Abstract
Congenital heart defects (CHDs) arise through various combinations of genetic and environmental factors. Our study explores how polymorphisms in the glutathione S-transferase (GST) genes affect the association between cigarette smoke exposure and CHDs. We analysed 299 mothers of children with CHDs and 284 mothers of children without any abnormalities who were recruited from six hospitals. The hair nicotine concentration (HNC) was used to quantify maternal smoke exposure and the maternal GSTT1 and GSTM1 and GSTP1 genes were sequenced. We found a trend of higher adjusted odds ratios with higher maternal HNC levels, suggesting a dose-response relationship between maternal smoke exposure and CHDs. The lowest HNC range associated with an increased risk of CHDs was 0.213–0.319 ng/mg among the mothers with functional deletions of GSTM1 or GSTT1and 0.319–0.573 ng/mg among the mothers with normal copies of GSTM1 and GSTT1. In addition, the adjusted odds ratio for an HNC of >0.573 ng/mg was 38.53 among the mothers with the GSTP1 AG or GG genotype, which was 7.76 (χ2 = 6.702, p = 0.010) times greater than the AOR in the mothers with GSTP1 AA genotype. Our study suggests that polymorphisms of maternal GST genes may modify the association of maternal smoke exposure with CHDs.
Similar content being viewed by others
Introduction
The aetiology of congenital heart defects (CHDs), which are among the most common birth defects with an estimated prevalence of 6–8% of live births, is largely an enigma1. Although there has been substantial progress in recent years in understanding the genetic and chromosomal risk factors for CHDs2, relatively few non-inherited, modifiable risk factors for CHDs are known. Increasing evidence from epidemiological studies over the last two decades indicates that maternal smoking during pregnancy is an important risk factor for CHDs3,4. The global estimated prevalence of daily smoking was 31.1% for men and 6.2% for women in 20125. Although most adult females in China do not smoke, more than half of the adult males are smokers, which results in approximately 300 million adult women exposed to the secondhand smoke6. Therefore, the study of the relationship between maternal smoke exposure and CHDs has great public health significance.
It is important to note that maternal smoke exposure identified in most previous studies was based on self-reporting by the women3,7. Self-reporting is particularly unreliable in ascertaining true smoke exposure because of the uncertainties in memory recall, the exposure to environmental tobacco smoke (ETS) and the social stigma of smoking during pregnancy8. Thus, it is important to identify a biological marker that objectively indicates maternal exposure to smoke. In recent years, hair nicotine analysis has emerged as a novel, non-invasive method to assess long-term exposure to tobacco smoke because nicotine can accumulate and remain stable in newly formed hair9,10,11.
Accumulating evidence suggests that the gene products responsible for activating and detoxifying xenobiotics can influence the effects of smoking on cancer, coronary artery disease and congenital anomalies (e.g., oral clefts)12,13,14. The glutathione S-transferases (GST), which are part of an important family of dimeric phase II metabolic enzymes involved in the detoxification of xenobiotics, might modulate the adverse effects of exogenous toxins15,16. At least four main classes of soluble GSTs have been identified in humans: alpha (A), mu (M), pi (P) and theta (T), which are encoded by2 genes (GSTA1,A2, located at 6p12), 5 genes (GSTM1-M5, located at 1p13.3), 1 gene (GSTP1, located at 11q13) and 2 genes (GSTT1,T2, located at 22q11.2), respectively. Functional polymorphisms have been reported in the GSTT1, GSTM1 and GSTP1 genes. These include deletions in GSTT1 and GSTM1, which can abrogate enzyme activity, as well as an I105 V polymorphism in GSTP1, which is thought to result in diminished enzyme activity. It has been proposed that mothers with such GSTT1, GSTM1 or GSTP1 polymorphisms are less capable of effectively detoxifying environmental xenobiotics (such as tobacco smoke by-products) that cross the placenta; as a result, these mothers and their foetuses are exposed to elevated toxin levels16. Therefore, we hypothesized that GST gene polymorphisms may increase the susceptibility to cigarette smoke-related human diseases, including CHDs. One study indicated that polymorphisms in GSTM1 and GSTT1can potentially modify the risk of toxicant exposure-associated CHDs17, but there is little direct evidence regarding whether maternal GST gene polymorphisms can modify the risk of smoke exposure-associated CHDs. More studies are required to confirm this hypothesis.
