Abstract
BiCh2-based compounds (Ch: S, Se) are a new series of layered superconductors and the mechanisms for the emergence of superconductivity in these materials have not yet been elucidated. In this study, we investigate the relationship between crystal structure and superconducting properties of the BiCh2-based superconductor family, specifically, optimally doped Ce1−xNdxO0.5F0.5BiS2 and LaO0.5F0.5Bi(S1−ySey)2. We use powder synchrotron X-ray diffraction to determine the crystal structures. We show that the structure parameter essential for the emergence of bulk superconductivity in both systems is the in-plane chemical pressure, rather than Bi-Ch bond lengths or in-plane Ch-Bi-Ch bond angle. Furthermore, we show that the superconducting transition temperature for all REO0.5F0.5BiCh2 superconductors can be determined from the in-plane chemical pressure.
Similar content being viewed by others
Introduction
Most superconductors with a high transition temperature (Tc) possess a layered crystal structure. Typical examples of layered high-Tc superconductors are Cu-oxide1 and Fe-based superconductors2; the crystal structures of these superconductors are composed of alternating superconducting layers (CuO2 or FeAs layers, respectively) and electrically-insulating spacer layers. The spacer layers are essential for the emergence of low-dimensional electronic states within the superconducting layers, which sometimes result in unconventional pairing mechanisms. One of the attractive features of the layered superconductors is the wide variety of crystal structures. New superconductors can be designed by stacking superconducting layers and various types of spacer layers; the superconducting properties depend on the type of the spacer layer. In fact, many Fe-based superconductors containing various types of spacer layers have been discovered, several of which attained high Tc2,3,4,5,6,7,8.
With this wide selection of related layered superconductors having various structures and properties, one can determine which crystal structure parameters are essential for the emergence of superconductivity in a given family of layered superconductors. It was found that Tc for the Fe-based family can be estimated from a crystal structure parameter, such as the As-Fe-As bond angle9 or the anion height from the Fe square lattice (anion = As, P, Se and Te)10. Changes in these parameters strongly affect Fermi surface configurations and pairing symmetry, subsequently affecting the superconducting properties of Fe-based superconductors11. In fact, clarification of which crystal structure parameter influences superconducting properties is one of the most important challenges for understanding the mechanisms of superconductivity in a new series of layered superconductors; this also provides a direct strategy to design new layered superconductors with high Tc.
In 2012, we reported layered superconductors composed of alternate stacks of BiS2 superconducting layers and various spacer layers12,13. Band calculations suggested that the parent compound of BiS2-based superconductors is an insulator containing Bi3+. Superconductivity is induced when electron carriers are doped into the BiS2 layers (in Bi-6p orbitals) by element substitution in the spacer layers12,14. For example, a parent compound REOBiS2 becomes a superconductor when O2− in the spacer layers is partially substituted by F− (namely, REO1−xFxBiS2)13. To date, 12 types of parent compounds of BiS2-based superconductors have been discovered: REOBiS2 (RE = La13, Ce15, Pr16, Nd17, Sm18, Yb19 and Bi20,21), AFBiS2 (A = Sr22,23, Eu24), Bi6O8S512, Bi3O2S325 and Eu3F4Bi2S426. Superconductivity was also observed in a BiSe2-based compound, LaO0.5F0.5BiSe227. Exploring new these compounds is a growing field of research in condensed matter physics.
As described above, electron-carrier doping is necessary for the emergence of superconductivity in the BiCh2 family. Some high-pressure (HP) studies, however, suggest that the crystal structure is also important for the emergence of superconductivity in these materials28,29,30,31,32. One such study investigated the effect of HP on Tc, in which LaO0.5F0.5BiS2 showed remarkable changes in superconductivity under HP. LaO0.5F0.5BiS2 did not exhibit bulk superconductivity (bulk SC), though it showed a filamentary (weak) superconductivity signal with a Tc of 2.5 K13. With the application of HP, LaO0.5F0.5BiS2 become a bulk superconductor and Tc drastically increases from 2.5 K to over 10 K13,28,29,30,31,32. Tc enhancement under HP has also been observed in other REO0.5F0.5BiS2 superconductors32. These facts strongly suggest that superconducting properties of BiCh2-based superconductors are correlated with changes in crystal structure, which is analogous to findings for the Fe-based family9,10.
