Abstract
Nematodes are known to be harmful to various crops, vegetables, plants and insects. The present study reports that, chitin upregulates the activity of chitinase (20%) and nematicidal potential (15%) of Pseudomonas aeruginosa. The chitinase gene (pachi) from P. aeruginosa was cloned and its nematicidal activity of pachi protein against Caenorhabditis elegans was studied. The mortality rate induced by pachi increased by 6.3-fold when in association with Cry21Aa from Bacillus thuringiensis. Pachi efficiently killed C. elegans in its native state (LC50 = 387.3 ± 31.7 μg/ml), as well as in association with Cry21Aa (LC50 = 30.9 ± 4.1 μg/ml), by degrading the cuticle, egg shell and intestine in a relatively short time period of 24 h. To explore the nematidal potential of chitinase, six fusion proteins were constructed using gene engineering techniques. The CHACry showed higher activity against C. elegans than others owing to its high solubility. Notably, the CHACry showed a synergistic factor of 4.1 versus 3.5 a mixture [1:1] of pachi and Cry21Aa. The present study has identified eco-friendly biological routes (e.g., mixed proteins, fusion proteins) with potent nematicidal activity, which not only can help to prevent major crop losses but also strengthen the agro-economy and increase gross crop yield.
Similar content being viewed by others
Introduction
Nematodes infect the root, stem and leaves of cultural crops, leading to plant wilting, chlorosis, reduced growth and ultimately death, resulting in major economic losses. In order to control nematodes, various chemicals have been used worldwide. These chemicals not only are toxic to the plants but also have various harmful effects on human health and the environment1. Gene engineering has opened new gateways to develop nontoxic bio-control agents that are effective in protecting from nematodiasis2. In the study of bio-control of nematodiasis, C. elegans has been used widely as an important model organism owing to its genetic manipulability and short life cycle3,4. A literature survey revealed that some nematophagous bacteria were effective in killing nematodes. For example, Bacillus thuringiensis, Pseudomonas and Xenorhabdus nematohilus, found in the rhizosphere of plants, showed high nematicidal activity5,6. The major virulence factors of nematophagous bacteria included microbial toxins, insecticidal crystal proteins (ICPs), hydrolytic enzymes and secondary metabolites, which killed the nematode(s) by various mechanisms. These proteins/enzymes acted on different body parts (cuticle, egg shell and intestine) and organs7,8. ICP was an important bio-control factor of Bacillus thuringiensis which has been used widely in bio-control of nematodiasis. The ICP was dissolved in the intestine and its C-terminal domain was cleaved by proteases to release the active region of the toxin9. The activated toxin was located at the brush border membrane vesicles and bound to its receptors, leading to epidermal cell rupture and pathogenic organism death10. In this study, Cry21Aa was tested on C. elegans and it showed higher nematicidal activity than Cry6Aa, Cry55Aa and Cry5Ba which have been reported as nematicidal factors11. The Cry21Aa consisted of N-terminal extension region, region of endotoxin N (14.9 kDa), region of delta-endotoxin C (28.8 kDa) and the C-terminus extension region. The toxin region of Cry21Aa (Cry) was composed of N-terminal extension region, endotoxin N and delta-endotoxin C. Chitinase was another important toxicity factor in bio-control of nematodiasis. Nematicidal chitinases were reported to be produced by various fungi, including Monacrosporium thaumasium12 and Clonostachys rosea13, which killed nematodes via egg shell digestion and cuticle hydrolysis7. As chitin was the main component of the egg shell and cuticle of nematodes and acted as a target for these nematicidal factors14, chitin and chitosan were incorporated into the soil to reduce nematode infection by inducing rhizobacteria to produce chitinase15. Pseudomonas was an important rhizobacteria strain, which could produce some hydrolases (protease and chitinase) in killing nematodes16,17,18.
To improve the nematicidal activity, many studies have been conducted on the synergistic effect(s) of two or more toxins against nematodes. For example, the cumulative effect of the Cry6A and Cry5B proteins [4:1] against C. elegans was significantly higher than their individual effects19. In another study, Cry6Aa, Cry55Aa and Cry5Ba were used in different sets and the Cry6Aa-Cry55Aa showed highest activity against M. incognita11. Recently, Luo et al. used the protease bmp1 as a synergistic factor to increase Cry5B activity (of ~7.9-fold) against C. elegans20. Moreover, not only mixed proteins but also fusion proteins have been widely applied as bio-control agents in agri-biotechnology to control pests. To improve the toxicity against adult aphids (M. persicae), the chitinase Bbchit1 was fused with the protease Pr1A and the result showed that insecticidal activity has been increased 2 times compared to Bbchit121. Additionally, the chitinase gene from tobacco (Nicotiana tabacum) was fused with cry1Ac gene from Bacillus thuringiensis and the insecticidal activity showed a remarkable increase of 11.3 times22.
While chitinase has been used widely in increasing ICP insecticidal activity, there are few reports about the use of chitinase as a factor in increasing ICP nematicidal activity. The objectives of the present study were (i) to investigate the synergistic effect of chitinase and ICP and (ii) to enhance the nematicidal activity by mixing or fusing the pachi from P. aeruginosa with Cry21Aa (Cry) to test their effects on eggs, larvae and mature nematodes and to explore the mode of action of nematicidal potential as a bio-control agent.