In 2009, we initiated a study program, “Gene-Environment Interaction on CHDs” (GEIOC), to explore how environmental and genetic factors interact to influence CHDs in human offspring. The current study is an important part of the GEIOC. We explored the association between maternal smoke exposure and the occurrence of foetal CHDs and evaluated whether this association is modified by polymorphisms in GSTT1, GSTM1, or GSTP1.
Results
The characteristics of the case and control groups are listed in Table 1. Maternal education level, alcohol drinking frequency and pre-pregnancy body mass index (ppBMI) were significantly different between the case and control groups. The maternal hair nicotine concentration (HNC) showed a left-skewed distribution in the case and control groups (Table 2). In the case group, the geometric mean (GM) of the HNC was 0.369 ng/mg, which was higher than that of the control group (0.171 ng/mg) (t = −9.65, p < .0001). A large variation in HNC was observed within each self-reporting category (Fig. 1). The mothers with an HNC of >0.319 ng/mg accounted for 34.75%, 41.32%, 50.00%, 57.58% and 50.00% of the unexposed nonsmokers, slight passively exposed nonsmokers, moderate passively exposed nonsmokers, severe passively exposed nonsmokers and active smokers, respectively. No significant differences in the frequencies of GST gene polymorphisms were observed between these two groups.
Compared with an HNC of ≤0.117 ng/mg, the adjusted odds ratios (AOR) for HNCs of >0.573 ng/mg, 0.319–0.573 ng/mg and 0.213–0.319 ng/mg were 6.83 (95% CI: 3.64, 12.80), 4.37 (95% CI: 2.42, 7.88) and 1.94 (95% CI: 1.09, 3.46), respectively. There also seemed to be a trend toward higher AOR with higher HNCs for each subcategory of CHDs (Table 3). Among the mothers with deletions in GSTM1 and/or GSTT1, the lowest HNC range associated with an increased risk of any CHDs was 0.213–0.319 ng/mg; in contrast, that range was 0.319–0.573 ng/mg among the mothers with normal copies of GSTM1 and GSTT1 (Table 4). Some AORs were higher among mothers without the favourable genotypes, but that was not a consistent finding across the GST genes and HNC levels. The AORs for the HNC levels of >0.213 ng/mg among the mothers with deletions in GSTM1 were higher than those among the mothers with normal copies of GSTM1. In addition, the AOR for an HNC of >0.573 ng/mg was 38.53 (95% CI: 8.17, 181.84) among the mothers with the GSTP1 AG or GG genotype. This AOR was 7.76 (χ2 = 6.702, p = 0.010) times greater than the AOR among the mothers with the GSTP1 AA genotype. However, no statistically significant interactions of GST gene polymorphisms and HNC levels were identified by likelihood ratio testing.
Discussion
We observed a trend of higher AOR with higher HNC level, suggesting a dose-response relationship between maternal smoke exposure during the first and second trimesters and foetal CHDs. In addition, mothers with deletions in GSTT1and/or GSTM1 tended to have a lower threshold of smoke exposure associated with an elevated risk of CHDs. These findings support our hypothesis that GSTs could modify the association between maternal smoke exposure and CHDs; however, the likelihood ratio analysis we performed did not support a direct interaction between HNCs and GST polymorphisms. This may be because there is no interaction, or perhaps the sample size was too small. Additionally, GST polymorphisms might be more or less likely to interact with HNC, for example, the mothers with an HNC of >0.573 ng/mg and a GSTP1 AG or GG genotype had a much higher risk of CHDs than the mothers with a genotype of GSTP1 AA and the same HNC level.