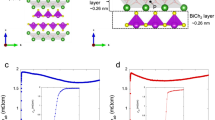
Furthermore, our recent studies concerning the effect of isovalent-substitution on superconductivity suggest that optimization of crystal structure is important for the emergence of bulk SC and the ability to attain a high Tc in optimally doped REO0.5F0.5BiCh2. We use the example of Ce1−xNdxO0.5F0.5BiS233 for following discussion. Since in this crystal structure, the valence of Ce and Nd are both 3+, electron carriers in these compounds are essentially the same: the formal valence of Bi is 2.5+. However, bulk SC is induced by the systematic substitution of Ce by Nd and Tc increases with increasing Nd concentration (x) as shown in Fig. 1a. The emergence of superconductivity in this material was explained by uniaxial lattice shrinkage along the a-axis and optimization of the lattice shrinkage ratio, c/a33. Another example of isovalent-substitution systems is LaO0.5F0.5Bi(S1−ySey)234. In this material, the S2− site within the superconducting layers is systematically substituted by Se2−. Therefore, the formal valence of Bi (2.5+) should not change with Se substitution. Bulk SC is induced by Se substitution and Tc increases with increasing Se concentration (y) as shown in Fig. 1b. Se substitution enhances the metallic conductivity of this system and induces bulk SC through lattice volume expansion34.
Superconductivity phase diagrams of Ce1−xNdxO0.5F0.5BiS2 and LaO0.5F0.5Bi(S1−ySey)2.
(a) Superconductivity phase diagrams of Ce1−xNdxO0.5F0.5BiS2. For x = 0 and 0.2, superconducting transition is not observed at T > 2 K. For 0.4 ≤ x ≤ 1, superconducting transition with a large shielding signal, with which we could regard the samples as a bulk superconductor (Bulk SC), is observed. Tc increases with increasing x. The inset figure shows a schematic of crystal structure of Ce1−xNdxO0.5F0.5BiS2. (b) Superconductivity phase diagrams of LaO0.5F0.5Bi(S1−ySey)2. For y = 0 and 0.1, superconducting transition is observed but their shielding signals are very small as a bulk superconductor (Filamentary SC). For y ≥ 0.2, superconducting transition with a large shielding signal is observed (Bulk SC). Tc increases with increasing y up to y = 0.5. The inset figure shows a schematic image of crystal structure of LaO0.5F0.5Bi(S1−ySey)2.
These experimental results obtained from HP studies on REO0.5F0.5BiS213,28,29,30,31,32 and isovalent-substitution studies on Ce1−xNdxO0.5F0.5BiS2 and LaO0.5F0.5Bi(S1−ySey)233,34, confirm that superconducting properties of the BiCh2 family are influenced by not only electron carrier concentration, but also crystal structure. Although previous studies suggest the importance of in-plane Bi-S distance18 and/or in-plane S-Bi-S angle35 on superconductivity, the universal relationship between crystal structure and superconductivity (and its Tc) in the BiCh2 family has not been clarified. In this study, we aim to determine a crystal structure parameter which universally explains the emergence of superconductivity and evolution of Tc in the BiCh2 family. We studied two isovalent-substitution systems, Ce1−xNdxO0.5F0.5BiS2 and LaO0.5F0.5Bi(S1−ySey)2, which exhibit similar superconductivity phase diagrams as shown in Fig. 1. To determine changes in crystal structure parameters and their relationship to superconductivity, we performed powder synchrotron X-ray diffraction (XRD) and Rietveld refinement for these two systems.
Here, we show that the structure parameter essential for the emergence of bulk SC in the BiCh2-based family is the in-plane chemical pressure, but not the Bi-Ch bond lengths or in-plane Ch-Bi-Ch angle. Furthermore, we show that Tc for REO0.5F0.5BiCh2 superconductors can be universally determined from the in-plane chemical pressure. We believe that the important role of in-plane chemical pressure in the evolution of superconductivity in REO0.5F0.5BiCh2 demonstrated here will be useful for designing new Bi-Ch-based layered superconductors with high Tc.
Results
Evolution of crystal structure parameters
We performed powder synchrotron XRD and Rietveld refinement analysis for Ce1−xNdxO0.5F0.5BiS2 and LaO0.5F0.5Bi(S1−ySey)2. Typical Rietveld refinement profiles for x = 0.6 and y = 0.5 are displayed in the supplementary information (Figure S1). Although minute impurity phases of the RE fluorides were detected, the structures of the main phase was refined using a tetragonal P4/nmm space group. The crystal structure parameters are plotted as a function of x (or y) in Fig. 2; the crystal structure data are listed in the supplementary information (Table S1). In Fig. 2, the data points for the samples showing bulk SC are highlighted with orange-filled circles. From Fig. 1, we note that Ce1−xNdxO0.5F0.5BiS2 and LaO0.5F0.5Bi(S1−ySey)2 exhibit similar superconductivity phase diagrams as a function of x or y. Therefore, if a crystal structure parameter for these two series changes similarly, the parameter should be considered essential for the emergence of superconductivity in these series.
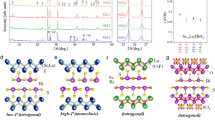
Crystal structure parameters for Ce1−xNdxO0.5F0.5BiS2 and LaO0.5F0.5Bi(S1−ySey)2.