Results
Cloning chitinase pachi
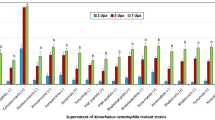
In this study, P. aeruginosa was selected for the bio-control of nematodiasis, using C. elegans as the model organism. The supernatant of lysate cell lost its nematicidal activity at high temperature and under high acidic or alkaline conditions (Fig. 1A,B), which indicated that proteins were related to the nematicidal activity of P. aeruginosa. Previous work has shown that P. aeruginosa exhibited high chitinase activity levels and in the present study, its nematicidal activity was enhanced by ~15% after the addition of 0.2% (m/v) colloid chitin in the growth medium (Fig. 1C), which indicated that chitinase would be a factor of nematicidal of P. aeruginosa. The chitinase pachi was cloned according to information from the P. aeruginosa genome (accession number: CP003149) and the sequencing results showed that pachi was similar to chitinase (99%, accession number: AFM65035)23. After being expressed in E. coli, pachi showed nematicidal activity and was selected for further experiments.
Chitinase as the potential toxin of P. aeruginosa against C. elegans.
(A) Effects of different temperatures on nematicidal activity P. aeruginosa. (B) Effects of different acidic and alkaline conditions on nematicidal activity of P. aeruginosa. (C) Effects of chitin on the chitinase activity and nematicidal activity of P. aeruginosa. The concentration of N-acetylglucosamine (NAG) indicated chitinase activity. Nematicidal activity was measured using L4 worms (~80 worms each well) at 20 °C.
Chitinases as high efficiency factors in bio-control
Figure 2 shows the phylogenetic analysis of the relationship among chitinases from different bacteria. Most chitinases were previously shown to have high antibiosis, antifungal and insecticidal activities, but few have been reported as to be virulent to nematodes7,24.
Phylogenetic analysis of the relationships among chitinases from different bacteria.
The phylogenetic tree was constructed using the neighbor-joining method (MEGA6.0). All chitinase sequences were obtained from GeneBank and PDB (http://www.rcsb.org/pdb/home/home.do) and the accession numbers of chitinase were listed in the form of “AAM48520”, except for pachi. These chitinases were reported to infect multiple phytopathogenic organisms such as fungi, insects and nematodes.
CHACry as a high nematicidal factor
In the present study, 6 fusion proteins were constructed and expressed in E. coli (Fig. 3A). The CHACry showed higher acitivity against C. elegans than others due to its high solubility (Fig. 3B) and thus it was selected for further experiments against C. elegans.
Schematic representation of construction, expression and nematicidal activity of fusion proteins.
(A) Fusion protein construction. (B) Fusion protein tested on C. elegans. (C) Fusion protein expression. Lane M: Standard protein molecular mass marker (10–225.0 kDa). Lane 2–7: Induced cell lysate supernatants of pGEX-6p-1, pGEX-6p-Cry21Aapachi, pGEX-6p-pachiCry21Aa, pGEX-6p-Crypachi, pGEX-6p-pachiCry, pGEX-6p-CryCHA and pGEX-6p-CHACry. The arrows point to target proteins.
Nematicidal activity analysis
The effects of the proteins on mortality rate, brood size and growth of nematodes were systematically studied using purified protein (Fig. 4B) and pachi showed a nematicidal factor of LC50 = 387.3 ± 31.7 μg/ml (Fig. 5A and Table 1). Synergism was reported in previous studies in which chitinase showed enhanced activity against nematodes11,19,25. In the present study, pachi was mixed with Cry21Aa in different ratios (Table 1); pachi at a ratio of 3:1 with Cry21Aa significantly increased nematicidal activity. This combination resulted in an approximately 6.3-fold reduction in the LC50 compared with the expected value (SF, 6.3). Moreover, the mortality rate induced by the protein mixture at a ratio of 3:1 (LC50, 30.9 ± 4.1 μg/ml) was higher than that of pachi and Cry21Aa individually (LC50, 78.7 ± 5.1 μg/ml) (Fig. 5A). The brood size test and growth bioassay were systematically performed with each sample using pachi, Cry21Aa, Cry21Aa: pachi at a 1:3 ratio and the CHACry fusion protein. In the brood size analysis, pachi showed high activity against C. elegans breeding (IC50, 106.1 ± 4.3 μg/ml) and the cumulative effect of pachi:Cry21Aa (3:1) (IC50, 27.9 ± 0.9 μg/ml) showed a SF of 3.2 (Table 2 and Fig. 5B). In growth bioassay studies, the larvae grew much slower in the system containing pachi added to Cry21Aa. The protein mixture displayed higher activity against larval growth (GC50, 24.4 ± 3.3 μg/ml) than pachi (GC50, 154.7 ± 5.2 μg/ml) and Cry21Aa (GC50, 44.8 ± 2.3 μg/ml) individually (Fig. 5C,D). Additionally, the fusion protein CHACry (LC50, 37.8 ± 2.2 μg/ml; SF, 4.0) showed higher activity against C. elegans than the Cry21Aa:pachi mixture[1:1] (LC50, 44.0 ± 3.9 μg/ml; SF, 3.5). The same efficacy was observed for the brood size (IC50, 35.0 ± 1.7 μg/ml) test and the growth bioassay (GC50, 26.7 ± 4.9 μg/ml).
