Abstract
The aim of this study was to investigate the effect of recombinant human endostatin (rh-Endo) in combination with radiation therapy (RT) on esophageal squamous cell carcinoma (ESCC) and explore the potential mechanisms. ECA109-bearing nude mice were administered RT and/or rh-Endo treatment. Tumor volume, survival, hypoxia and vascular parameters were recorded during the treatment schedule and follow-up as measures of treatment response. ESCC cell lines (ECA109 and TE13) and human umbilical vein endothelial cells (HUVECs) were developed to investigate the outcomes and toxicities of rh-Endo and RT in vitro. Hypoxia inducible factor-1α (HIF-1α) and vascular endothelial growth factor (VEGF) were also evaluated. In vivo studies of ECA109-bearing xenografts showed that rh-Endo improved the radioresponse, with normalization of tumor vasculature and a reduction in hypoxia. In vitro studies showed that rh-Endo did not radiosensitize ESCC cell lines but did affect endothelial cells with a time- and dose-dependent manner. Studies of the molecular mechanism indicated that the improved radioresponse might be due to crosstalk between cancer cells and endothelial cells involving HIF and VEGF expression. Our data suggest that rh-Endo may be a potential anti-angiogenic agent in ESCC especially when combined with RT. The improved radioresponse arises from normalization of tumor vasculature and a reduction in hypoxia.
Similar content being viewed by others
Introduction
Esophageal cancer (EC) is the eighth most common cancer and the sixth most common cause of cancer-related death in the world and esophageal squamous cell carcinoma (ESCC) constitutes the major histopathologic subtype1. Although radiotherapy (RT) plays an important role in the nonsurgical management of ESCC, radioresistance accounts for a high recurrence rate and poor 5-year survival2. In order to reduce the mortality, the development of novel therapeutic strategies is essential.
Angiogenesis is an essential event to allow small, established tumors to grow beyond a critical size of a few millimeters. It is thought that without the necessary microenvironment for neovascularization, tumor growth is arrested3. Endostatin is the 20 kDa C-terminal fragment of collagen XVIII, a proteoglycan mainly found in the basement membrane around blood vessels. Endostatin was first described in 1997 by Judah Folkman’s group and this rapidly drove media attention and Wall Street enthusiasm to EntreMed, the company that licensed its rights4. Preclinical and clinical studies reported that recombinant endostatin expressed in bacteria was able to cause the regression of several types of tumor without the induction of resistance and with virtually no signs of toxicity. However, EntreMed abandoned phase III clinical trials because it was unable to provide sufficient quantities of endostatin. Despite halted progress in the western world, endostatin development was pursued in China and recombinant human endostatin (rh-Endo, under the trade name of Endostar) has been approved by the China Food and Drug Administration since 2005. Furthermore, the National Comprehensive Cancer Network (NCCN) Guidelines (Chinese version) have suggested the use of rh-Endo in combination with chemotherapy in patients with non-small cell lung cancer (NSCLC). In addition, several studies have reported that rh-Endo is effective against metastatic melanoma and head and neck cancer5,6,7.
Although previous studies have indicated that rh-Endo may have benefits, it is yet to be established whether rh-Endo might be useful in the treatment of ESCC, particularly when used in combination with RT. The present study utilized both in vivo and in vitro experiments to investigate the effects of rh-Endo in combination with RT on ESCC and explore potential mechanisms.
Results
rh-Endo enhances the radioresponse in ESCC xenografts
In the mouse model of ESCC, combined treatment with rh-Endo and irradiation (IR) was more effective at delaying tumor growth than rh-Endo or IR alone (Fig. 1a) (P < 0.05). Images of representative cases are shown in Fig. 1b. We further analyzed the time required for the tumor to double in size in each of the treatment groups. The doubling times for the ECA109 tumor in the rh-Endo alone group (5.01 ± 0.66 days) and IR alone group (4.67 ± 1.65 days) were not significantly different from that in the control group (4.07 ± 0.56 days). In contrast, combined treatment with rh-Endo and IR extended the doubling time to 6.79 ± 0.81 days (P < 0.05 compared with the control and IR alone groups; Fig. 1c). Intraperitoneal injection of rh-Endo enhanced the response of ECA109 xenografts to IR with an enhancement factor of 2.93 (see Table 1 for details). In addition, there was a trend toward improved survival in mice administered both rh-Endo and IR, although statistical significance was not attained (Fig. 1d).
rh-Endo improves the radioresponsiveness of ESCC in vivo.
