Abstract
Lycorine is reported to be a multifunctional compound. We previously showed that lycorine is an HCV inhibitor with strong activity. Further research on the antivirus mechanism indicated that lycorine does not affect the enzymes that are indispensable to HCV replication but suppresses the expression of Hsc70 in the host cell to limit HCV replication. However, due to the cytotoxicity and apoptosis induction of lycorine, lycorine is unsafe to be a anti-HCV agent for clinical application. As a result of increasing interest, its structure was optimized for the first time and a novel series of lycorine derivatives was synthesized, all of which lost their cytotoxicity to different degrees. Structure-activity analysis of these compounds revealed that disubstitution on the free hydroxyl groups at C1 and C2 and/or degradation of the benzodioxole group would markedly reduce the cytotoxicity. Furthermore, an α, β-unsaturated ketone would improve the HCV inhibitory activity of lycorine. The C3-C4 double bond is crucial to the anti-HCV activity because hydrogenation of this double bond clearly weakened HCV inhibition.
Similar content being viewed by others
Introduction
Hepatitis C virus (HCV) is seriously harmful to human health and is one of the most common causes of chronic hepatitis worldwide1,2,3. Several potent HCV inhibitors have been developed4,5,6,7, but resistance to them has developed due to the high variability of HCV. There is therefore a need for alternative HCV drugs, which are in development8,9,10.
Lycorine (1, Fig. 1a) is a multifunctional benzylphenethylamine alkaloid11,12. We previously reported that it has good activity against HCV. Upon investigating the anti-HCV mechanisms of lycorine, we found that it works by down-regulating the level of intracellular Hsc70 protein (Fig. 1b,c) in a dose-dependent fashion, as shown by the positive correlation between them (Fig. 1d). Lycorine was formerly known as an antitumour agent. Previous reports showed that it exhibits good cytotoxicity and apoptosis induction activities in cancer cell lines13,14,15. As a result, although lycorine is clearly an inhibitor of HCV, it poses safety issues. We therefore investigated the structure-activity relationships of lycorine analogues to identify a related compound with comparable levels of HCV inhibition but less cytotoxicity. Here, we describe the structural optimization process of lycorine and present an evaluation of its derivatives’ anti-HCV effects and toxicity in vitro. We also analysed their structure-activity relationships (SAR).
Hsc70 Down-regulation and anti-HCVactivity of lycorine.
(a) The structure of lycorine; (b) The intracellular HCV core protein in Huh 7.5 cells decreased in a dose-dependent fashion following 72 h of treatment with compound 1 (lycorine) and the Hsc70 expression of Huh 7.5 cells also decreased; (c)The intracellular concentration of the Hsc70 mRNA; (d) The correlation between the inhibition of Hsc70 expression and the anti-HCV activity of lycorine. All mean and standard deviation data were obtained from quintuplicate experiments (N = 5).
Results and Discussion
Design and Synthesis of Lycorine Derivatives
As shown in Fig. 2, our initial attempts at modifying the structure of 1 focused on manipulating the C-1 and C-2 substituents (green-labelled) and the C3-C4 double bond (blue-labelled). In addition, we investigated the effects of modifying the properties of the nitrogen atom at the 5-position of the lycorine skeleton (red-labelled) and the benzodioxole group (yellow-labelled). Fifteen lycorine derivatives were ultimately synthesized, bearing various combinations of these modifications.