Accumulating evidence indicates that maternal smoke exposure during pregnancy is potentially associated with an increased risk of CHDs. A recent meta-analysis found a positive association between maternal smoking during pregnancy and CHD risk as a group (RR: 1.11, 95% CI:1.02, 1.21)3. In addition, a dose response was observed between the parental smoking category (light, medium and heavy) and the CHD subtype, e.g., septal defects, conotruncal defects and left ventricular outflow tract obstructions7,18,19. Our study confirmed that maternal smoke exposure during pregnancy is associated with an increased risk of CHDs. Moreover, the degree of the increased risk of CHDs in our study was much higher than in other studies3,4,20. A very local study population and relatively strict inclusion and exclusion criteria for test cases and controls used in our study, compared to other studies, likely contribute to this difference. Additionally, the method of smoke exposure measurement and the selection of an exposure reference may contribute to the difference. Almost all of the previous epidemiological studies on the correlation between smoke exposure and CHDs used self-reporting to measure smoking exposure. Smoking mothers are likely to under-report or conceal their smoking habits due to social unacceptability or embarrassment and may not accurately recall the amount of smoking even if they have no reservations about reporting it21. It is difficult for nonsmoking mothers to accurately measure their exposure to secondhand smoking (SHS), mainly due to the various determinants of SHS, e.g., the duration and frequency of exposure and the concentrations of SHS influenced by the amount of smoking and air exchange22,23. Although it has been shown that women accurately report their smoking behaviour on birth certificates in some populations, in one study, the Spearman rho correlation coefficient between infant nicotine levels and maternal-reported cigarettes per day was only 0.54 in the third trimester24. The Montreal Prematurity Study in Canada also found a low degree of correlation (coefficient: 0.22–0.36) between the maternal HNC and the self-reported number of cigarettes smoked per day in the first and second trimesters25. A low accuracy of self-reporting is also illustrated by our finding that a considerable proportion of mothers with a high HNC were present in the unexposed nonsmokers category. If the ‘nonsmokers’ identified through self-reporting are used as the reference group, this group likely contains many participants who are actually exposed to ETS or SHS. Therefore, the risk of CHDs associated with smoking would be underestimated.
Cigarette smoke contains more than 4,000 harmful chemicals, of which approximately 70 are considered “carcinogens”. There is a large amount of evidence suggesting that maternal smoke exposure may seriously affect normal foetal development and increase the risks of low birth weight, premature labour and congenital anomalies4,26. Although the mechanisms by which smoke causes CHDs have not yet been clearly elucidated, there are some clues to help us interpret these untoward effects. First, the main smoke by-products, nicotine and carbon monoxide, cross the placental barrier and induce vasoconstriction, resulting in foetal hypoxia20. Several animal models have indicated that chronic foetal hypoxia most likely leads to myocardial and ventricle dilation, cardiomyocyte hypertrophy, myocardial hypoplasia and impaired foetal heart maturation due to increases in the percentage and size of binucleated cardiomyocytes that cannot be differentiated27. Second, nicotine can inhibit the expression of the cardiac differentiation marker genes α-actin, desmin and cTn1, indicating that nicotine can depress early foetal heart development. Third, tobacco smoke not only causes DNA damage to both spermatozoa and oocytes but also causes epigenetic changes. These include alterations in the normal levels of DNA methylation, which can cause subtle changes in gene expression, which is a possible predisposition to congenital anomalies. However, the mechanisms of how male or female germ-cell mutations and epigenetic changes cause CHDs have not yet been clarified20.
The variation in the risk of CHDs associated with exposure to toxic agents among different populations suggests that different individuals may have different susceptibilities to the effects of cigarette smoke. In particular, polymorphisms in the GST genes have been extensively studied for smoking-associated diseases. A significant combined effect of maternal smoking and deletions in GSTM1 or GSTT1 in the infant or mother was observed on cleft lip/palate (CL/P) or oral cleft development in case-control studies in the US and the Netherlands14,28,29; however, no gene-smoking interaction effects have been identified in other studies30,31. A recent case-parent study showed that children with deletions in GSTM1 and GST1 whose parents were exposed to toxins had a higher risk of CHDs than did children with normal copies of GSTM1 and GST117. A significant interaction or joint effect between smoke exposure and the GSTP1 genotype was reported on CL/P32; however, conflicting results were observed in another study33. Our study suggests that the polymorphisms in the GST genes might influence the association between maternal smoke exposure and CHDs. GSTM1, which is predominantly expressed in the liver, is the primary gene that detoxifies polycyclic aromatic hydrocarbons, the main compounds in cigarette smoke. GSTT1 may play a more global role than GSTM1, as the former detoxifies many types of exogenous compounds and is expressed more broadly, i.e. in erythrocytes, lungs, kidney, brain, skeletal muscle, heart and small intestine34. Mothers with deletions in both GSTM1 and GSTT1 will have a complete loss of both enzymes. In this case, the capacity for xenobiotic detoxification in the body is weakened and more toxic agents will likely cross the placental barrier, impairing foetal heart development. The findings of our study appear to confirm this point because the mothers with deletions in GSTM1 or GSTT1 tended to have a lower risk threshold of smoke exposure compared to the mothers with normal copies of GSTM1 and GSTT1. However, this finding needs to be confirmed by studies with larger group sizes. For GSTP1, the I105V polymorphism leads to decreased enzyme activity. GSTP1 appears to be important in the detoxification of compounds in cigarette smoke because the gene is widely expressed in the lungs and placenta in the mother and increases in expression in the embryo at 8 and 12 gestational weeks.