Recombinant plasmid construction and SDS-PAGE analysis of protein expression.
(A) Fusion protein construction and partial restriction enzymatic site. (B) SDS-PAGE analysis of recombination protein expression in E. coli and purification. The arrows point to target proteins. Lane 1: The standard protein molecular mass marker (14.4–116.0 kDa). Lane 2–5: Induced cell lysate supernatants of pGEX-6p-1, pGEX-6p-CHACry, pGEX-6p-Cry21Aa and pGEX-6p-pachi harbored in E. coli. Lane 6-8: Induced cell lysate sediments of pGEX-6p-CHACry, pGEX-6p-Cry21Aa and pGEX-6p-pachi harbored in E. coli. Lane 9–11: Purified CHACry, Cry21Aa and pachi proteins.
Pachi enhances Cry21Aa activity against C. elegans.
(A) Mortality analysis was performed on L4 worms. (B) Brood size tests were performed on L4 worms. (C) Growth assays were performed on L1 larva. Error bars represent the standard deviation from the average values within three parallel experiments; (D) The effects of growth inhibition. All images were captured at 10X magnification under a Zeiss LSM 510 confocal microscope.
Effects of pachi on egg shell, cuticle and intestine
In the present study, we observed that pachi acts effectively on the egg shell and cuticle to kill nematodes. Fresh eggs were incubated with pachi at 20 °C and 37 °C for different time periods (2, 4, 6, 8, 10, 12 h). The egg surface began to show roughness and irregularities after 6 h (at 20 °C), whereas eggs incubated with the control (PBS) were smooth and remained formed after 6 h (Fig. 6A). The egg shells were destroyed in 4 h when incubated with pachi at 37 °C and disappeared after 12 h of incubation, whereas eggs incubated in the PBS control remained unaffected (Fig. 6B).
Similarly, the nematode cuticle was critically destroyed after incubation with pachi at 37 °C for 24 h, with hardly any pieces of whole cuticle observed under a microscope after 48 h (Fig. 6D). In contrast, the cuticle remained in its original state after 48 h of incubation in PBS (Fig. 6C). Furthermore, most ICPs were able to destroy the nematode intestine8. After incubation with pachi for 24 h, the intestine was digested and destroyed, leading to nematode death (Fig. 7C). The intestine shrunk and became difficult to identify after incubation with Cry21Aa for 36 h, whereas the cuticle remained unaffected (Fig. 7B). Moreover, the ability of Cry21Aa to destroy the intestine of nematodes was enhanced by pachi (Fig. 7D). When the catalytic domain of pachi was fused with the Cry21Aa toxin region, the intestine and the cuticles were destroyed after 60 h of incubation at 37 °C. This result was probably due to each protein killing the nematodes by different mechanisms. Here, the degradation of the cuticle and intestine demonstrated that pachi damaged the skin and intestine, whereas the ICP acted only on the intestine of the nematode.
Discussion
In various previous studies, P. aeruginosa was reported to cause disease in insects, nematodes and mice18,26,27,28. In the present study, we attempted to explore new nematicidal agents from P. aeruginosa. Chitinase from P. aeruginosa has been selected not only because of the regulation of its nematicidal activity by colloidal chitin (Fig. 1C) but also the scarcity of reports on chitinase as a bio-control agent for nematodes (Fig. 2). The chitinase pachi showed a high potential to kill nematodes, bio-control broods and inhibit growth through digestion of the cuticle, egg shells and intestine of C. elegans. Moreover, the chitinase pachi enhanced Cry21Aa activity against C. elegans.
Chitin played an important role in the formation of the egg shell and cuticle of C. elegans by acting as an important barrier to protect nematodes from infection by pathogenic microorganisms14. Chitinase, due to its high chitin-degrading activity, has been used as a bio-control agent against phyto-pathogenic bacteria, fungi, insects and nematodes (Fig. 2). Several bacteria such as Bacillus, Serratia, Pseudomonas and Streptomyces could use chitin as a carbon source and infect hosts using chitinase29,30,31,32. The antifungal mechanism of chitinase was due to the hydrolysis of chitin in the cell wall, leading to cell lysis24. Chitinase could also destroy the intestine and cuticle of insects by digesting chitin, leading to gut shrinkage and osmotic pressure imbalance33. In this study, pachi effectively destroyed the C. elegans cuticle and intestine, resulting in the death of nematodes (Figs 6C,D and 7). Pachi also displayed high activity for the digestion of egg shells and the inhibition of egg hatching, which was supported by a study carried out by Gan et al.34. In the study of Mercer et al.35, the chitinase increased hatch rates of nematode eggs but caused the death of juveniles instantly. In the present study, chitin upregulated the activity of chitinase of P. aeruginosa and increased its nematicidal activity. Chitin could act as important carbon source and signal molecules to upregulate production of chitinase. However, thus far, the information about the nematicidal mechanism of chitinase is very limited in the literature7. The complex structure and multi-component system of chitin has made elusive the understanding of whether the mechanism of action of chitinase is different or the same in fungi, insects and nematodes.