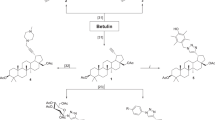
The first lycorine derivatives were prepared by acetylating the C-1 and C-2 hydroxyl groups of the parent compound. Controlled acetylation was achieved by varying the quantities of the reagents used (acetic anhydride and DMAP) and the reaction time, yielding 1, 2-di-O-acetyllycorine (1a) and 2-O-acetyllycorine (1c). Compound 1a was then hydrolysed to obtain 2-O-acetyllycorine (1d)13,16. Compounds 1c and 1d bear similar structural features, although they differ in having an acetoxy group at the C-1 or C-2 position, respectively. This difference was confirmed by comparing the 1H and 13C NMR data with relevant references13,16. Compound 1a was first oxidized using PhIO and NBu4I to afford 1,2-diacetyl-6-carbonyllycorine (2b). The acetyl groups of 2b were then cleaved to yield 2a, which was further etherified with methyl iodide to give 2c16. Subsequent reduction of the C-6 carbonyl of 2c afforded 1, 2-dimethoxylycorine (1b). The benzodioxole group of 1a and 1c was degraded using BBr3 to afford compounds 3 and 3a, respectively16. In addition, lycorine was selectively oxidized by Jones agents to afford 7, followed by etherification to get compounds 7a–c. Lycorine hydrochloride was transformed to dihydrolycorine hydrochloride (4) by hydrogenation, followed by reaction with NaOH to furnish compound 5 (Fig. 3).
Synthesis of lycorine derivatives.
Reagents and conditions: (a) Ac2O (3.0 eq), DMAP, py, r.t., 8 h, 78%; (b) Ac2O (1.0 eq), py, r.t., 2 h, 40%; (c) 10% HCl, 60 °C, 1.5 h, 40%; (d) PhIO, NBu4I, CH3CN/H2O 9:1, r.t., 6 h, 75%; (e) K2CO3, MeOH/H2O 9:1, 60 °C, 4 h, 90%; (f) NaH, MeI, THF, r.t., 24 h, 80%; (g) LAH, THF, −78 °C, 6 h, 75%; (h) BBr3, CH2Cl2, −78 °C, 6 h, 75% for 3, 60% for 3a; (i) 10% Pd/C, H2, H2O, r.t., 24 h; (j) NaOH, H2O, 95%; (k) Burgess reagent, DMF, 50 °C, 2 h, 70%; (l) Jones reagent, r.t., 8 h, 60%; (m) Ac2O, py, 60 °C, 8 h, 72%; (n) propionic anhydride, py, 60 °C, 8 h, 65%; (o) Butyric anhydride, py, 60 °C, 12 h, 70%.
The Anti-HCV Activity of Lycorine Derivatives
All 15 analogues were examined to determine their effects on HCV replication in Huh 7.5 cells (EC50) and their cytotoxicity (CC50) was assessed in vitro to determine their selective index (SI) values (Table 1) using Cyclopenta [c]pyrrole-1-carboxamide (VX-950), which is an inhibitor of the HCV NS3/4A protease, as a positive control17. The data indicate that these compounds lost their cytotoxicity to different degrees and some of these compounds, such as 1a and 3, exhibited reduced cytotoxicity while possessing levels of anti-HCV activity similar to that of lycorine.
SAR Analysis of the Anti-HCV Effects of Lycorine Derivatives
The SAR study initially focused on the effects of modifying the C-1 and C-2 hydroxyl groups of lycorine by examining compounds 1a–d. The disubstituted derivative 1a and lycorine exhibited similar levels of anti-HCV activity, but the other three analogues, 1b, 1c and 1d, exhibited weaker anti-HCV activities, to varying degrees. These results suggest that diacetyl derivatives have stronger anti-HCV activity than monoacetylated or dimethylated ones, so the hydroxyl groups at C-1 and C-2 could be crucial to the cytotoxicity of lycorine (Fig. 4a).
SAR analysis of lycorine derivatives.
(a) Disubstitution, especially the diacetylation of the free hydroxyl groups at C-1, C-2, should markedly reduce the cytotoxicity; (b) Oxidization of the basic nitrogen atom of lycorine reduces its anti-HCV activity; (c) Degradation of the benzodioxole group reduces the cytotoxicity. The SI value of 3 is greater than 50. (d) An α, β-unsaturated ketone would improve the HCV inhibitory activity of lycorine. The CC50 value of compound 2a, 2b, 7a, 7b and 7c are higher than 80 μM. The SI values of compound 3 and 7b are higher than 80. All mean and standard deviation data were obtained from quintuplicate experiments (N = 5).