Our analysis of the association between maternal smoke exposure, identified by the biomarker HNC and the risk of foetal CHDs may overcome the bias introduced by the subjectivity of self-reporting in previous studies. We have assessed the potential effects of the GST polymorphisms on the association between maternal smoke exposure and CHDs. Our findings provide important clues to follow in the investigation of the mechanisms of CHD development. However, there are also some limitations in our study. First, the groupings based on HNCs were empirical because there were insufficient objective criteria for establishing reference values; mainly due to a deficiency in the distribution of HNC in the unexposed nonsmoker, as well as active and passive smoking in the Chinese population. Second, the modest group sizes used in our study may have been responsible for the unstable results, such as the wide 95% CI for AOR and limited the power required to detect the association between maternal smoke exposure and CHDs among the mothers with different GST genotypes. Third, the specific inclusion and exclusion criteria and a very local population in our study may limit the extrapolation of our findings and make direct comparisons to other studies difficult.
Conclusion
In conclusion, our study suggests that cigarette smoke exposure during pregnancy is associated with an increased risk of foetal CHDs. This association may be modified by polymorphisms in the GST genes. These findings are important for the development of public health strategies and interventions to encourage women of childbearing age, pregnant women and their families, friends and colleagues to quit smoking.
Methods
Study population and sampling
This GEIOC study used a case-control design. Pregnant women with a gestational age of 14–28 weeks were recruited from six tertiary maternal and child hospitals with the qualification of prenatal screening and diagnosis in China. Foetuses were screened using a systematic ultrasonic examination and targeted echocardiography. Mothers whose foetuses were diagnosed with CHDs but lacked any extra-cardiac abnormalities and mothers whose foetuses were diagnosed without any anomalies were initially chosen as the cases and controls, respectively. Each live birth was examined within seven days to confirm the prenatal diagnosis. Autopsy was the preferred method to confirm the prenatal diagnoses for cases of terminated foetus. If an autopsy was not possible, the static and dynamic echocardiography images were reviewed by ultrasound specialists for the final diagnosis. Additionally, all live births were followed up for three months to confirm their final diagnoses. The details regarding the recruitment process have been well-described in two published reports7,35,36. Multiple exclusion criteria were used for the study: (1) a woman with multiple foetation; (2) a mother with a child who was selected as a control but exhibited congenital abnormalities after birth; (3) a mother with a child with heart abnormalities but an unclear diagnosis; and (4) CHD cases associated with a known syndrome or genetic abnormality. Additionally, isolated cardiomyopathies, a single umbilical artery and rhythm disorders (i.e., atrioventricular blocks and Wolff-Parkinson-White syndrome) were not considered to be CHDs.
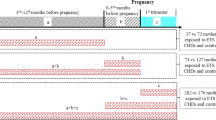
During the study period from Feb. 2010 to Oct. 2012, 627 cases and 689 controls that were compliant with the inclusion criteria were initially recruited into the GEIOC program. During recruitment, each pregnant mother was interviewed using a structured questionnaire. Several types of biological samples (e.g., maternal hair, maternal blood, urine, amniotic fluid and placenta) were collected during pregnancy or postpartum after obtaining the mother’s consent. Thus, an epidemiological and biological databank for CHD cases and controls was established. In the present study, the participants were chosen from this databank. Due to the exclusion criteria, 267 cases and 69 controls were excluded from the study. Additionally, 61 cases and 336 controls were excluded due to a lack of maternal hair or blood samples. Therefore, 299 cases and 284 controls were included in the study. The flowchart of case and control inclusion and exclusion is shown in Fig. 2.
The study was approved by the Medical Ethics Committee of Sichuan University (No. 2010004). Written informed consent was obtained from all subjects. All experiments involving human subjects and tissues were performed in accordance with guidelines approved by Sichuan University.