ECA109 xenograft-bearing male BALB/c mice were divided into four treatment groups (n = 6): control; IR alone; rh-Endo alone; and a combination of rh-Endo and irradiation. 10 days after inoculation of ECA109 cells (1 × 106 cells/mouse), the mice were irradiated with a single fraction of 8-Gy X-rays. (a) Measurements of tumor size. Data represent the mean tumor volume; error bars represent the SD. **P < 0.01 vs. the control group; #P < 0.05 vs. the rh-Endo group. (b) Representative images of ECA109 xenograft-bearing mice. (c) Comparison of tumor doubling times between the four groups. *P < 0.05. (d) Kaplan-Meier survival curves for the mice in the four groups.
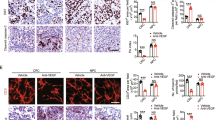
rh-Endo normalizes ESCC vascularization
Immunohistochemistry (IHC) staining with an antibody directed against CD31 and calculation of micro-vessel density (MVD) were used to detect changes in tumor vascularization and organization on day 10 (D10), day 16 (D16) and day 22 (D22) after transplantation of ECA109 cells. In addition, functional vessels, new vessels and pericyte coverage were assessed using IHC of leptin, CD105 and α-SMA and the effects of rh-Endo and/or IR treatment were determined on D22. Measurements of CD31 immunoreactivity showed a notable reduction in the vascularity of the ECA109 xenografts after treatment with rh-Endo for 6 days (D16) or 12 days (D22) and an even greater effect of combined therapy (Fig. 2a,b). In further analysis, it was observed that treatment with rh-Endo caused a greater reduction of new vessels than functional vessels, as well as an increase in pericyte coverage (Fig. 2c,d).
rh-Endo normalizes ESCC vascularization in vivo.
Tumor tissues from mice in the four treatment groups were removed and stained with anti-CD31, anti-CD105, anti-Lectin and anti-α-SMA antibodies on days 10, 16 and 22 (D10, D16 and D22) after transplantation of ECA109 cells. (a) Representative images of CD31 immunostaining (used as a microvessel marker). (b) Microvessel densities determined from CD31 immunostaining in the various experimental groups. (c) Representative images showing immunostaining for CD105 (a marker of new vessels), Lectin (a marker of functional vessels) and α-SMA (a marker of pericyte coverage). (d) Integral optical densities (IODs) of the various experimental groups (upper, CD105; middle, Lectin; lower, α-SMA) on D22 (the end of the treatment period). *P < 0.05 vs. IR group, **P < 0.01 vs. IR group.
rh-Endo improves ESCC tissue hypoxia
Interestingly, treatment with rh-Endo significantly improved tumor hypoxia on D22 (i.e. 12 days after treatment), as shown by immunofluorescence (IF) experiments in the ESCC mouse model (Fig. 3A). These data indicate the tumor hypoxia modulation effect of rh-Endo in cells was similar with that in animal models.
rh-Endo improves ESCC tissue hypoxia in vivo.
Immunofluorescence assessment of tumor tissues were conducted on D22 (the end of the treatment period). (A) Pimonidazole staining showed an improvement of hypoxia in the rh-Endo + IR group. (B) Quantify analysis of CD31 and pimonidazole. *P < 0.05 vs. control, **P < 0.01 vs. control.
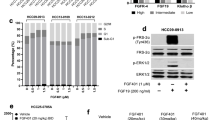
rh-Endo does not improve radiosensitivity in vitro
To determine whether rh-Endo affected ESCC cells directly and whether the improved radioresponse was due to radiation-induced tumor cell death, a cell counting kit (CCK8) assay and colony formation analysis were used. Although higher concentrations of rh-Endo (400 μg/mL and higher) exerted small inhibitory effects on the growth of ESCC cell lines, overall there were no clear time- and dose-dependent effects, as is usually the case for radiosensitizers (Fig. 4a,b). However, rh-Endo did inhibit the growth of human umbilical vein endothelial cells (HUVECs), even at the lowest concentration (50 μg/mL) used (Fig. 4c, P < 0.05). Flow cytometry experiments revealed that rh-Endo (at concentrations that did not affect growth of ESCC cell lines) induced apoptosis of HUVECs in vitro in a dose-dependent manner (Fig. 4f), but did not induce apoptosis of the ESCC cell lines ECA109 and TE13 (Fig. 4d,e). In colony formation assays, rh-Endo (at concentrations that did not affect growth of ESCC cell lines) also had no effect on ECA109 and TE13 cells, with DDP used as a positive control (Fig. 4g,h). These data suggest that the target of rh-Endo is endothelial cells rather than tumor cells and that the alleviation of hypoxia was not due to tumor cell killing.
rh-Endo does not improve radiosensitivity of EC cell lines in vitro but does affect endothelial cells.