In this study, two different properties proteins, pachi obtained from P. aeruginosa and Cry21Aa from B. thuringiensis, functioned together as a nematicidal agent. Previous studies showed that chitinase could enhance the insecticidal activity of B. thuringiensis by penetrating the peritrophic membrane barrier in the larval midgut and aiding the ICPs to bind their receptors on epithelial cell membranes36. To enhance its large-scale application and use as an insecticidal agent, B. thuringiensis was used as a host for co-expressing several chitinases from Bacillus licheniformis, Bacillus circulans, Pseudomonas maltophilia, Bacillus sphaericus, Aeromonoas hydrophila, Serratia marcescens and Nicotiana tabacum22,37,38,39,40,41. Moreover, nematodes had a similar peritrophic membrane structure and composition as insects42. Therefore, as reported, the chitinase pachi also could enhance the toxicity of Cry21Aa against C. elegans by the similar modes of insecticidal.
Another attractive property was that the CHACry fusion protein showed higher activity than each individual protein. Six fusion proteins were constructed and tested on C. elegans, but the CHACry showed highest activity and solubility. A previous study indicated that C-terminal extension region was related to solubility of protein and the CHA had higher solubility than pachi23. Recently, many fusion proteins have been constructed and co-expressed to enhance relative activity and more data about such fusion proteins would facilitate the access to the binding sites, improve their stability and broaden the insect-resistance spectrum43,44. Fusion venoms were well known to resist proteolytic activity in insects, alter the shape of the original protein crystals and alter receptor binding sites present in the midgut of insects45. Previous studies also showed that the ICPs from Bacillus expressed in E. coli easily formed inclusion bodies and lowered the activity due to the dissolution process that was necessary for ICP activation46. Our previous study indicated that solubility of chitinase was increased after removing the chitin binding domain23. In the present study, the catalytic domain of pachi was fused with the toxin region of Cry21Aa and the CHACry fusion protein (~50% soluble) showed better solubility than Cry21Aa, which increased Cry21Aa activity against C. elegans (Fig. 4B). Additionally, each toxin had a unique mechanism of killing hosts and damaging the host cell11. Many researchers believed that the ICP damaged the intestine of the host and that chitinases hydrolyzed the cuticle and egg shell35,47. Our results revealed that the chitinase showed high intestinal digestion activity, which would protect the ICP from proteolysis and help the ICP bind to its receptor. Moreover, the chitinase also showed high cuticle degradation activity and provided a new way for the ICP to enter into the intestine of the nematode. However, the mechanism of synergy by which a biopesticide entered the nematode and became resistant to insecticidal attack remained to be elucidated.
Previous studies indicated that P. aeruginosa PA14 work in two different modes to kill C. elegans. At low salt concentration medium, PA14 showed a mild infection and killed the nematodes in 72–96 h (‘slow killing’) via production of hydrolytic enzyme, like protease, which was due to the mass accumulation of bacteria in the intestine and enzymatic degradation. But at high salt medium, PA14 produced diffusible toxins like cyanongen, phenazines and pycoyanin and killed nematodes within a short time period (‘fast killing’) by inhibiting metabolic pathways48. In this study, P. aeruginosa killed C. elegans also by producing hydrolytic enzyme (chitinase) and toxin. It was interesting to note that the strain (P. aeruginosa) did not produce protease, which was different from some previous studies17, while chitinase played a vital role in nematicidal activity. Recent studies of Pseudomonas infection in nematodes were based upon the quorum sensing system (QS)49. It was a complex cell-to-cell signaling system allowing the bacteria to sense and regulated their own cell densities. There were three types of secretion systems (type I, type II and type III) in QS of P. aeruginosa to secrete extracellular hydrolytic enzyme into the cytoplasm of hosts50. Type II secretion system regulated the production of extracellular hydrolases, like elastase, alkaline phosphatase, phospholipase C and chitinase etc.51. In the present study, the genome analysis of P. aeruginosa predicted that some extracellular hydrolases (like serine protease, collagenase, phospholipase C, chitinase) might play an important role in killing nematodes. The mechanisms of different hydrolases in killing nematodes would be an interesting topic in future studies.
Materials and Methods
Strains and culture
The strains and plasmids used in this study are listed in Supplementary data Table 1. The bacterial strain was isolated from a mud soil sample from South Lake near Huazhong Agricultural University, Wuhan, China. The culture was grown in nutrient agar medium containing peptone 1%, yeast extract 0.5%, NaCl 1% and agar 1.5% (m/v). The selected bacterial strain was identified as P. aeruginosa by 16S rRNA sequencing. Escherichia (E.) coli strains were maintained in Luria-Bertani medium containing ampicillin (100 μg/ml). All bacterial strains were kept in 20% (v/v) glycerol suspension at −80 °C. The C. elegans N2 wild-type strain was provided by the Caenorhabditis Genetics Center (CGC) and maintained on nematode growth medium (NGM) agar plates with E. coli OP50 as its food at 20 °C and stored at −80 °C.