Case classification
The CHD cases were classified into six subtypes based on the anatomic lesion as follows: (1) septal, including atrial septal defects, ventricular septal defects and endocardial cushion defects; (2) conotruncal, including transposition of the great arteries, tetralogy of Fallot, truncus arteriosus and double outlet right ventricle; (3) right-sided obstructive, including pulmonary valve stenosis, pulmonary atresia, tricuspid atresia and Ebstein anomalies; (4) left-sided obstructive, including aortic valve stenosis, hypoplastic left heart syndrome and variants, coarctation of the aorta and interrupted aortic arch; (5) anomalous venous return, including total and partial anomalous pulmonary or systematic venous return; and (6) others, including single ventricle, heterotaxia and other cardiac structural abnormalities.
Exposure Measurements
Self-reported measurements: Information on maternal smoke exposure was collected through specific questions, including whether the pregnant woman or her husband smoked and their average daily smoking amount, whether other smokers were physically near the pregnant woman and their average daily smoking amount and whether the mother actively avoided others or environments that exposed her to smoke. The subjects were classified based on the answers into five groups: (1) unexposed nonsmokers, which referred to nonsmoking mothers who reported that they had no exposure to smoking environments; (2) slight passively exposed nonsmokers, which referred to nonsmoking mothers who reported that they were exposed to smoking environments but actively avoided these areas; (3) moderate passively exposed nonsmokers, which referred to nonsmoking mothers who reported that they were exposed to smoking environments with <10 cigarettes per day and did not actively avoid these environments; (4) severe passively exposed nonsmokers, which referred to non-smoking mothers who reported that they were exposed to smoking environments with ≥10 cigarettes per day and did not actively avoid these environments; and (5) active smokers.
Biomarker measurements: Maternal hair samples were collected soon after the questionnaire was collected. Pencil-width locks of hair were cut from the posterior vertex of the scalp using a fine pair of scissors, placed in individually labelled sterile envelopes and stored at −20 °C until analysis. Because the average hair growth rate is approximately 1 cm per month, approximately 3- to 4-cm-long segments were cut close to the scalp in the first and second trimesters. Before analysis, the hair samples were washed with ethanol, dichloromethane and distilled water in turn to remove any environmental contamination and then dried overnight. The hair analysis was conducted in a single-blinded manner in which the testers did not know the identities of the samples. The hair nicotine analysis was performed using a Gas Chromatograph-Mass Spectrometer (GC-MS). After being cut and minced into 1- to 2-mm-long pieces, 0.01–0.05 g of hair sample was accurately weighed on an analytical balance, placed in a test tube, mixed with 2 ml of a 1.0 mol/L NaOH solution and incubated at 37 °C overnight (12 hours). The digestion solution was extracted with 2.0 ml of dichloromethane by vortex mixing for 1 min. The extract was centrifuged and 1.5 ml of the organic phase was transferred into a polypropylene tube containing 500 μl of methanol/25 mM HCl. The sample was evaporated to dryness under a stream of nitrogen at 40 °C. The residue was reconstituted in 200 μl of methylene chloride containing 2 μg/ml of quinoline (the internal standard) and 1 μl of the solution was injected into the GC-MS. The HNC results were expressed in nanograms of nicotine per milligram of hair, with a detection limit of 0.01 ng/mg. Further details regarding the hair nicotine analysis are described elsewhere37.