(a) Lattice constant of a for Ce1−xNdxO0.5F0.5BiS2 and LaO0.5F0.5Bi(S1−ySey)2 is plotted as a function of x (or y). Data points for the samples showing Bulk SC in Fig. 1 are highlighted with orange filled circles in (a–e). (b) Lattice constant of c for Ce1−xNdxO0.5F0.5BiS2 and LaO0.5F0.5Bi(S1−ySey)2 is plotted as a function of x (or y). (c) Evolution of Ch1-Bi-Ch1 angle for Ce1−xNdxO0.5F0.5BiS2 and LaO0.5F0.5Bi(S1−ySey)2 as a function of x (or y). (d) Se occupancy at the in-plane Ch1 site in LaO0.5F0.5Bi(S1−ySey)2 as a function y. (e) Evolution of three different Bi-Ch distances in Ce1−xNdxO0.5F0.5BiS2 and LaO0.5F0.5Bi(S1−ySey)2 as a function of x (or y). The right image describes the Bi, Ch1 and Ch2 sites and three Bi-Ch distances: Bi-Ch1 (in-plane), Bi-Ch1 (inter-plane) and Bi-Ch2.
Figure 2a,b show the x (or y) dependences of the lattice constants a and c for Ce1−xNdxO0.5F0.5BiS2 and LaO0.5F0.5Bi(S1−ySey)2, respectively. In Ce1−xNdxO0.5F0.5BiS2, both lattice constants decrease with increasing x due to increasing concentration of Nd3+ (with an ionic radius of 112 pm, assuming a coordination number of 8), which is smaller than Ce3+ (with an ionic radius of 114 pm). Conversely, in LaO0.5F0.5Bi(S1−ySey)2, both lattice constants increase with increasing y due to the increase of Se2− (with an ionic radius of 198 pm, assuming a coordination number of 6) concentration, which is larger than S2− (with an ionic radius of 184 pm). These contrasting changes in lattice constants suggest that the evolution of superconductivity in these two series cannot be explained by simple lattice contraction or expansion.
Figure 2c shows the x (or y) dependences of Ch1-Bi-Ch1 angle for Ce1−xNdxO0.5F0.5BiS2 and LaO0.5F0.5Bi(S1−ySey)2. The Ch1-Bi-Ch1 angle is an indicator of the flatness of the Bi-Ch1 plane. Since electrons in the hybridized Bi-6p/Ch-p orbitals (S-3p or Se-4p) are essential for the emergence of superconductivity in BiCh2-based superconductors, a flatter Bi-Ch1 plane should facilitate the emergence of bulk SC with high Tc. A study of the crystal structure of CeO1−xFxBiS2 with different F concentrations demonstrated that a flatter Bi-S1 plane resulted in higher superconducting properties36. In Ce1−xNdxO0.5F0.5BiS2, the Ch1-Bi-Ch1 angle approaches 180° with increasing x, which indicates that the Bi-S1 plane becomes flatter with Nd substitution and superconductivity is induced. In contrast, in LaO0.5F0.5Bi(S1−ySey)2, the dependence of the Ch1-Bi-Ch1 angle on Se concentration decreases with increasing y, leading to distortion of the Bi-Ch1 plane. Therefore, the Ch1-Bi-Ch1 angle (i.e. the flatness of the Bi-Ch1 plane) cannot explain the evolution of superconductivity in these BiCh2-based superconductors. The contrasting changes in the in-plane structure (flatness) may be due to the difference in Ch2− ions at the Ch1 site. Figure 2d shows the y dependence of Se occupancy at the Ch1 site, indicating that Se ions selectively occupy the in-plane Ch1 site. At x = 0.5, approximately 90% of Ch1 sites are occupied with Se. Recently, a similar site selectivity of Se in LaO1−xFxBiSSe single crystals was reported37. The preferential occupation of Se2−, which has a larger ionic radius, in the two-dimensional Bi-Ch1 plane may cause distortion.
Figure 2e shows the x (or y) dependences of the three different Bi-Ch distances. Figure 2e (right) demonstrates the BiCh2 layer structure, where one Bi ion is coordinated by six Ch ions. The shortest Bi-Ch distance is the Bi-Ch2 distance along the c-axis. The Bi-Ch2 distance in Ce1−xNdxO0.5F0.5BiS2 decreases slightly with increasing x. In contrast, the Bi-Ch2 distance in LaO0.5F0.5Bi(S1−ySey)2 increases with increasing y. Therefore, this indicates that Bi-Ch2 distance cannot be correlated to the evolution of superconductivity within these two series. The Bi-Ch1 (in-plane) distance in Ce1−xNdxO0.5F0.5BiS2 decreases with increasing x. In contrast, that in LaO0.5F0.5Bi(S1−ySey)2 increases with increasing y. Thus, the in-plane Bi-Ch1 distance cannot explain the evolution of superconductivity. The longest Bi-Ch distance is Bi-Ch1 (inter-plane), which roughly corresponds to the inter-layer distance of two BiCh2 layers. The Bi-Ch1 (inter-plane) distance in Ce1−xNdxO0.5F0.5BiS2 does not change much upon Nd substitution, while that in LaO0.5F0.5Bi(S1−ySey)2 shows a noticeable increase with increasing y. Therefore, the Bi-Ch1 (inter-plane) distance cannot typically explain the evolution of superconductivity.