Chitinase activity
P. aeruginosa was collected and resuspended in PBS buffer (final OD600 ~ 3.0), followed by ultrasonic disruption. The supernatant was selected to test its chitinase and nematicidal activity. Chitinase activity was measured according to method described by Chen et al.23, while nematicidal activity was measured according to the method of Bischof et al.52.
Cloning of gene pachi and Cry21Aa
Genomic DNA extracted from P. aeruginosa was used as a template to amplify pachi using the primers pachi-f and pachi-r (Supplementary data Table 2). The Cry21Aa gene was amplified from the plasmid pHT304-Cry21Aa using the primer pair Cry21Aa-f and Cry21Aa-r. The pachi gene was digested by EcoR I and Xho I and cloned into the pGEX-6p-1 vector to construct the pGEX-6p-pachi expression vector, whereas Cry21Aa was digested by BamH I and Xho I to construct the pGEX-6p-Cry21Aa expression vector. The recombinant plasmids were then transformed into E. coli BL21 (DE3) cells.
Construction of fusion proteins
The pGEx-6p-1 plasmid was used as the template to construct the pGEX-6p-H plasmid by inserting an additional Hind III restriction enzyme site between BamH I and EcoR I using the primer pair 6p-H-f and 6p-H-r. Rapid polymerase chain reaction (PCR)-based site-directed mutagenesis was used to add the two restriction enzyme sites. Fusion proteins were constructed by using over-lapping PCR. The CHACry (3087 bp; 114.5 kDa; 3 bp for termination codon) was constructed by fusing the catalytic domain of pachi (1026 bp; 37.7 kDa) with the toxin region of Cry21Aa (Cry) (2058 bp; 76.8 kDa). The Cry contained the N-terminus extension region, endotoxin N and delta-endotoxin C. Cloning of the Cry21Aa N-terminus was directed by using the primer pair Cry21Aa-f2 and Cry21Aa-r2. The catalytic domain (CHA) of pachi was cloned with the primer pair CHA-f and CHA-r. Finally, the recombinant CHACry gene was cloned with the primer pair CHA-f and Cry21Aa-r2 by overlapping PCR. Recombinant pGEX-6p-CHACry was transformed into E. coli BL21 (DE3) cells to express the CHACry fusion protein.
Protein expression and purification
E. coli BL21 (DE3) cells harboring pGEX-6p-pachi, named DE3/pGEX-6p-pachi, were inoculated into LB broth (ampicillin; 100 μg/ml) with shaking at 37 °C for 12 h. The seed culture was used to inoculate production broth (v/v, 2/100) and growth was induced by adding IPTG (1 mM) after 2–3 h. The IPTG-induced production broth was incubated at 18 °C for 14 h with shaking (250 rpm/min).
Finally, the induced cells were collected by centrifugation, resuspended in phosphate-buffered saline (PBS) buffer (NaCl 0.8%, KCl 0.02%, Na2HPO4 0.14%, KH2PO4 0.03%; pH 7.0) and homogenized using a high-pressure homogenizer (NS100IL 2K, Niro Soavi, Germany). The target proteins (Cry21Aa, pachi and CHACry) were purified using a glutathione S-transferase (GST) Gene Fusion System (GE Healthcare, USA) and eluted from the GST tag by 3C proteases (PreScission, Pharmacia). The molecular weight was analyzed by SDS-PAGE with 10% polyacrylamide gels. The protein concentration was measured by the Bradford method using bovine serum albumin (BSA) as a standard.
Quantitative analysis of nematicidal activity
The purified proteins were used for bioassays including quantitative mortality tests, brood size assays and growth analyses. The bioassay procedures and 50% lethal/inhibition/growth concentration (LC50/IC50/GC50) evaluations were undertaken according to the method of Bischof et al.52.
Cuticle and egg shell digestion
The cuticle was separated from the nematode body as described by Cox et al.14. Cuticles (eggs) was incubated with pachi at 20 °C and 37 °C for various time periods. The L4 worms were incubated with pachi, Cry21Aa and the CHACry fusion protein at 20 °C and 37 °C for various time periods. Pachi was used to study complete digestion at 1 mg/ml for the cuticle and at 200 μg/ml for the egg shell and intestine. The effects of Cry21Aa on the intestine were assessed at a final concentration of 150 μg/ml, while CHACry was assessed at 100 μg/ml. Pachi (1 mg/ml) was used as an effective nematicidal dose to ensure the complete killing of C. elegans.
Synergistic activity assays for pachi and Cry21Aa in L4 worms of the N2 strain
The synergistic factor was calculated by the formula of Tabashnik et al.53: 1/LC50(m) = Ra/LC50(a) + Rb/LC50(b), where Ra and Rb indicate the percentage of toxin A and toxin B proteins used in the final mixture; LC50(a) and LC50(b) represent the LC50 values for each toxin and LC50(m) is the expected theoretical value of LC50 calculated from the formula above. The real LC50 value was calculated from the bioassay for the observed toxicity of the mixture. Synergism was indicated with a SF value of greater than 1.
Statistical analysis
Independent experiments were repeated at least three times. All of the data obtained were analyzed using GraphPad Prism 5.0 and Excel 2003 software for figures and LC50 values. All of the values were expressed as the mean values ± standard deviation, with the statistical significance set at p < 0.05.