Genotyping
Genomic DNA was extracted from peripheral blood leukocytes using the QIAamp DNA Blood Mini Kit (Qiagen, Germany) according to the manufacturer’s instructions. The GSTT1 and GSTM1 genotypes were determined using GSTT1-specific primers (forward: 5′ TTCCTTACTGGTCCTCACATCTC 3′; reverse: 5′ TCACCGGATCATGGCCAGCA 3′) or GSTM1-specific primers (forward: 5′ GAACTCCCTGAAAAGCTAAAGC 3′; reverse: 5′ CTTGGGCTCAAATATACGGTGG 3′) using a multiplex PCR approach. As an internal control, the β-globin gene was amplified in the same reaction (forward: 5′ CAACTTCAT CCACGTTCACC 3′; reverse: 5′ GAAGAGCCAAGGACAGGTAC 3′). The PCR reaction was performed in a final volume of 25 μl containing 1 μl (20 ng) of purified genomic DNA, 1 μl of forward primer, 1 μl of reverse primer (5 pmol of each), 2 μl of 2.5 mM dNTPs, 2.5 μl of 10 × buffer, 2 μl of 25 mM MgCl2 and 0.2 μl of HSTaq DNA Polymerase (TaKaRa Corp., Dalian, China). The PCR procedure used the following conditions: 95 °C for 3 min, followed by 30–36 cycles of 95 °C for 30 s, 64 °C for 45 s and 72 °C for 1 min, with a final extension at 72 °C for 5 min. The amplification was performed with a thermal Cycler C1000 (Bio-Rad, CA, USA). The β-globin internal standard product was 268 bp. The GSTM1 and GSTT1 products were 215 bp and 480 bp, respectively. A 292 bp fragment containing the GSTP1 polymorphism was amplified (forward: 5′ATCCTTCCACGCACATCCTCT3′; reverse: 5′ AAGCCCCTTTCTTTGTTCAGC3′) in the same procedure except that an annealing temperature of 59 °C was used. The product was then digested with HpyCH4IV (NEB, USA) to detect the three genotypes: AA (292 bp fragment), AG (292, 215 and 77 bp fragments) and GG (215 and 77 bp fragments). All PCR products and digestion fragments were electrophoresed on a 3% agarose gel containing GELVIEW and the results were visualized on the Bio-Rad Gel-Doc 1000 apparatus (Bio-RAD, CA, USA).
Data analysis
A case-control analysis was performed to assess the associations between maternal smoke exposure and CHDs as well as the effect of GST gene polymorphisms on the associations. The potential confounders were those correlated with both the main determinant and CHDs. These confounders included maternal age (<25, 25–34, or ≥35years), maternal education(<12, 12–15, or ≥16 years), gestational age (14–22 or 23–28 weeks) and maternal drinking (often (≥1 time(s)/week), occasional(<1 time/week), or never) during the three months prior to the first trimester and maternal ppBMI (<18.5, 18.5–24.0, or ≥24.0). Based on the percentiles of the HNCs (P20, P40, P60 and P80) for the combined sample, the smoke exposure was divided into 5 levels: ≤0.117, 0.117–0.213, 0.213–0.319, 0.319–0.573 and >0.573 ng/mg.
T-test was used to test the difference in the logarithm of HNC between the cases and controls. Differences in the proportions between the cases and controls regarding potential factors and GST genotypes were tested using the Chi-square test. Multivariable dichotomous logistic models were used to assess the adjusted associations between CHDs and HNC categories. The models were expressed as: Logit(P) = HNC + {adjusters} + ε. In the models, the presence or absence of any CHD or its subtype was the dependent variable and the HNC level was the main independent variable. HNC was set as a categorical variable and the ≤0.117 ng/mg level was set as the reference group. The potential confounders were chosen as adjusters and added as categorical variables into the model. The results of these models are shown in Table 3. In addition, the adjusted associations between any CHDs and HNC level among GSTM1, GSTT1 and GSTP1 polymorphisms were evaluated by logistic models. These models were expressed as: Logit(P) = HNC + GST + GST*HNC + {adjusters} + ε. The significance of the GST*HNC terms was tested by hierarchical likelihood ratio testing. The results of these models are shown in Table 4.
All of the statistical analyses were performed using SAS 9.0 software (SAS Institute, Cary, NC, USA). Two-tailed values of P<0.05 and 95% CIs excluding 1.00 were considered to be statistically significant.
Additional Information
How to cite this article: Li, X. et al. Modification of the association between maternal smoke exposure and congenital heart defects by polymorphisms in glutathione S-transferase genes. Sci. Rep. 5, 14915; doi: 10.1038/srep14915 (2015).
References
Hoffman, J. I. & Kaplan, S. The incidence of congenital heart disease. J Am Coll Cardiol 39, 1890–900 (2002).
Richards, A. A. & Garg, V. Genetics of congenital heart disease. Curr Cardiol Rev 6, 91–7 (2010).
Lee, L. J. & Lupo, P. J. Maternal smoking during pregnancy and the risk of congenital heart defects in offspring: a systematic review and metaanalysis. Pediatr Cardiol 34, 398–407 (2012).
Hackshaw, A., Rodeck, C. & Boniface, S. Maternal smoking in pregnancy and birth defects: a systematic review based on 173 687 malformed cases and 11.7 million controls. Hum Reprod Update 17, 589–604 (2011).