Although we expected the Ch1-Bi-Ch1 angle or one of the Bi-Ch distances to exhibit similar behaviour in Ce1−xNdxO0.5F0.5BiS2 and LaO0.5F0.5Bi(S1−ySey)2, we could not identify any clear correlation between them. However, the assumption that the in-plane Bi-Ch1 distance should, to a certain extent, correlate with the evolution of superconductivity stems from the facts that the superconductivity is induced in the Bi-Ch1 plane and superconducting properties change remarkably with change in the crystal structure without altering the electron carrier concentration (F concentration). Therefore, we introduce the concept of in-plane chemical pressure to discuss the relationship between in-plane structure and the evolution of superconductivity in Ce1−xNdxO0.5F0.5BiS2, LaO0.5F0.5Bi(S1−ySey)2 and other REO0.5F0.5BiCh2 superconductors.
Influence of in-plane chemical pressure on superconductivity
Figure 3a shows schematics of compression or expansion of the Bi-Ch plane caused by Nd or Se substitution. In Ce1−xNdxO0.5F0.5BiS2, Bi-Ch1 planes are compressed owing to a decrease in the volume of spacer layers with increasing Nd concentration. The compression of the Bi-Ch1 plane results in an enhancement of the packing density of Bi2.5+ and S2− ions within the superconducting plane: this is the so-called in-plane chemical pressure. In LaO0.5F0.5Bi(S1−ySey)2, the in-plane Bi-Ch1 distance increases with increasing occupancy of Se at the Ch1 site. However, the increase of the in-plane Bi-Ch1 distance is smaller than that expected from the difference in the ionic radii of S2− and Se2− because the composition of the spacer layer (LaO) remains constant in LaO0.5F0.5Bi(S1−ySey)2. Therefore, the packing density of Bi2.5+ and Ch2− ions in the superconducting plane is enhanced. This situation is similar to the enhancement of in-plane chemical pressure in Ce1−xNdxO0.5F0.5BiS2. In order to compare the magnitude of in-plane chemical pressure in the two series, we define in-plane chemical pressure using equation (1).
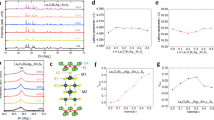
Influence of in-plane chemical pressure to crystal structure and superconductivity in Ce1−xNdxO0.5F0.5BiS2 and LaO0.5F0.5Bi(S1−ySey)2.
(a) Schematics of changes in crystal structure with increasing in-plane chemical pressure in Ce1−xNdxO0.5F0.5BiS2 and LaO0.5F0.5Bi(S1−ySey)2. In Ce1−xNdxO0.5F0.5BiS2, the volume of spacer layer decreases with increasing Nd concentration (x) and Bi-S plane is compressed; hence, in-plane chemical pressure is enhanced. In LaO0.5F0.5Bi(S1−ySey)2, the volume of superconducting Bi-Ch1 layer increases with increasing Se concentration (y). However, the expansion of Bi-Ch1 plane is smaller than that expected from the ionic radii of S2− and Se2− because the composition of the spacer layer (LaO layer) remains constant; hence, in-plane chemical pressure is enhanced as well as in Ce1−xNdxO0.5F0.5BiS2. (b) In-plane chemical pressure of Ce1−xNdxO0.5F0.5BiS2 and LaO0.5F0.5Bi(S1−ySey)2, calculated using equation (1), are plotted as a function of x (or y). In both systems, bulk SC is induced with increasing chemical pressure. The dashed line at an in-plane chemical pressure of ~1.011 is an estimated boundary of Bulk-SC and non-SC regions.

RBi is the ionic radius of Bi2.5+. Here, we assume that the ionic radius of Bi2.5+ is 104.19 pm, which is obtained from the average of the six Bi-S bonds (four in-plane Bi-S1 bonds, one inter-plane Bi-S1 bond and one Bi-S2 bond) determined from the structure analysis of a single crystal of LaO0.54F0.46BiS235. RCh1 is the ionic radius of the chalcogen at the Ch1 site: 184 and 198 pm for S2− and Se2−, respectively. In the case of LaO0.5F0.5Bi(S1−ySey)2, we calculated an average value for RCh1 using the occupancy of Se at the Ch1 site. The Bi-Ch1 (in-plane) distances were obtained from the Rietveld refinement (Fig. 2e). We note that the chemical pressure derived from ionic radii is a simplified estimation and it cannot describe the exact orbital overlap between Bi and Ch. Nevertheless, the estimation appears very useful to discuss the relationship between crystal structure and superconductive properties of these systems.