The nitrogen atoms in alkaloids are known to have profound effects on their biological activities. The oxidized lycorine derivatives 2a–c were therefore prepared to determine how the conversion of the parent compound’s basic nitrogen into an amide would affect its biological activity. All three derivatives had substantially lower anti-HCV activity than lycorine itself, regardless of their C-1 and C-2 substituents. The basic nitrogen at the N-5 position thus seems be very important to the anti-HCV activity (Fig. 4b).
Notably, the two derivatives with a degraded benzodioxole group (3 and 3a) were substantially less cytotoxic than the parent compounds. Compared to 1a, compound 3 lost a large portion of its cytotoxicity, while its anti-HCV activity remained nearly constant. A similar phenomenon can be observed in the comparisons of 1a with 3a. It appears that the benzodioxole group is another key site of cytotoxicity whose removal has little effect on HCV inhibition (Fig. 4c).
α, β-unsaturated ketones are widely considered to be crucial to the bioactivities of compounds, so we designed lycorine derivatives with an α, β-unsaturated ketone substructure (compounds 7, 7a, 7b and 7c) by the oxidation of the hydroxyl at the C-2 position. An anti-HCV bioassay indicated that the unsaturated ketone could indeed enhance the anti-HCV activity of compounds, such as 7. However, when the hydroxyl at the C-1 position was substituted by a methyl (7a), ethyl (7b) or allyl (7c) group, the cytotoxicity decreased and the anti-HCV activity was degraded (Fig. 4d). It can be inferred that an α, β-unsaturated ketone would improve the HCV inhibitory activity of lycorine.
The importance of the C3-C4 double bond was then investigated. Hydrogenation of this double bond noticeably alters the electron distribution within ring D of lycorine and may thereby affect its anti-HCV activity. The comparison of lycorine and compound 5 indicates that a compound without the double bond, such as 5, does not show anti-HCV activity. It thus seems that the C3-C4 double bond is crucial to the anti-HCV activity. The aromatization of ring D (compound 6) also causes a loss of the anti-HCV activity of lycorine.
Evaluation of the Apoptosis Induction Activity of Lycorine Derivatives
As mentioned above, some scientists reported that lycorine exhibits significant antitumour activity correlated with apoptosis15. We analyzed the anti-HCV activities of modified lycorine using qRT-PCR under the circumstance with the cells no cytotoxicity judged with CPE method and confirmed with MTT staining method after 72 hours treatment with derivatives. If there is no cytotoxicity with CPE or MTT method after 72 hours treatment with compounds, there is no apoptosis which will more or less show cytotoxicity that can be detected with those methods. To confirm this speculate, we added three of good HCV inhibitors 1a, 3 and 7 at the concentration of 2 times of EC50 into HCV-infected Huh 7.5 cells. At that dose, they showed no cytotoxicity with CPE method (Lane CPE) and also no apoptosis confirmed with apoptoticbody staining method (Lane Apoptoticbody staining). But, they still showed strongly anti-HCV activity (Lane HCV core staining). These results indicated that lycorine derivatives could not induce Huh 7.5 cell apoptosis (Fig. 5).
Conclusions
The cytotoxic lycorine could become a safe and efficient HCV inhibitor through appropriate modifications. According to the processes of the structure-activity relationship optimization of lycorine derivatives, we conclude that the C3-C4 double bond and the basic nitrogen at N-5 position are crucial to the anti-HCV activity. The benzodioxole group and the hydroxyl groups at C-1 and C-2 seem to be very important to the cytotoxicity. The disubstitution, especially the diacetylation of the free hydroxyl groups at C-1 and C-2 and/or the degradation of the benzodioxole group, should markedly reduce the cytotoxicity of the lycorine derivatives. However, this is accompanied by a variable reduction in the anti-HCV activity. The addition of an α, β-unsaturated ketone would improve the HCV inhibitory activity. As the C3-C4 double bond is crucial to the anti-HCV activity, the hydrogenation of this double bond would clearly weak the HCV inhibition. These findings provide constructive guidance for further structural modifications of lycorine.