Ng, M. et al. Smoking prevalence and cigarette consumption in 187 countries, 1980–2012. JAMA 311, 183–92 (2014).
Xiao, L., Yang, Y., Li, Q., Wang, C. X. & Yang, G. H. Population-based survey of secondhand smoke exposure in China. Biomed Environ Sci 23, 430–6 (2011).
Deng, K. et al. Periconceptional paternal smoking and the risk of congenital heart defects: a case-control study. Birth Defects Res A Clin Mol Teratol 97, 210–6 (2013).
DeLorenze, G. N., Kharrazi, M., Kaufman, F. L., Eskenazi, B. & Bernert, J. T. Exposure to environmental tobacco smoke in pregnant women: the association between self-report and serum cotinine. Environ Res 90, 21–32 (2002).
Avila-Tang, E. et al. Assessing secondhand smoke using biological markers. Tob Control 22, 164–71 (2013).
Benowitz, N. L., Hukkanen, J. & Jacob, P. 3rd . Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol. 29–60 (2009), 10.1007/978-3-540-69248-5_2.
Florescu, A. et al. Reference values for hair cotinine as a biomarker of active and passive smoking in women of reproductive age, pregnant women, children and neonates: systematic review and meta-analysis. Ther Drug Monit 29, 437–46 (2007).
Koh, W. P. et al. Glutathione S-transferase (GST) gene polymorphisms, cigarette smoking and colorectal cancer risk among Chinese in Singapore. Carcinogenesis 32, 1507–11 (2011).
Elhasid, R. et al. Influence of glutathione S-transferase A1, P1, M1, T1 polymorphisms on oral busulfan pharmacokinetics in children with congenital hemoglobinopathies undergoing hematopoietic stem cell transplantation. Pediatr Blood Cancer 55, 1172–9 (2010).
Lammer, E. J., Shaw, G. M., Iovannisci, D. M. & Finnell, R. H. Maternal smoking, genetic variation of glutathione s-transferases and risk for orofacial clefts. Epidemiology 16, 698–701 (2005).
Salinas, A. E. & Wong, M. G. Glutathione S-transferases--a review. Curr Med Chem 6, 279–309 (1999).
Murdzoska, J. et al. In utero smoke exposure and role of maternal and infant glutathione s-transferase genes on airway responsiveness and lung function in infancy. Am J Respir Crit Care Med 181, 64–71 (2009).
Cresci, M. et al. Maternal and paternal environmental risk factors, metabolizing GSTM1 and GSTT1 polymorphisms and congenital heart disease. Am J Cardiol 108, 1625–31 (2011).
Alverson, C. J., Strickland, M. J., Gilboa, S. M. & Correa, A. Maternal smoking and congenital heart defects in the Baltimore-Washington Infant Study. Pediatrics 127, e647–53 (2011).
Malik, S. et al. Maternal smoking and congenital heart defects. Pediatrics 121, e810–6 (2008).
Gianicolo, E. A., Cresci, M., Ait-Ali, L., Foffa, I. & Andreassi, M. G. Smoking and congenital heart disease: the epidemiological and biological link. Curr Pharm Des 16, 2572–7 (2010).
Florescu, A. et al. Methods for quantification of exposure to cigarette smoking and environmental tobacco smoke: focus on developmental toxicology. Ther Drug Monit 31, 14–30 (2009).
Leonardi-Bee, J., Smyth, A., Britton, J. & Coleman, T. Environmental tobacco smoke and fetal health: systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed 93, F351–61 (2008).
Salmasi, G., Grady, R., Jones, J. & McDonald, S. D. Environmental tobacco smoke exposure and perinatal outcomes: a systematic review and meta-analyses. Acta Obstet Gynecol Scand 89, 423–41 (2010).
Searles Nielsen, S., Dills, R. L., Glass, M. & Mueller, B. A. Accuracy of prenatal smoking data from Washington State birth certificates in a population-based sample with cotinine measurements. Ann Epidemiol 24, 236–9 (2014).
Koren, G., Blanchette, P., Lubetzky, A. & Kramer, M. Hair nicotine:cotinine metabolic ratio in pregnant women: a new method to study metabolism in late pregnancy. Ther Drug Monit 30, 246–8 (2008).