The calculated in-plane chemical pressure is plotted as a function of x (or y) in Fig. 3b. For both systems, the in-plane chemical pressure increases with increasing x (or y). Surprisingly, the chemical pressure at which bulk SC is induced is similar (above ~1.011) in both Ce1−xNdxO0.5F0.5BiS2 and LaO0.5F0.5Bi(S1−ySey)2. Based on these experimental facts, we suggest that the emergence of bulk SC in these systems can be explained by the increase of in-plane chemical pressure. Enhancement of the in-plane chemical pressure would enhance the overlap of Bi-6p and Ch-p orbitals, which would result in an increase of the metallic conductivity and induce bulk SC in the REO0.5F0.5BiCh2 family.
Discussion
Figure 4 shows the plot of Tc for Ce1−xNdxO0.5F0.5BiS2 and LaO0.5F0.5Bi(S1−ySey)2 as a function of in-plane chemical pressure. To clarify a general tendency of superconductivity in the REO0.5F0.5BiCh2 family, we added data points of Nd0.8Sm0.2O0.5F0.5BiS2 and Nd0.6Sm0.4O0.5F0.5BiS2 (from this study), PrO0.5F0.5BiS216 and single crystals of NdO0.5F0.5BiS238, SmO0.5F0.5BiS218 and LaO0.5F0.5BiSe2 (y = 1)39. Interestingly, data points for all the REO0.5F0.5BiS2-type compounds are located on a single curve bounding the blue region. Notably, the data points for CeO0.5F0.5BiS2 and SmO0.5F0.5BiS2, which do not exhibit superconductivity, are located to the left of the boundary (in-plane chemical pressure <1.011). These facts suggest that the emergence of superconductivity and the Tc of REO0.5F0.5BiS2-type materials simply depend on the magnitude of in-plane chemical pressure. According to the Bardeen-Cooper-Schrieffer (BCS) theory involving electron-phonon mechanisms40, the enhancement of Tc can be explained by an increase of phonon frequency and/or an enhancement of the density of states at the Fermi level. Usually, in metals, an increase in the orbital overlap should decrease the density of states because of an increase of bandwidth. Therefore, the increase in the density of state cannot simply explain the enhancement of Tc in this series. Although phonon frequency may be enhanced with increasing in-plane chemical pressure, further experimental and theoretical investigations are needed to elucidate the mechanisms of the enhancement of Tc with increasing in-plane chemical pressure.
Relationship between Tc and degree of in-plane chemical pressure in REO0.5F0.5BiCh2.
The data of Tc and the in-plane chemical pressure of Ce1−xNdxO0.5F0.5BiS2 and LaO0.5F0.5Bi(S1−ySey)2 are plotted with those of Nd0.8Sm0.2O0.5F0.5BiS2 and Nd0.6Sm0.4O0.5F0.5BiS2 (from this study), PrO0.5F0.5BiS216 and single crystals of NdO0.5F0.5BiS238, SmO0.5F0.5BiS218 and LaO0.5F0.5BiSe2 (y = 1)39. The curves for REO0.5F0.5BiS2 (blue) and LaO0.5F0.5Bi(S1−ySey)2 (red) lie on different regions.
A further inspection of Fig. 4 reveals that Tc for LaO0.5F0.5Bi(S1−ySey)2 and LaOBiSe2, shown by the red region, are clearly lower than those for the REO0.5F0.5BiS2-type series. An important observation is that Tc vs. in-plane chemical pressure plots for REO0.5F0.5BiS2 and LaO0.5F0.5Bi(S1−ySey)2 lie in different regions. For the LaO0.5F0.5Bi(S1−ySey)2 compounds, in-plane chemical pressure is relatively higher than that in REO0.5F0.5BiS2, but their Tc’s are noticeably lower than those of REO0.5F0.5BiS2. As described above, Se preferably occupies the in-plane Ch1 site. Therefore, it can be considered that superconductivity in LaO0.5F0.5Bi(S1−ySey)2 is induced in the Bi-(S,Se) or Bi-Se plane. From the perspective of the BCS theory, the lower Tc in the Bi-Se plane than in the Bi-S plane can be explained by a lower phonon frequency in the firmer owing to the larger atomic number of Se. To confirm the above assumption and the mechanisms of superconductivity in the BiCh2-based superconductor family, further studies of other REO0.5F0.5BiSe2 superconductors, such as CeO0.5F0.5BiSe2 or NdO0.5F0.5BiSe2, are needed for comparison. From Fig. 4, we conclude that a higher Tc should be obtained for the Bi-S plane with a higher in-plane chemical pressure.