Image analysis by confocal microscopy
To study the effect of each protein on nematodes and their body parts, images were captured using a 20X objective lens with confocal laser scanning microscopy on a Zeiss LSM 510 microscope (CLSM; Zeiss LSM 510) imaging. Mixture of diethyl ether/ethanol absolute (1:1) was used as anaesthetic treatment to keep worms static during image capture. All pictures were processed using Photoshop 7.0 software and the worm sizes were calculated using ImageJ 2.4.1.7.
Nucleotide sequence accession number
The nucleotide sequences of Pseudomonas aeruginosa 16S and pachi have been submitted to GeneBank under accession numbers KR007310 (16S) and KR007311 (pachi).
Additional Information
How to cite this article: Chen, L. et al. Enhanced nematicidal potential of the chitinase pachi from Pseudomonas aeruginosa in association with Cry21Aa. Sci. Rep. 5, 14395; doi: 10.1038/srep14395 (2015).
References
Lifshitz, M. & Gavrilov, V. Central nervous system toxicity and early peripheral neuropathy following dermal exposure to methyl bromide. ClinToxicol 38, 799–801 (2000).
Opperman, C. H., Acedo, G. N., Saravitz, D. M., Skantar, A. M., Song, W. et al. Bioengineering resistance to sedentary endoparasitic nematodes. Advances in Molecular Plant Nematology 221–230 (Springer, 1994).
Kaletta, T. & Hengartner, M. O. Finding function in novel targets: C. elegans as a model organism. Nat Rev Drug Discov 5, 387–398 (2006).
Leung, M. C., Williams, P. L., Benedetto, A., Au, C., Helmcke, K. J. et al. Caenorhabditis elegans: an emerging model in biomedical and environmental toxicology. Toxicol Sci 106, 5–28 (2008).
Li, B., Xie, G. L., Soad, A. & Coosemans, J. Suppression of Meloidogyne javanica by antagonistic and plant growth-promoting rhizobacteria. J Zhejiang Univ Sci B 6, 496–501 (2005).
Samaliev, H. Y., Andreoglou, F. I., Elawad, S. A., Hague, N. G. & Gowen, S. R. The nematicidal effects of the bacteria Pseudomonas oryzihabitans and Xenorhabdus nematophilus on the root-knot nematode Meloidogyne javanica. Nematology 2, 507–514 (2000).
Yang, J., Liang, L., Li, J. & Zhang, K. Nematicidal enzymes from microorganisms and their applications. Appl Microbiol Biotechnol 97, 7081–7095 (2013).
Wei, J. Z., Hale, K., Carta, L., Platzer, E., Wong, C. et al. Bacillus thuringiensis crystal proteins that target nematodes. P NATL ACAD SCI 100, 2760–2765 (2003).
Boonserm, P., Mo, M., Angsuthanasombat, C. & Lescar, J. Structure of the functional form of the mosquito larvicidal Cry4Aa toxin from Bacillus thuringiensis at a 2.8-angstrom resolution. J Bacteriol 188, 3391–3401 (2006).
Bravo, A., Gomez, I., Conde, J., Munoz-Garay, C., Sanchez, J. et al. Oligomerization triggers binding of a Bacillus thuringiensis Cry1Ab pore-forming toxin to aminopeptidase N receptor leading to insertion into membrane microdomains. BBA-Biomembranes 1667, 38–46 (2004).
Soares, F. E. D. F., Queiroz, J. H. D., Araújo, J. V. D., Queiroz, P. V., Gouveia, A. d. S. et al. Nematicidal action of chitinases produced by the fungus Monacrosporium thaumasium under laboratorial conditions. Biocontrol Sci Techn 25, 1–17 (2015).
Ahmed, M., Laing, M. & Nsahlai, I. Use of Clonostachys rosea against sheep nematodes developing in pastures. Biocontrol Sci Techn 24, 389–398 (2014).
Cox, G. N., Kusch, M. & Edgar, R. S. Cuticle of Caenorhabditis elegans: its isolation and partial characterization. J Cell Biol 90, 7–17 (1981).
Radwan, M. A., Farrag, S. A. A., Abu-Elamayem, M. M. & Ahmed, N. S. Extraction, characterization and nematicidal activity of chitin and chitosan derived from shrimp shell wastes. Biol Fert Soils 48, 463–468 (2011).
Singh, N., Kumar, S., Bajpai, V. K., Dubey, R. C., Maheshwari, D. K. et al. Biological control of Macrophomina phaseolina by chemotactic fluorescent Pseudomonas aeruginosa PN1 and its plant growth promotory activity in chir-pine. Crop Prot 29, 1142–1147 (2010).
Ali, N. I., Siddiqui, I. A., Shahid Shaukat, S. & Zaki, M. Nematicidal activity of some strains of Pseudomonas spp. Soil Biol Biochem 34, 1051–1058 (2002).
Yorgey, P., Rahme, L. G., Tan, M. W. & Ausubel, F. M. The roles of mucD and alginate in the virulence of Pseudomonas aeruginosa in plants, nematodes and mice. Mol Microbiol 41, 1063–1076 (2001).