(a–c) rh-Endo did not inhibit the growth of ECA109 and TE13 cells at lower concentrations (200 μg/mL and below) but did inhibit the growth of HUVECs at these lower concentrations. *P < 0.05 vs. 0 μg/mL at the same time point; **P < 0.01 vs. 0 μg/mL at the same time point. (d–f) Flow cytometric analysis revealed that rh-Endo did not induce apoptosis of ECA109 cells and TE13 cells, but did affect the apoptosis of HUVECs. **P < 0.01. (g,h) Clonogenic assays showed that rh-Endo did not affect the radioresponsiveness of ESCC cells in vitro, whereas DDP improved the sensitivity to radiation.
The improved radioresponse with rh-Endo is associated with decreased expression of HIF-1α and VEGF
VEGF expression was evaluated both in vivo and in vitro. In the animal models, serum VEGF-A was not affected by any of the treatments (Fig. 5a). Meanwhile, the supernatant VEGF-A differed in the medium of ESCC cell lines but not in HUVECs normalized to cell numbers (Fig. 5b). To future evaluate VEGF and HIF-1α expressions in tumor tissues, experiments were performed to detect CD31/VEGF and CD31/HIF-1α. Both VEGF and HIF-1α expressions in the vascular area were reduced when rh-Endo was combined with IR (Fig. 5c–f).
The improved radioresponse with rh-Endo is associated with decreased expression of HIF-1α and VEGF.
(a) Serum VEGF-A levels measured in vivo in the four experimental groups. (b) Supernatant levels of VEGF-A from ECA109 cells, TE13 cells and HUVECs normalized to cell numbers. (c,d) Immunofluorescence assessment of VEGF and HIF-1α in the four experimental groups on D22. (e,f) Quantity analysis of c and d. *P < 0.05 vs. control or 0 μg/mL, **P < 0.01 vs. control or 0 μg/mL.
Disscussion
RT is a well-established therapeutic modality in oncology. It provides survival benefits in several cancer types, including EC8. rh-Endo is approved by the China Food and Drug Administration and its use as an anti-angiogenic agent in advanced NSCLC is suggested in the NCCN guidelines (Chinese version)9. Moreover, clinical trials and retrospective studies have demonstrated an anti-tumor effect of rh-Endo in metastatic melanoma, head and neck cancer, breast cancer and gastric cancer10. However, there remains debate as to whether rh-Endo can be used in EC.
This is the first comprehensive report of the use of rh-Endo with RT in EC. To explore the intrinsic mechanism underlying the contribution of angiogenesis inhibition to the radioresponse in EC, we used in vivo studies of xenografts and in vitro cell line models. Translational experiments indicated that the improved radioresponse with rh-Endo might be attributable to tumor vasculature remodeling and hypoxia reduction, possibly through regulation of the HIF/VEGF pathway. rh-Endo does not kill EC cells directly, but likely affects crosstalk between endothelial cells and cancer cells, such as VEGF-A expression, which directly affects endothelial cell proliferation11.
The intrinsic mechanisms underlying the contribution of angiogenesis inhibitors to the radioresponse remain unclear, despite a considerable amount of research in recent years. Some authors have suggested that angiogenesis inhibitors and RT synergistically enhance the radioresponse of tumor growth in solid tumors11,12,13. Importantly, there is evidence in different tumors that angiogenesis inhibitors can induce vascular normalization14,15. Notwithstanding these findings, new insights are needed to explain the potential mechanism that may be operative in the improved radiation response with angiogenesis inhibitors.