Finally, we briefly discuss the HP phase of LaO0.5F0.5BiS2, which shows the highest Tc among BiCh2-based superconductors13,28,29,30,31,32. T. Tomita et al. reported that the crystal structure of LaO0.5F0.5BiS2 above 0.7 GPa was monoclinic31. In the monoclinic structure, the Bi-S1 plane is distorted into a zigzag chain with a Bi-S1 distance of 2.72 Å; the zigzag chains are connected with a Bi-S1 distance of 3.03 Å. In addition, we have previously reported the in-plane anisotropy of the upper critical field within the Bi-S1 plane in an HP phase of LaO0.5F0.5BiS2, implying the emergence of quasi-one-dimensional superconducting states in the distorted plane41. Calculating the value of chemical pressure by substituting a Bi-S1 distance of 2.72 Å in equation (1) yields chemical pressure of 1.06. In this case, the chemical pressure is not the in-plane chemical pressure but instead is the quasi-one-dimensional chemical pressure. A chemical pressure of 1.06 and Tc of 10 K roughly lie on the extrapolated Tc-chemical pressure curve for the other REO0.5F0.5BiS2 superconductors shown in Fig. 4. This suggests that the enhancement of quasi-one-dimensional chemical pressure, but not in-plane chemical pressure, is responsible for the evolution of superconductivity in REO0.5F0.5BiCh2 compounds. To further discuss and understand the relationship between chemical pressure and superconductivity in the BiCh2 family, investigations of crystal structure and superconducting properties of new BiS2-based, BiSe2-based and/or BiTe2-based compounds with various spacer layers are needed.
In summary, we analysed the crystal structure of the optimally doped BiCh2-based superconductors, Ce1−xNdxO0.5F0.5BiS2 and LaO0.5F0.5Bi(S1−ySey)2 using powder synchrotron XRD. We investigated the relationship between the crystal structure and the superconducting properties for these compounds. We found that an enhancement of the in-plane chemical pressure could induce bulk SC in both systems. Furthermore, we revealed that Tc for the REO0.5F0.5BiCh2 superconductors could be determined by the magnitude of in-plane chemical pressure and type of Ch element that composes the superconducting Bi-Ch planes. In addition, we extended our results to include a monoclinic phase (HP phase) of LaO0.5F0.5BiS2. We suggested that the enhancement of the quasi-one-dimensional chemical pressure within a Bi-Ch chain, but not of the in-plane (two-dimensional) chemical pressure, is required for high Tc in BiCh2-based superconductors.
Methods
Polycrystalline samples of Ce1−xNdxO0.5F0.5BiS2 and LaO0.5F0.5Bi(S1−ySey)2 used in this study were prepared using solid state reaction. The detailed synthesis procedures were described in previous reports33,34. Powder synchrotron X-ray powder diffraction measurements were performed at room temperature at the BL02B2 experimental station of SPring-8 (JASRI; Proposal No. 2014B1003, 2014B1071 and 2015A1441). The wavelength of the radiation beam was 0.49542(4) Å. We have performed the Rietveld refinement (RIETAN-FP42) for Ce1−xNdxO0.5F0.5BiS2 and LaO0.5F0.5Bi(S1−ySey)2 using a typical structure model of REOBiCh2-based superconductor with a tetragonal space group of P4/nmm35,37. Contributions from impurity phases of rare-earth fluorides (REF3) and/or Bi2S3 are included in Rietveld refinement. In addition, we have analysed a crystal structure for two related compounds of Nd0.8Sm0.2O0.5F0.5BiS2 and Nd0.6Sm0.4O0.5F0.5BiS2 to enrich the discussion part33. The obtained crystal structure parameters are summarized in Table S1. The schematic images of crystal structure were drawn using VESTA43.
Additional Information
How to cite this article: Mizuguchi, Y. et al. In-plane chemical pressure essential for superconductivity in BiCh2-based (Ch: S, Se) layered structure. Sci. Rep. 5, 14968; doi: 10.1038/srep14968 (2015).
References
Bednorz, J. G. & Müller, K. A. Possible high Tc superconductivity in the Ba−La−Cu−O system. Z. Physik B Condensed Matter 64, 189–193 (1986).
Kamihara, Y. et al. Iron-Based Layered Superconductor La[O1−xFx]FeAs (x = 0.05–0.12) with Tc = 26 K. J. Am. Chem. Soc. 130, 3296–3297 (2008).
Chen, X. H. et al. Superconductivity at 43 K in SmFeAsO1−xFx . Nature 453, 761–762 (2008).
Ren, Z. A. et al. Superconductivity at 55 K in Iron-Based F-Doped Layered Quaternary Compound Sm[O1−xFx] FeAs. Chinese Phys. Lett. 25, 2215 (2008).
Rotter, M., Tegel, M. & Johrendt, D. Superconductivity at 38 K in the Iron Arsenide (Ba1−xKx)Fe2As2 . Phys. Rev. Lett. 101, 107006 (1–4) (2008).