Methods
Chemical
Reaction procedures of lycorine derivatives
1,2-Di-O-Acetyllycorine (1a)
Lycorine (30 mg 0.1 mmol) was added to a solution of pyridine (3 mL), Ac2O (30 mg, 0.3 mmol) and DMAP (20 mg). The solution was stirred at room temperature for 8 h under N2 and then poured into ice-cold water (50 mL). The mixture was extracted with EtOAc (30 mL) twice and washed with saturated NaHCO3 followed by brine and then concentrated after separation by silica gel column chromatography to afford compound 1a with a yield of 78%.
2-O-Acetyllycorine (1c)
Lycorine (33 mg 0.1 mmol) was added into a solution of pyridine (3 mL) and Ac2O (8 mg, 0.08 mmol). The solution was stirred at room temperature for 2 h under N2 and then poured into ice-cold water (50 mL) with vigorous stirring. The mixture was extracted with EtOAc (30 mL) twice, washed with saturated NaHCO3 and brine and then concentrated following separation by silica gel column chromatography to afford compound 1c with a yield of 40%.
1-O-Acetyllycorine (1d)
A solution of 1a (37 mg, 0.1 mmol) in 5 mL of 10% HCl was heated at 60 °C for 1.5 h. Then, the reaction mixture was treated with NaHCO3 solution until a basic pH was reached and extracted several times with CH2Cl2. The organic phases were dried over MgSO4, concentrated and purified by silica gel column chromatography to obtain 1-O-acetyllycorine (1d) (18 mg, 40%).
1,2-Di-O-Acetyl-6-carbonyl-lycorine (2b)
Tetrabutylammonium iodide (TBAI, 37 mg, 0.1 mmol) and ISB (308 mg, 1.4 mmol) were dissolved in MeCN/H2O 9:1 (10 mL). Compound 1a (0.5 mmol) was subsequently added to the mixture, which was stirred for 6 h at room temperature and then diluted with toluene (100 mL). The organic layer was washed with sodium hyposulphite solution (2 × 10 mL) and saturated brine (2 × 10 mL). It was then concentrated under reduced pressure to yield the crude product, which was purified by silica gel column chromatography to afford 1,2-di-O-acetyl-6-carbonyl-lycorine (2b) with a yield of 75%.
6-Carbonyllycorine (2a)
A solution of compound 2b (34.3 mg, 0.1 mmol) and CH3OH/H2O (9:1, 10 mL) was added to K2CO3 (41.4 mg, 0.3 mmol). The mixture was stirred for 4 h at 60 °C and then diluted with EtOAc (30 mL). The organic layer was washed with NH4Cl solution (2 × 10 mL) and saturated brine (2 × 10 mL) and concentrated following separation by silica gel column chromatography to afford 6-carbonyllycorine (2a) with a yield of 90%.
1,2-Di-O-Methyl-6-carbonyllycorine (2c)
Compound 2a (30 mg, 0.1 mmol) was dissolved in dry THF (10 mL), after which NaH (50 mg, 2 mmol) and CH3I (1 mmol) were added. The mixture was stirred at room temperature for 24 h and then quenched with water (50 mL) in an ice bath. The reaction solution was evaporated to remove THF and extracted with CH2Cl2 (30 mL) twice. The organic layer was washed with saturated NaHCO3 and then brine, dried over MgSO4, filtered and concentrated. The residue was purified by column chromatography to afford 1,2-di-O-methyl-6-carbonyllycorine (2c), with a yield of 80%.
1,2-Di-O-Methyllycorine (1b)
Compound 2c (33 mg, 0.1 mmol) was dissolved in THF (5 mL). The reaction solution was then cooled to −78 °C and LiAlH4 (7.6 mg, 0.2 mmol) was added. The mixture was stirred for 2 h and diluted in H2O (10 mL). The solution was extracted with CH2Cl2 (15 mL) twice. The organic layer was washed with brine, concentrated and then purified by column chromatography to give 1,2-di-O-methyllycorine (1b), with a yield of 80%.