Yu, Z., Luo, H., Xiong, J., Zhou, Q., Xia, L. et al. Bacillus thuringiensis Cry6A exhibits nematicidal activity to Caenorhabditis elegans bre mutants and synergistic activity with Cry5B to C. elegans. Lett Appl Microbiol 58, 511–519 (2014).
Peng, D., Chai, L., Wang, F., Zhang, F., Ruan, L. et al. Synergistic activity between Bacillus thuringiensis Cry6Aa and Cry55Aa toxins against Meloidogyne incognita. Microbial Biotechnol 4, 794–798 (2011).
Luo, X., Chen, L., Huang, Q., Zheng, J., Zhou, W. et al. Bacillus thuringiensis metalloproteinase Bmp1 functions as a nematicidal virulence factor. Appl Environ Microb 79, 460–468 (2013).
Fang, W., Feng, J., Fan, Y., Zhang, Y., Bidochka, M. J. et al. Expressing a fusion protein with protease and chitinase activities increases the virulence of the insect pathogen Beauveria bassiana. J Invertebr Pathol 102, 155–159 (2009).
Ding, X., Luo, Z., Xia, L., Gao, B., Sun, Y. et al. Improving the insecticidal activity by expression of a recombinant cry1Ac gene with chitinase-encoding gene in acrystalliferous Bacillus thuringiensis. Curr Microbiol 56, 442–446 (2008).
Chen, L., Chen, J., Kumar, A. & Liu, Z. Effects of domains modification on the catalytic potential of chitinase from Pseudomonas aeruginosa. Int J Biol Macromol 78, 266–272 (2015).
Ntalli, N. G., Ferrari, F., Giannakou, I. & Menkissoglu-Spiroudi, U. Synergistic and antagonistic interactions of terpenes against Meloidogyne incognita and the nematicidal activity of essential oils from seven plants indigenous to Greece. Pest Manag Sci 67, 341–351 (2011).
Rahme, L., Stevens, E., Wolfort, S., Shao, J., Tompkins, R. et al. Common virulence factors for bacterial pathogenicity in plants and animals. Science 268, 1899–1902 (1995).
Rahme, L. G., Tan, M. W., Le, L., Wong, S. M., Tompkins, R. G. et al. Use of model plant hosts to identify Pseudomonas aeruginosa virulence factors. P Natl Acad Sci 94, 13245–13250 (1997).
Jander, G., Rahme, L. G. & Ausubel, F. M. Positive correlation between virulence of Pseudomonas aeruginosa mutants in mice and insects. J Bacteriol 182, 3843–3845 (2000).
Huang, C., Wang, T., Chung, S. & Chen, C. Identification of an antifungal chitinase from a potential biocontrol agent, Bacillus cereus 28-9. J Biochem Mol Biol 38, 82–88 (2005).
Kalbe, C., Marten, P. & Berg, G. Strains of the genus Serratia as beneficial rhizobacteria of oilseed rape with antifungal properties. Microbiol Res 151, 433–439 (1996).
Gaffney, T. D., Lam, S. T., Ligon, J., Gates, K., Frazelle, A. et al. Global regulation of expression of antifungal factors by a Pseudomonas fluorescens biological control strain. Mol Plant Microbe In 7, 455–463 (1994).
Mahadevan, B. & Crawford, D. L. Properties of the chitinase of the antifungal biocontrol agent Streptomyces lydicus WYEC108. Enzyme Microb Tech 20, 489–493 (1997).
Theis, T. & Stahl, U. Antifungal proteins: targets, mechanisms and prospective applications. Cell Mol Life Sci 61, 437–455 (2004).
Qin, Y., Ying, S.-H., Chen, Y., Shen, Z.-C. & Feng, M.-G. Integration of insecticidal protein Vip3Aa1 into Beauveria bassiana enhances fungal virulence to Spodoptera litura larvae by cuticle and per os infection. Appl Environ Microb 76, 4611–4618 (2010).
Gan, Z., Yang, J., Tao, N., Liang, L., Mi, Q. et al. Cloning of the gene Lecanicillium psalliotae chitinase Lpchi1 and identification of its potential role in the biocontrol of root-knot nematode Meloidogyne incognita. Appl Microbiol Biot 76, 1309–1317 (2007).
Mercer, C., Greenwood, D. & Grant, J. Effect of plant and microbial chitinases on the eggs and juveniles of Meloidogyne hapla Chitwood (Nematoda: Tylenchida). Nematologica 1, 227–236 (1992).
Pardo-Lopez, L., Munoz-Garay, C., Porta, H., Rodríguez-Almazán, C., Soberón, M. et al. Strategies to improve the insecticidal activity of Cry toxins from Bacillus thuringiensis. Peptides 30, 589–595 (2009).
Tantimavanich, S., Pantuwatana, S., Bhumiratana, A. & Panbangred, W. Cloning of a chitinase gene into Bacillus thuringiensis subsp. aizawai for enhanced insecticidal activity. J Gen Appl Microbiol 43, 341–347 (1997).
Wiwat, C., Lertcanawanichakul, M., Siwayapram, P., Pantuwatana, S. & Bhumiratana, A. Expression of chitinase-encoding genes from Aeromonas hydrophila and Pseudomonas maltophilia in Bacillus thuringiensis subsp. israelensis. Gene 179, 119–126 (1996).