Wang, X. C. et al. The superconductivity at 18 K in LiFeAs system. Solid State Commun. 148, 538–540 (2008).
Ogino, H. et al. Superconductivity at 17 K in (Fe2P2)(Sr4Sc2O6): a new superconducting layered pnictide oxide with a thick perovskite oxide layer. Supercond. Sci. Technol. 22, 075008 (1–4) (2009).
Zhu, X. et al. Transition of stoichiometric Sr2VO3FeAs to a superconducting state at 37.2 K. Phys. Rev. B 79, 220512 (1–4) (2009).
Lee, C. H. et al. Effect of Structural Parameters on Superconductivity in Fluorine-Free LnFeAsO1−y (Ln = La, Nd). J. Phys. Soc. Jpn. 77, 083704 (1–4) (2008).
Mizuguchi, Y. et al. Anion height dependence of Tc for the Fe-based superconductor. Supercond. Sci. Technol. 23, 054013 (1–5) (2010).
Kuroki, K. et al. Pnictogen height as a possible switch between high-Tc nodeless and low-Tc nodal pairings in the iron-based superconductors. Phys. Rev. B 79, 224511 (1–16) (2009).
Mizuguchi, Y. et al. BiS2-based layered superconductor Bi4O4S3 . Phys. Rev. B 86, 220510 (1–5) (2012).
Mizuguchi, Y. et al. Superconductivity in Novel BiS2-Based Layered Superconductor LaO1−xFxBiS2 . J. Phys. Soc. Jpn. 81, 114725 (1–5) (2012).
Usui, H., Suzuki, K. & Kuroki, K. Minimal electronic models for superconducting BiS2 layers. Phys. Rev. B 86, 220501 (1–5) (2012).
Xing, J. et al. Superconductivity Appears in the Vicinity of an Insulating-Like Behavior in CeO1−xFxBiS2 . Phys. Rev. B 86, 214518 (1–5) (2012).
Jha, R. et al. Synthesis and superconductivity of new BiS2 based superconductor PrO0.5F0.5BiS2 . J. Supercond. Nov. Magn. 26, 499–502 (2013).
Demura, S. et al. BiS2-based superconductivity in F-substituted NdOBiS2 . J. Phys. Soc. Jpn. 82, 033708 (1–3) (2013).
Thakur, G. S. et al. Synthesis and properties of SmO0.5F0.5BiS2 and enhancement in Tc in La1−ySmyO0.5F0.5BiS2 . Inorg. Chem. 54, 1076–1081 (2015).
Yazici, D. et al. Superconductivity of F-substituted LnOBiS2 (Ln = La, Ce, Pr, Nd, Yb) compounds. Philos. Mag. 93, 673 (1–8) (2012).
Okada, T. et al. Topotactic Synthesis of a new BiS2-based superconductor Bi2(O,F)S2 . Appl. Phys. Express 8, 023102 (1–4) (2015).
Shao, J. et al. Superconductivity in BiO1−xFxBiS2 and possible parent phase of Bi4O4S3 superconductor. Supercond. Sci. Technol. 28, 015008 (1–6) (2015).
Lin, X. et al. Superconductivity induced by La doping in Sr1−xLaxFBiS2 . Phys. Rev. B 87, 020504 (1–4) (2013).
Jha, R., Tiwari, B. & Awana, V. P. S. Appearance of bulk superconductivity under hydrostatic pressure in Sr0.5RE0.5FBiS2 (RE = Ce, Nd, Pr and Sm) compounds. J. Appl. Phys. 117, 013901 (1–7) (2015).
Zhai, H. F. et al. Possible Charge-density wave, superconductivity and f-electron valence instability in EuBiS2F. Phys. Rev. B 90, 064518 (1–9) (2014).
Phelan, W. A. et al. Stacking Variants and Superconductivity in the Bi-O-S System. J. Am. Chem. Soc. 135, 5372–5374 (2013).
Zhai, H. F. et al. Anomalous Eu Valence State and Superconductivity in Undoped Eu3Bi2S4F4 . J. Am. Chem. Soc. 136, 15386–15393 (2014).
Maziopa, A. K. et al. Superconductivity in a new layered bismuth oxyselenide: LaO0.5F0.5BiSe2 . J. Phys.: Condens. Matter 26, 215702 (1–5) (2014).
Deguchi, K. et al. Evolution of superconductivity in LaO1−xFxBiS2 prepared by high pressure technique. EPL 101, 17004 (p1–p5) (2013).
Mizuguchi, Y. et al. Stabilization of High-Tc Phase of BiS2-Based Superconductor LaO0.5F0.5BiS2 Using High-Pressure Synthesis. J. Phys. Soc. Jpn. 83, 053704 (1–4) (2014).