1,2-Di-O-Acetyllycorine-8,9-diphenol (3)
Compound 1a (70 mg, 0.2 mmol) was dissolved in 10 mL CH2Cl2. The reaction solution was then cooled to −78 °C and BBr3 (120 μL, 0.3 mmol) was added. The mixture was then stirred for 6 h and diluted in 50 mL saturated NaHCO3, after which it was extracted twice with CH2Cl2 (20 mL). The organic layer was then washed with brine and concentrated. The residue was purified by column chromatography using chloroform-methanol (15:1) as the eluent to give 1,2-di-O-acetyllycorine-8,9-diphenol (3) as a pale yellow powder (49.5 mg, yield 75%).
2-O-Acetyllycorine-8,9-diphenol (3a)
Compound 3a was synthesized from 1c (66 mg, 0.02 mmol) by the same procedure as 3. The crude product was purified by column chromatography using chloroform-methanol (30:1) as the eluent to give 2-O-acetyllycorine-8,9-diphenol (3a) as a pale yellow powder (38.5 mg, yield 60%).
α-Dihydrolycorine (5)
A solution of lycorine hydrochloride (60 mg, 0.02 mmol) and 10% Pd/C (60 mg) in H2O (10 mL) was stirred under an atmosphere of H2 for 40 h to afford 4. The solution was alkalized by NaOH (in solution, 1 M/L) and then filtered and the cake was purified by column chromatography to afford α-dihydrolycorine (5) as a colourless solid (25 mg, yield 95%).
5,7-dihydro-4H-[8,9]dioxolo[4,5-j]pyrrolo[3,2,1-de]phenanthridine (6)
A solution of lycorine (30 mg, 0.01 mmol) and Burgess reagent (24 mg, 0.1 mmol) in DMF (2 mL) was stirred under an atmosphere of H2 at 50 °C for 2 h to afford 4. The solution was then evaporated to remove DMF and purified by column chromatography to afford 5,7-dihydro-4H-[8,9]dioxolo[4]pyrrolo[2,3]phenanthridine (6) as a colourless solid (20 mg, yield 80%).
2-Oxolycorine (7)
Lycorine (30 mg, 0.01 mmol) was dissolved in 5 ml THF, to which Jones reagent (1 mL) was added. The mixture was then stirred at room temperature for 8 h, quenched with water (5 mL) and neutralized with NaHCO3. The reaction solution was evaporated to remove THF and extracted with CH2Cl2 (15 mL) twice. The organic layer was concentrated and purified by column chromatography to afford 2-oxolycorine (7) (18.5 mg, yield 65%).
Preparation of compounds 7a–c
Compound 7 (30 mg, 0.1 mmol) was dissolved in dry THF (10 mL), after which NaOH (10 mg, 0.25 mmol) and alkylating agents (methyl iodide or ethylbromide or allyl bromide, 1 mmol) were added. The mixture was stirred at room temperature for 24 h and then quenched with water (50 mL) in an ice bath. The reaction solution was evaporated to remove THF and extracted with CH2Cl2 (30 mL) twice. The organic layer was washed with saturated NaHCO3 and then brine, dried over MgSO4, filtered and concentrated. The residue was purified by column chromatography to afford 7a, 7b and 7c.
1-methoxy-2-carbonyl-lycorine (7a)
6 mg, yield 20%.
1-ethoxy-2-carbonyl-lycorine (7b)
9.4 mg, yield 30%.
1-allyloxy-2-carbonyl-lycorine (7c)
6.6 mg, yield 20%.
Bioactivity Assay
Cell Culture
Huh 7.5 human liver cells were cultured in Dulbecco’s modified Eagle medium (DMEM, Invitrogen, CA) supplemented with 10% inactivated foetal bovine serum (Invitrogen) and 1% penicillin-streptomycin (Invitrogen). Cells were digested with 0.05% trypsin-ethylene diamine tetraacetic acid (EDTA) (Invitrogen) and split twice a week.