Leonardi-Bee, J., Britton, J. & Venn, A. Secondhand smoke and adverse fetal outcomes in nonsmoking pregnant women: a meta-analysis. Pediatrics 127, 734–41 (2011).
Patterson, A. J. & Zhang, L. Hypoxia and fetal heart development. Curr Mol Med 10, 653–66 (2010).
van Rooij, I. A. et al. Smoking, genetic polymorphisms in biotransformation enzymes and nonsyndromic oral clefting: a gene-environment interaction. Epidemiology 12, 502–7 (2001).
Shaw, G. M. et al. Risks of human conotruncal heart defects associated with 32 single nucleotide polymorphisms of selected cardiovascular disease-related genes. Am J Med Genet A 138, 21–6 (2005).
Hartsfield, J. K., Jr. et al. Analysis of the EPHX1 113 polymorphism and GSTM1 homozygous null polymorphism and oral clefting associated with maternal smoking. Am J Med Genet 102, 21–4 (2001).
Hozyasz, K. K., Mostowska, A., Surowiec, Z. & Jagodzinski, P. P. [Genetic polymorphisms of GSTM1 and GSTT1 in mothers of children with isolated cleft lip with or without cleft palate]. Przegl Lek 62, 1019–22 (2005).
Shi, M. et al. Orofacial cleft risk is increased with maternal smoking and specific detoxification-gene variants. Am J Hum Genet 80, 76–90 (2007).
Ramirez, D. et al. Maternal smoking during early pregnancy, GSTP1 and EPHX1 variants and risk of isolated orofacial clefts. Cleft Palate Craniofac J 44, 366–73 (2007).
Landi, S. Mammalian class theta GST and differential susceptibility to carcinogens: a review. Mutat Res 463, 247–83 (2000).
Li, X. et al. The association between periconceptional folic acid supplementation and congenital heart defects: a case-control study in China. Prev Med 56, 385–9 (2013).
Liu, Z. et al. Association between maternal exposure to housing renovation and offspring with congenital heart disease: a multi-hospital case-control study. Environ Health 12, 25 (2013).
Man, C. N., Ismail, S., Harn, G. L., Lajis, R. & Awang, R. Determination of hair nicotine by gas chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 877, 339–42 (2009).
Acknowledgements
We thank the obstetricians, paediatricians, pathologists, experimental technicians and other participants involved in this project for recruiting the case and control mothers and collecting the data. We thank all of the participating families for their cooperation and for providing personal information. Funding: This study was funded by the National “Twelfth Five-Year” Plan for Science & Technology Support (grant ID: 2014BAI06B01), the National Science Foundation for Young Scholars of China (grant ID: 81502818), the National Basic Research Program of China (grant ID: 2010CB529502), the Program for Changjiang Scholars and Innovative Research Team in University (grant ID: IRT0935) and the National Science Foundation (grant ID: 81273086). The funders had no role in the study design, data analysis, data interpretation, or writing of the report.
Author information
Authors and Affiliations
Contributions
J.Z. conceived the study. X.L., Z.L. and Y.D. conducted the experiment, analysed the data and wrote the manuscript. S.L., X.T., Y.L., J.Y., J.L. and X.C. diagnosed the CHD cases and collected the biological samples. D.M., N.L., Y.W. and K.D. provided comments on the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Li, X., Liu, Z., Deng, Y. et al. Modification of the association between maternal smoke exposure and congenital heart defects by polymorphisms in glutathione S-transferase genes. Sci Rep 5, 14915 (2015). https://doi.org/10.1038/srep14915
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep14915
This article is cited by
-
Association of maternal phthalates exposure and metabolic gene polymorphisms with congenital heart diseases: a multicenter case-control study
BMC Pregnancy and Childbirth (2024)
-
Biomarkers for isolated congenital heart disease based on maternal amniotic fluid metabolomics analysis
BMC Cardiovascular Disorders (2022)
-
Association between maternal smoke exposure and congenital heart defects from a case–control study in China
Scientific Reports (2022)
-
Association between prepregnancy body mass index and risk of congenital heart defects in offspring: an ambispective observational study in China
BMC Pregnancy and Childbirth (2020)
-
Assessment of interaction between maternal polycyclic aromatic hydrocarbons exposure and genetic polymorphisms on the risk of congenital heart diseases
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.