Kotegawa, H. et al. Pressure Study of BiS2-Based Superconductors Bi4O4S3 and La(O,F)BiS2. J. Phys. Soc. Jpn. 81, 103702 (1–4) (2012).
Tomita, T. et al. Pressure-Induced Enhancement of Superconductivity and Structural Transition in BiS2-Layered LaO1−xFxBiS2 . J. Phys. Soc. Jpn. 83, 063704 (1–4) (2014).
Wolowiec, C. T. et al. Enhancement of superconductivity near the pressure-induced semiconductor–metal transition in the BiS2-based superconductors LnO0.5F0.5BiS2 (Ln = La, Ce, Pr, Nd). J. Phys.: Condens. Matter 25, 42220 (1–6) (2013).
Kajitani, J. et al. Chemical pressure effect on superconductivity of BiS2-based Ce1−xNdxO1−yFyBiS2 and Nd1−zSmzO1−yFyBiS2 . J. Phys. Soc. Jpn. 84, 044712 (1–6) (2015).
Hiroi, T. et al. Evolution of superconductivity in BiS2-based superconductor LaO0.5F0.5Bi(S1−xSex)2 . J. Phys. Soc. Jpn. 84, 024723 (1–4) (2015).
Miura, A. et al. Crystal structures of LaO1−xFxBiS2 (x ~ 0.23, 0.46): effect of F doping on distortion of Bi-S plane. J. Solid State Chem. 212, 213–217 (2014).
Miura, A. et al. Structure, Superconductivity and Magnetism of Ce(O,F)BiS2 Single Crystals. Cryst. Growth Des. 15, 39–44 (2015).
Tanaka, M. et al. Site Selectivity on Chalcogen Atoms in Superconducting La(O,F)BiSSe. Appl. Phys. Lett. 106, 112601 (1–5) (2015).
Nagao, M. et al. Structural Analysis and Superconducting Properties of F-Substituted NdOBiS2 Single Crystals. J. Phys. Soc. Jpn. 82, 113701 (1–4) (2013).
Tanaka, M. et al. First single crystal growth and structural analysis of superconducting layered bismuth oxyselenide; La(O,F)BiSe2 . J. Solid State Chem. 219, 168–172 (2014).
Bardeen, J., Cooper, L. N. & Schrieffer, J. R. Theory of Superconductivity. Phys. Rev. 108, 1175–1204 (1957).
Mizuguchi, Y. et al. Anisotropic upper critical field of BiS2-based superconductor LaO0.5F0.5BiS2 . Phys. Rev. B 89, 174515 (1–7) (2014).
Izumi, F. & Momma Three-Dimensional Visualization in Powder Diffraction. Solid State Phenom. 130, 15–20 (2007).
Momma, K. & Izumi, F. VESTA: a three-dimensional visualization system for electronic and structural analysis. J. Appl. Crystallogr. 41, 653–658 (2008).
Acknowledgements
The authors would like to thank Dr. N. L. Saini of Sapienza University of Rome, Dr. K. Kuroki of Osaka University and Dr. Y. Takano of National Institute for Materials Science for fruitful discussion. This work was partly supported by Grant-in-Aid for Young Scientist (A): 25707031 and Grant-in-Aid for challenging Exploratory Research: 26600077 and 15K14113. The synchrotron X-ray diffraction experiments were performed under proposals of JASRI; Proposal No. 2014B1003 and 2014B1071.
Author information
Authors and Affiliations
Contributions
Y.M. and A.M. planned the research. Y.M., J.K., T.H. and O.M. prepared polycrystalline samples and studied superconducting properties. Y.M., A.M., K.T., N.K., E.M., C.M. and Y.K. performed synchrotron XRD and crystal structure analysis. Y.M. wrote the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Mizuguchi, Y., Miura, A., Kajitani, J. et al. In-plane chemical pressure essential for superconductivity in BiCh2-based (Ch: S, Se) layered structure. Sci Rep 5, 14968 (2015). https://doi.org/10.1038/srep14968
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep14968
This article is cited by
-
Possible pairing mechanism switching driven by structural symmetry breaking in BiS2-based layered superconductors
Scientific Reports (2021)
-
Evolution of two bulk-superconducting phases in Sr0.5RE0.5FBiS2 (RE: La, Ce, Pr, Nd, Sm) by external hydrostatic pressure effect
Scientific Reports (2020)
-
Superconductivity in a New Layered BiSe2-Based Compound LaO1-xBiSe2
Journal of Superconductivity and Novel Magnetism (2020)
-
Bulk superconductivity in a four-layer-type Bi-based compound La2O2Bi3Ag0.6Sn0.4S5.7Se0.3
Scientific Reports (2019)
-
Superconductivity, electronic phase diagram, and pressure effect in Sr1−xPrxFBiS2
Science China Physics, Mechanics & Astronomy (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.