Aberrant tumor vasculature is hyperpermeable and tortuous and confers compromised blood supply to tumor tissues leading to a hypoxic and acidic tumor microenvironment16,17. We observed vascular normalization in ESCC xenografts 5 days after therapy with rh-Endo. As might be expected, we observed the most significant delay in tumor growth when IR was given on day 5, with an effect superior to that of rh-Endo alone, IR alone and IR performed 1 day before or after rh-Endo (data not shown) in mice. In further studies, rh-Endo was found to affect both CD105 and lectin, markers of new vessels and functional vessels. In contrast to the reduced MVD, we observed an increase in the amount of pericytes and their endothelial coverage 5 days after rh-Endo treatment (IHC of α-SMA). An increased number of pericytes and restored close pericyte-endothelial cell interactions have been observed almost universally in studies showing vascular normalization18.
Hypoxic tumors have long been demonstrated to possess higher radiation resistance and numerous modalities have attempted to address this by improving the oxygen supply18. In our study, pimonidazole was employed to validate the hypoxic change and rh-Endo was found to reduce overall tumor hypoxia from day 5. Excitingly, the modulation of hypoxia on day 5 after rh-Endo treatment provides a basis for the design of an rh-Endo/IR combination schedule in future clinical practice.
It is currently not clear how anti-angiogenic treatment normalizes the aberrant tumor vasculature and the underlying mechanism involving hypoxic changes. In vitro studies were conducted to determine whether rh-Endo radiosensitizes ESCC cells directly and these showed no radiosensitization effect in ESCC cell lines. However, rh-Endo was able to influence the proliferation and apoptosis of endothelial cells directly. In the molecular analysis, we detected HIF/VEGF expression changes in the tissues after treatment with rh-Endo and this was enhanced by irradiation. Treatment did not affect serum VEGF-A expression in xenografts and HUVECs, but did affect VEGF-A secretion by ESCC cell lines. VEGF-A is well established as a regulator of the proliferation, migration and survival of endothelial cells19. This suggests that VEGF secreted by cancer cells may have an impact on endothelial cells, which can further contribute to change in tumor vasculature and hypoxia20.
There are some limitations to this study. First, the ECA109 cell line was chosen because it is highly proliferative, representative and is resistant to many therapies. However, the beneficial effects of rh-Endo on IR need to be confirmed in other cell lines. Second, a single large-dose of IR was employed to exclude the potential confounding effects of conventional fractionated IR. However, the use of a single 8-Gy dose, selected on the basis of previous studies21,22, differs from regimens that are used clinically. Therefore, the optimal combination of rh-Endo with conventional IR warrants further investigation. Third, measurements of tumor volume were made only up to D22; although this observation period was based on that used in previous investigations23,24, additional research is warranted to determine the effects of rh-Endo and IR on tumor volume over extended periods. Fourth, additional studies in eligible patients with ESCC are needed to explore changes in tumor hypoxia and vasculature after treatment with rh-Endo and RT. Fifth, although there appeared to be a trend for improved survival in mice treated with both rh-Endo and IR, statistical significance was not attained. It may be that our study was underpowered (n = 6 per group) to detect any real differences in survival between groups; thus additional studies with larger sample sizes are merited.
These findings indicate that rh-Endo is a potential anti-angiogenic agent in ESCC especially when combined with RT. The improved radioresponse may arise from normalization of the tumor vasculature and a reduction in tumor hypoxia25,26. Our data support the emerging notion that the HIF/VEGF pathway may be involved in the improved radioresponse. However, clinical studies are needed to further confirm these findings.
Materials and Methods
Cell lines, cell culture and reagents
ESCC cell lines ECA109 and TE13 were obtained from Shanghai Institute of Cell Biology (Shanghai, China) and were maintained in DMEM medium (Gibco, Life Technologies, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Hyclone, GE Healthcare, Little Chalfont, UK), 1% penicillin/streptomycin (Invitrogen, Life Technologies). Cells were maintained in an incubator at 37 °C, in an atmosphere containing 5% CO2. HUVECs were isolated from human umbilical cord veins using a standard procedure described previously27 and grown in EBM-2 medium with SingleQuots™ (Lonza, Walkersville, MD, USA) containing VEGF and other growth factors.
Cells in the IR group were subjected to a 2, 4, 6 or 8 Gy of X-ray irradiation from a medical linear accelerator (Elekta Precise) at room temperature.
anti-CD31, anti-CD105, anti-CD31, anti-CD105, anti-lectin, anti-α-SMA, anti-VEGF, anti-HIF-1α (Abcam), fluorescein (FITC)-conjugated anti-mouse IgG and horseradish peroxidase (HRP)-conjugated anti-rabbit IgG (Jackson ImmunoResearch, West Grove, PA, USA). rh-Endo was provided by Simcere Pharmaceuticals (Nanjing, China).