HCV Infection and Treatment
The Huh 7.5 cells were seeded into 96-well plates (Costar) at a density of 3 × 104 cells/cm2; after 24 hours of incubation, the cells were infected with HCV viral stock (chimeric HCV FL-J6/JFH/JC1 virus strain, approximately 45 IU/cell) and simultaneously treated with either one of the compounds in solution or with solvent only as a control. After 72 hours inoculation, the culture medium was removed and the intracellular RNA was extracted using the RNeasy Mini Kit (Qiagen). Additionally, Total intracellular proteins were also extracted using the Cyto-Buster Protein Extraction Reagent (Novagen) with a 1 mM protease inhibitor cocktail (Roche Applied Science). The intracellular levels of the HCV mRNA and the internal control gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA were quantified using an AgPath-ID™ One-Step RT-PCR Kit (Applied Biosystems). The fluorescent signal was detected using a 7500-fast real time PCR system (Applied Biosystems). The HCV core protein was detected by western blotting (see below).
Cytotoxicity Assay
50 μL of Huh 7.5 cells at a density of 2 × 105 cells/mL was plated into 96-well plates. Fresh culture medium containing test compounds at various concentrations was added 24 hours later. Cytotoxicity was evaluated using a tetrazolium (MTT) assay after 72 hours of treatment.
Western Blotting
The extracted total protein or viral lysates were denatured by adding loading buffer (5 × 250 mM tris-HCl, pH 6.8, 5% dithiothreitol, 10% SDS, 0.5% bromophenol blue, 50% glycerol), followed by boiling the lysates for 5 minutes at 100 °C. Proteins were analysed by SDS-PAGE and then transferred to nitrocellulose membranes using an electroblotter (Bio-Rad Laboratories). The membranes were blocked by 5% non-fat dry milk in TBS-T solution (20 mM Tris [pH 7.4], 150 mM NaCl, 0.1% Tween-20) for 1 hour and then washed three times for 10 minutes each in the TBS-T solution. Membrane samples were probed with a monoclonal antibody specific to the HCV core protein (diluted to 1 μg/mL; Abcam, Ltd.) or Hsc70 (diluted to 0.2 μg/mL; Santa Cruz Biotechnology, Inc., Santa Cruz, CA). As a control, probing experiments were performed using a polyclonal antibody against actin (diluted to 0.2 μg/mL; Santa Cruz Biotechnology, Inc.). After being washed with TBS-T, the membranes were incubated for one hour at room temperature with the appropriate secondary antibody: goat anti-mouse for the HCV core protein, goat anti-rat for Hsc70 and goat anti-rabbit for actin (ZSGB-BIO, China). The protein signal was visualized and captured using Immobilon Western Chemiluminescent HRP Substrate ECL working solution (Millipore Inc.) and a ChemiDoc™ XRS gel imager system (Bio-Rad, CA).
Additional Information
How to cite this article: Chen, D. et al. Design, Synthesis and Structure-Activity Relationship Optimization of Lycorine Derivatives for HCV Inhibition. Sci. Rep. 5, 14972; doi: 10.1038/srep14972 (2015).
References
Gordon, C. P. & Keller, P. A. Control of hepatitis C: a medicinal chemistry perspective. J. Med. Chem. 48, 1–20 (2004).
Huang, Z., Murray, M. G. & Secrist, J. A. Development of therapeutics for chronic HCV infection. Antiviral. Res. 71, 351–362 (2006).
Houghton, M. & Abrignani, S. Prospects for a vaccine against the hepatitis C virus. Nature. 436, 961–966 (2005).
Leung, D., Abbenante, G. & Fairlie, D. P. Protease inhibitors: current status and future prospects. J. Med. Chem. 43, 305–341 (2000).
Upasana, R. & Saumitra, D. Interplay between NS3 protease and human La protein regulates translation-replication switch of hepatitis C virus. Sci. Rep. 1, 1, 10.1038/srep00001 (2011).