Driss, F., Kallassy-Awad, M., Zouari, N. & Jaoua, S. Molecular characterization of a novel chitinase from Bacillus thuringiensis subsp. kurstaki. J Appl Microbiol 99, 945–953 (2005).
Okay, S., Tefon, B., Özkan, M. & Özcengiz, G. Expression of chitinase A (chiA) gene from a local isolate of Serratia marcescens in Coleoptera-specific Bacillus thuringiensis. J Appl Microbiol 104, 161–170 (2008).
Cai, Y., Yan, J., Hu, X., Han, B. & Yuan, Z. Improving the insecticidal activity against resistant Culex quinquefasciatus mosquitoes by expression of chitinase gene chiAC in Bacillus sphaericus. Appl Environ Microb 73, 7744–7746 (2007).
Borgonie, G., Claeys, M., Vanfleteren, J., Waele, D. D. & Coomans, A. Presence of peritrophic-like membranes in the intestine of three bacteriophagous nematodes (Nematoda: Rhabditida). Fundam Appl Nematol 18, 227–233 (1995).
Pyati, P., Fitches, E. & Gatehouse, J. A. Optimising expression of the recombinant fusion protein biopesticide omega-hexatoxin-Hv1a/GNA in Pichia pastoris: sequence modifications and a simple method for the generation of multi-copy strains. J Ind Microbiol Biot 41, 1237–1247 (2014).
Tajne, S., Boddupally, D., Sadumpati, V., Vudem, D. R. & Khareedu, V. R. Synthetic fusion-protein containing domains of Bt Cry1Ac and Allium sativum lectin (ASAL) conferred enhanced insecticidal activity against major lepidopteran pests. J Biotechnol 1, 71–75 (2014).
Yan, F., Cheng, X., Ding, X., Yao, T., Chen, H. et al. Improved insecticidal toxicity by fusing Cry1Ac of Bacillus thuringiensis with Av3 of Anemonia viridis. Curr Microbiol 68, 604–609 (2014).
Fahnert, B., Lilie, H. & Neubauer, P. Inclusion bodies: formation and utilisation. Physiological Stress Responses in Bioprocesses 93–142 (Springer, 2004).
Wang, F., Liu, Y., Zhang, F., Chai, L., Ruan, L. et al. Improvement of crystal solubility and increasing toxicity against Caenorhabditis elegans by asparagine substitution in block 3 of Bacillus thuringiensis crystal protein Cry5Ba. Appl Environ Microb 78, 7197–7024 (2012).
Tan M. W., A. F. M. Caenorhabditis elegans a model genetic host to study Pseudomonas aeruginosa pathogenesis. Curr Microbiol 3, 29–34 (2000).
Venturi, V. Regulation of quorum sensing in Pseudomonas. FEMS Microbiol Rev 30, 274–291 (2006).
Kipnis, E., Sawa, T. & Wiener-Kronish, J. Targeting mechanisms of Pseudomonas aeruginosa pathogenesis. Med Maladies Infect 36, 78–91 (2006).
Folders, J. et al. Characterization of Pseudomonas aeruginosa chitinase, a gradually secreted protein. J Bacteriol 183, 7044–7052 (2001).
Bischof, L. J., Huffman, D. L. & Aroian, R. V. Assays for toxicity studies in C. elegans with Bt crystal proteins. C. elegans. 139–154 (Springer, 2006).
Tabashnik, B. E. Evaluation of synergism among Bacillus thuringiensis toxins. Appl Environ Microb 58, 3343–3346 (1992).
Acknowledgements
This work was supported by grants from National Basic Research Program (973) of China (2013CB127504) and the China 948 Program of Ministry of Agriculture (2011-G25).
Author information
Authors and Affiliations
Contributions
L.C. designed the experiments and wrote the manuscript. L.C. and J.C. performed the experiments. L.C. analyzed the data. H.J., Q.C., J.C., A.K., G.W., Z.L. and M.S. contributed reagents/materials/analysis tools.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Chen, L., Jiang, H., Cheng, Q. et al. Enhanced nematicidal potential of the chitinase pachi from Pseudomonas aeruginosa in association with Cry21Aa. Sci Rep 5, 14395 (2015). https://doi.org/10.1038/srep14395
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep14395
This article is cited by
-
Plant chitinases: Types, structural classification, antifungal potential and transgenic expression in plants for enhanced disease resistance
Plant Cell, Tissue and Organ Culture (PCTOC) (2024)
-
Nematicidal effects of a peptidase (NlpC/P60) from the phytopathogen Pseudomonas syringae MB03 on the model nematode Caenorhabditis elegans and plant-parasitic Meloidogyne incognita
Molecular Biology Reports (2022)
-
Thermophilic Chitinases: Structural, Functional and Engineering Attributes for Industrial Applications
Applied Biochemistry and Biotechnology (2021)
-
Serratia sp., an endophyte of Mimosa pudica nodules with nematicidal, antifungal activity and growth-promoting characteristics
Archives of Microbiology (2021)
-
TOXiTAXi: a web resource for toxicity of Bacillus thuringiensis protein compositions towards species of various taxonomic groups
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.