Xenografts
All animal experiments were approved by the Ethics Committee of Nanjing Medical University and performed in accordance with the guidelines of the National Institutes of Health and Nanjing Medical University. Male BALB/C nude mice (age: 8 weeks; weight: 19.04 ± 1.59 g) were provided by Nanjing Medical University Animal Center and xenografts were established in each mouse by subcutaneous injection of 0.1 mL of ECA109 cells (1 × 107 cells/mL) into the right proximal hind leg.
After 9 days of incubation, when the tumors had reached approximately 100–150 mm3 in volume, the animals were assigned into 1 of 4 groups (n = 6 per group): (1) vehicle (saline, D10–D22), (2) rh-Endo (D10–D22), (3) IR (D16), (4) rh-Endo (D10–D22) +IR (D16). rh-Endo was administered by injection via the caudal vein at a dose of 15 mg/kg, once daily for 13 days. The control (vehicle) group was administered similar volumes of physiologic saline. Tumors were irradiated with X-rays (8 Gy dose, 2 Gy/min) on D16, using an RS-2000 biological irradiator. The selection of a single dose of 8-Gy of X-rays was made on the basis of previous studies using single doses of 2–20 Gy21,22. Tumor growth was measured every day until D25 and the tumor volume was calculated according to the formula: Tumor volume = (length [L] × width [W])2/2. Mice were raised until they died naturally and survival time was recorded.
Based on the previous results, another 72 xenografts were allocated into the same 4 groups (n = 18 per group) as described above. Six mice in each group were killed on day 10 (before rh-Endo), day 16 (before IR) and day 22 (after the treatment schedule). Pimonidazole (Hypoxyprobe kit, #HP1-100; Chemicon International, Schwalbach, Germany), a hypoxia marker and Hoechst 33342 (B2261; Sigma-Aldrich, St. Louis, MO, USA), a perfusion marker, were injected 60 min before the mouse was killed.
Immunohistochemistry (IHC)
Paraffin-embedded tumor tissue sections were deparaffinized in xylene, rehydrated in graded ethanol and rinsed twice with phosphate-buffered saline (PBS). Endogenous peroxidase activity was blocked by incubating sections with 3% hydrogen peroxide in the dark for 15 min. The sections were then incubated overnight at 4 °C with polyclonal antibody to CD31, CD105, lectin or α-SMA (diluted 1:500). After washing, slides were incubated with HRP-conjugated anti-rabbit secondary antibody (diluted 1:100) for 1 h at room temperature. Finally, the slides were visualized by incubation with 3, 3′-diaminobenzidine (DAB) (Dako, Hamburg, Germany) and counterstained with hematoxylin (37%). The sections were analyzed under an Axiovert A1 light microscope (Zeiss, Jena, Germany). The intensity of the immunoreactivity in the tumor was evaluated using Image-Pro Plus 6.0 software. Mean intensity was calculated by dividing the integral optical density (IOD) by the area28. The microvessel density was calculated as a ratio of microvessel number and area of the picture with Image pro-plus 6.0 software.
Immunofluorescence (IF)
The tissues were fixed with acetone and permeabilized with 0.1% Triton X-100 in PBS for 5 min at room temperature. The tissues were then incubated with antibodies against Hoechst, CD31, pimonidazole, HIF-1α and VEGF (diluted 1:500) at 4 °C overnight and then with FITC-conjugated secondary antibody (diluted 1:100) for 1 h at room temperature. After washing in PBS, cells were incubated with 0.25 mg/mL DAPI for 3 min. The images were examined using a laser scanning confocal microscope (LSM510, Zeiss).
Cell viability assay
Cell proliferation was measured using a CCK8 assay. Cells were plated at a concentration of 5 × 103 cells/well in 96-well plates. The medium was removed and replaced with fresh medium with or without rh-Endo after 24 h. A CCK8 cell proliferation and cytotoxicity assay kit (Obio Technology, Shanghai, China) was used after 12, 24 and 48 h. The absorbance was measured at a wavelength of 490 nm.
Flow cytometric analysis
ECA109, TE13 and HUVEC cells were fixed in 2% paraformaldehyde and then stained with an Annexin V-FITC Apoptosis Kit (Keygene Biotechnology, Nanjing, China). The cells were analyzed by flow cytometry using FACSan with Cell-Quest software (BD Biosciences, San Jose, CA, USA)29.