Salah, E. D. R., Abdel, M. A. R., El-Halawany, A. M., Hattori, M. & Abdel, S. E. HCV-NS3/4A protease inhibitory iridoid glucosides and dimeric foliamenthoic acid derivatives from anarrhinum orientale. J. Nat. Prod. 74, 943–948 (2011).
Salam, K. A. et al. Inhibition of hepatitis C virus NS3 helicase by manoalide. J. Nat. Prod. 75, 650–654 (2012).
Guan, L. & Disney, M. D. Recent advances in developing small molecules targeting RNA. ACS Chem. Biol. 7, 73–86 (2011).
Czarniecki, M. Small molecule modulators of toll-like receptors. J. Med. Chem. 51, 6621–6626 (2008).
David, S. C. et al. Hepatitis C virus antigenic convergence. Sci. Rep. 2, 267, 10.1038/srep00267 (2012).
Gabrielsen, B. et al. Antiviral (RNA) activity of selected amaryllidaceae isoquinoline constituents and synthesis of related substances. J. Nat. Prod. 55, 1569–1581 (1992).
Ieven, M. et al. Plant antiviral agents.3 Isolation of alkaloids from clivia miniata regel (amaryllidaceae). J. Nat. Prod. 45, 564–573 (1982).
Lamoral, T. D. et al. Lycorine, the main phenanthridine amaryllidaceae alkaloid, Exhibits significant antitumor activity in cancer cells that display resistance to proapoptotic stimuli: an investigation of structure-activity relationship and mechanistic insight. J. Med. Chem. 52, 6244–6256 (2009).
Antonio, E. et al. Biological evaluation of structurally diverse amaryllidaceae alkaloids and their synthetic derivatives: discovery ofnovel leads for anticancer drug design. Planta Med. 52, 501–507 (2009).
Jerald, N. J. et al. Cytotoxicity studies of lycorine alkaloids of the Amaryllidaceae. Nat. Prod. Commun. 9, 1193–1210 (2014).
Toriizuka, Y. et al. New lycorine-type alkaloid from Lycoris traubii and evaluation of antitrypanosomal and antimalarial activities of lycorine derivatives. Bioorg. Med. Chem. 16, 10182–10189 (2008).
Lin, C. et al. In vitro resistance studies of hepatitis C virus serine protease inhibitors, VX-950 and BILN 2061: structural analysis indicates different resistance mechanisms. J. Biol. Chem. 279, 17508–17514 (2004).
Acknowledgements
We thank Dr. Weiguang Wang (Kunming Institute of Botany, Chinese Academy of Sciences, China) for providing help on the purification of the compounds throughout this research work. This research was supported financially by the National Natural Science Foundation of China (81402828 and 21432010) and the “Xi Bu Zhi Guang” Foundation of the Chinese Academy of Sciences.
Author information
Authors and Affiliations
Contributions
D.C. and C.J. designed and performed the chemical synthesis under the guidance of X.H.; J.C. purified all the compounds; Z.P., J.C., J.Y. and J.J. evaluated the anti-HCV activities and other bio-activities of all compounds; D.C. and X.H. wrote the manuscript with advice from all of the authors; X.H. guided all aspects of this study.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Chen, D., Cai, J., Cheng, J. et al. Design, Synthesis and Structure-Activity Relationship Optimization of Lycorine Derivatives for HCV Inhibition. Sci Rep 5, 14972 (2015). https://doi.org/10.1038/srep14972
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep14972
This article is cited by
-
Searching for plant-derived antivirals against dengue virus and Zika virus
Virology Journal (2022)
-
In Vitro Inhibition of Alphaviruses by Lycorine
Virologica Sinica (2021)
-
An Explorer of Chemical Biology of Plant Natural Products in Southwest China, Xiaojiang Hao
Natural Products and Bioprospecting (2018)
-
Tylophorine Analogs Allosterically Regulates Heat Shock Cognate Protein 70 And Inhibits Hepatitis C Virus Replication
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.