mimiviridae: clusters of orthologous genes, reconstruction of gene repertoire evolution and proposed expansion of the giant virus family. Virol J 10, 106 (2013).
Yutin, N., Wolf, Y. I. & Koonin, E. V. Origin of giant viruses from smaller DNA viruses not from a fourth domain of cellular life. Virology, 38–52 (2014).
Lovalvo, D. et al. A geothermal-linked biological oasis in Yellowstone Lake, Yellowstone National Park, Wyoming. Geobiology 8, 327–336 (2010).
Gaia, M. et al. Broad spectrum of mimiviridae virophage allows its isolation using a mimivirus reporter. PLoS One 8, e61912 (2013).
Fischer, M. G. & Suttle, C. A. A virophage at the origin of large DNA transposons. Science 332, 231–234 (2011).
Gaia, M. et al. Zamilon, a novel virophage with Mimiviridae host specificity. PLoS One 9, e94923 (2014).
Krupovic, M., Bamford, D. H. & Koonin, E. V. Conservation of major and minor jelly-roll capsid proteins in Polinton (Maverick) transposons suggests that they are bona fide viruses. Biol Direct 9, 6 (2014).
Claverie, J. M. Giant virus in the sea: Extending the realm of Megaviridae to Viridiplantae. Commun Integr Biol 6, e25685 (2013).
Acknowledgements
This work was supported partially by the National Natural Science Foundation of China (41376135, 31570112), Doctoral Fund of Ministry of Education of China (20133104110006), Innovation Program of Shanghai Municipal Education Commission (14ZZ144), China and the Special Fund for the Development of Science and Technology of Shanghai Ocean University (2015).
Author information
Authors and Affiliations
Contributions
S.Y. and Y.W. designed this study. W.Z. assembled the sequences and analyzed the data. J.Z. assembled preliminary sequences. T.L. advised on statistical analyses. Y.Y. and Y.P. modified the manuscript. W.Z. and Y.W. wrote the manuscript. Y.W. analyzed the data. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Zhang, W., Zhou, J., Liu, T. et al. Four novel algal virus genomes discovered from Yellowstone Lake metagenomes. Sci Rep 5, 15131 (2015). https://doi.org/10.1038/srep15131
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep15131
This article is cited by
-
Significant Differences in Planktonic Virus Communities Between “Cellular Fraction” (0.22 ~ 3.0 µm) and “Viral Fraction” (< 0.22 μm) in the Ocean
Microbial Ecology (2023)
-
Giant virus biology and diversity in the era of genome-resolved metagenomics
Nature Reviews Microbiology (2022)
-
Ecogenomics reveals viral communities across the Challenger Deep oceanic trench
Communications Biology (2022)
-
The genome of a prasinoviruses-related freshwater virus reveals unusual diversity of phycodnaviruses
BMC Genomics (2018)
-
Hidden diversity of soil giant viruses
Nature Communications (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.