Clonogenic survival assay
ECA109 and TE13 cells were seeded onto six-well dishes. After overnight culture, the cells were treated with rh-Endo (50 μg/mL or 200 μg/mL) for 24 h. Cells treated with 10 μM cisplatin (DDP) were used as positive controls and a negative control group was also used. Cells were then irradiated with 6MV X-rays at doses of 0, 2, 4, 6 or 8 Gy at 4.5 Gy/min. The cells were then cultured in a 5% CO2 incubator at 37 °C for 10 days. The colonies were fixed and stained with crystal violet to count the number of colonies (>50 cells/colony).
VEGF-A assay
The amount of VEGF-A in the culture medium and serum of xenografts was measured with a commercial ELISA kit (R&D Systems, Minneapolis, MN, USA).
Data analysis
The mean ± standard deviation (SD) from triplicate assays was calculated and differences between treatment groups were determined using the ANOVA test. Statistical analysis was performed using STATA 11.0 software (StataCorp, College Station, TX, USA) and Prism 5.0 software (GraphPad, La Jolla, CA, USA). P < 0.05 was considered statistically significant.
Additional Information
How to cite this article: Zhu, H. et al. Recombinant human endostatin enhances the radioresponse in esophageal squamous cell carcinoma by normalizing tumor vasculature and reducing hypoxia. Sci. Rep. 5, 14503; doi: 10.1038/srep14503 (2015).
References
Jemal, A. et al. Global cancer statistics. CA Cancer J Clin 61, 69–90 (2011).
D'Journo, X. B. & Thomas, P. A. Current management of esophageal cancer. J Thorac Dis 6, Suppl 2 S253–264 (2014).
Jain, R. K. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med 7, 987–989 (2001).
Zhuo, W. et al. Endostatin inhibits tumour lymphangiogenesis and lymphatic metastasis via cell surface nucleolin on lymphangiogenic endothelial cells. J Pathol 222, 249–260 (2010).
Jiang, L. P. et al. N-terminal modification increases the stability of the recombinant human endostatin in vitro. Biotechnol Appl Biochem 54, 113–120 (2009).
Al-Rawi, S., Meehan-Andrews, T., Bradley, C. & Al-Rawi, J. Novel benzoxazines as inhibitors of angiogenesis. Invest New Drugs 33, 45–52 (2015).
Xu, X. et al. Endostar, a modified recombinant human endostatin, suppresses angiogenesis through inhibition of Wnt/beta-catenin signaling pathway. PloS One 9, e107463 (2014).
Javle, M. & Hsueh, C. T. Updates in Gastrointestinal Oncology—insights from the 2008 44th annual meeting of the American Society of Clinical Oncology. J Hematol Oncol 2, 9 (2009).
Chu, T. Q. et al. Can determination of circulating endothelial cells and serum caspase-cleaved CK18 predict for response and survival in patients with advanced non-small-cell lung cancer receiving endostatin and paclitaxel-carboplatin chemotherapy? a retrospective study. J Thorac Oncol 7, 1781–1789 (2012).
Cui, C. et al. A phase II, randomized, double-blind, placebo-controlled multicenter trial of Endostar in patients with metastatic melanoma. Mol Ther 21, 1456–1463 (2013).
Bussink, J., Kaanders, J. H. & van der Kogel, A. J. Microenvironmental transformations by VEGF- and EGF-receptor inhibition and potential implications for responsiveness to radiotherapy. Radiother Oncol 82, 10–17 (2007).
Sonveaux, P. Provascular strategy: targeting functional adaptations of mature blood vessels in tumors to selectively influence the tumor vascular reactivity and improve cancer treatment. Radiother Oncol 86, 300–313 (2008).
Mandac, I. & Kolonic, S. O. Lenalidomide induced good clinical response in a patient with multiple relapsed and refractory Hodgkin's lymphoma. J Hematol Oncol 3, 20 (2010).
Escorcia, F. E. et al. Selective killing of tumor neovasculature paradoxically improves chemotherapy delivery to tumors. Cancer Res 70, 9277–9286 (2010).
Loriot, Y. et al. BMS-690514, a VEGFR and EGFR tyrosine kinase inhibitor, shows anti-tumoural activity on non-small-cell lung cancer xenografts and induces sequence-dependent synergistic effect with radiation. Br J Cancer 103, 347–353 (2010).
Jain, R. K. A new target for tumor therapy. N Engl J Med 360, 2669–2671 (2009).
Sun, W. Angiogenesis in metastatic colorectal cancer and the benefits of targeted therapy. J Hematol Oncol 5, 63 (2012).
Dewhirst, M. W., Cao, Y. & Moeller, B. Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nat Rev Cancer 8, 425–437 (2008).
Olsson, A. K., Dimberg, A., Kreuger, J. & Claesson-Welsh, L. VEGF receptor signalling—in control of vascular function. Nat Rev Mol Cell Biol 7, 359–371 (2006).
Ji, Y. et al. Signaling pathways in the development of infantile hemangioma. J Hematol Oncol 7, 13 (2014).
Granton, P. V. et al. A longitudinal evaluation of partial lung irradiation in mice by using a dedicated image-guided small animal irradiator. Int J Radiat Oncol Biol Phys 90, 696–704 (2014).
Shuryak, I., Smilenov, L. B., Kleiman, N. J. & Brenner, D. J. Potential reduction of contralateral second breast-cancer risks by prophylactic mammary irradiation: validation in a breast-cancer-prone mouse model. PLoS One 8, e85795 (2013).
Perez, B. A. Assessing the radiation response of lung cancer with different gene mutations using genetically engineered mice. Front Oncol 3, 72 (2013).
Kimple, R. J. et al. Enhanced radiation sensitivity in HPV-positive head and neck cancer. Cancer Res 73, 4791–4800 (2013).
Goel, S. et al. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev 91, 1071–1121 (2011).
Harris, A. L. Hypoxia—a key regulatory factor in tumour growth. Nat Rev Cancer 2, 38–47 (2002).
Jaffe, E. A., Nachman, R. L., Becker, C. G. & Minick, C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest 52, 2745–2756 (1973).
Jaffe, E. A., Nachman, R. L., Becker, C. G. & Minick C. R. Nuclear PKM2 expression predicts poor prognosis in patients with esophageal squamous cell carcinoma. Pathol Res Pract 209, 510–515 (2013).
Meng, M. B. et al. Enhanced radioresponse with a novel recombinant human endostatin protein via tumor vasculature remodeling: experimental and clinical evidence. Radiother Oncol 106, 130–137 (2013).
Acknowledgements
This work was supported by the Natural Science Foundation of China (No. 81272504, No. 81472809), Innovation Team [No. LJ201123 (EH11)], A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) (JX10231801), grants from Key Academic Discipline of Jiangsu Province “Medical Aspects of Specific Environments”, Research and Innovation Project for College Graduates of Jiangsu Province (KYLX_0955). The methods were carried out in accordance with the approved guidelines.
Author information
Authors and Affiliations
Contributions
Conceived and designed the study strategy: X.C.S. Acquisition of data: statistical analysis and interpretation of data H.C.Z., X.Y., Y.Q.D., J.L. and L.J. Drafting or revision of the manuscript: H.C.Z., X.Y., L.L.Z. and Q.Q. Reference collection and data management: H.Z., X.C.C., Y.H.Y. and Y.Y. Wrote the manuscript: H.C.Z. and X.C.S.; Prepared the tables and figures: Z.M.L., M.L.Y., X.F.Z. and H.Y.C. Study supervision: X.C.S. All authors read and approved the final manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Zhu, H., Yang, X., Ding, Y. et al. Recombinant human endostatin enhances the radioresponse in esophageal squamous cell carcinoma by normalizing tumor vasculature and reducing hypoxia. Sci Rep 5, 14503 (2015). https://doi.org/10.1038/srep14503
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep14503
This article is cited by
-
Role of vascular endothelial growth factor in radiotherapy resistance to esophageal squamous cell carcinoma
Journal of Cancer Research and Clinical Oncology (2023)
-
The tumour immune microenvironment in oesophageal cancer
British Journal of Cancer (2021)
-
Recombinant human vascular endostatin injection to synchronize craniospinal radiotherapy for the treatment of recurrent medulloblastoma in children: A retrospective clinical study*
Oncology and Translational Medicine (2021)
-
Targeting hallmarks of cancer to enhance radiosensitivity in gastrointestinal cancers
Nature Reviews Gastroenterology & Hepatology (2020)
-
Anti-tumor effect of endostatin in a sleep-apnea mouse model with tumor
Clinical and Translational Oncology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.