Abstract
European expansion and contact with indigenous populations led to catastrophic depopulation primarily through the introduction of novel infectious diseases to which native peoples had limited exposure and immunity. In the Amazon Basin such contacts continue to occur with more than 50 isolated indigenous societies likely to make further contacts with the outside world in the near future. Ethnohistorical accounts are useful for quantifying trends in the severity and frequency of epidemics through time and may provide insight into the likely demographic consequences of future contacts. Here we compile information for 117 epidemics that affected 59 different indigenous societies in Greater Amazonia and caused over 11,000 deaths between 1875 and 2008, mostly (75%) from measles, influenza and malaria. Results show that mortality rates from epidemics decline exponentially through time and, independently, with time since peaceful contact. The frequency of documented epidemics also decreases with time since contact. While previous work on virgin soil epidemics generally emphasizes the calamity of contacts, we focus instead on improvements through time. The prospects for better survivorship during future contacts are good provided modern health care procedures are implemented immediately.
Similar content being viewed by others
Introduction
European colonization of the New World brought catastrophic depopulation to indigenous societies1,2,3,4,5,6,7,8,9. Many of the same external threats of epidemics, violence and displacement continue to adversely affect indigenous populations to this day6,10,11. Some of the smallest and most vulnerable human groups are the more than 50 isolated indigenous societies in Amazonia who have limited contact with the outside world12,13. Little scientific attention has been paid to the likely demographic outcomes of future contacts14, but such an endeavor is important given the precariousness of their situation12,15,16,17.
The introduction of novel infectious diseases was arguably the most devastating consequence of European colonization8,9,18. At the time of early colonization, many indigenous populations of the Americas had no prior exposure to pathogens that had become common in Europe. Such populations are often referred to as virgin soil populations2. In such populations, epidemics caused by acute infectious pathogens (called virgin soil epidemics) can be extremely severe, resulting in high mortality and complete social disruption. In small indigenous communities in Amazonia and elsewhere, this situation occurred not only at the time of initial European colonization, but repeatedly, albeit relatively rarely and widely dispersed in time over the succeeding centuries4,5,6.
The rarity of epidemics in isolated populations is well understood. In 1957, a statistician, Maurice Bartlett, examined several time series of measles epidemics from 19 towns in England and Wales and showed that there was a relationship between the mean time between epidemics and the size of a population19. Based on his results, he proposed 3 fundamental measles epidemic patterns. Type I waves occurred in large cities of over 250,000 residents and were characterized by constant, but small numbers of cases and occasional larger epidemic outbreaks. Type II waves occurred in populations between about 10,000 and 250,000 people and consisted of discrete but regular epidemics which were separated by time spans where no measles cases were present. Type III waves were characteristic of small populations and consisted of sporadic epidemics whenever a disease was introduced but an inability to maintain a disease without exogenous introductions. The size of these populations was so small that outbreaks would spread quickly through the population, but the rapid depletion of susceptible individuals would break epidemic chains and result in local extinction of the disease20,21.
In a test of Bartlett’s ideas, Black22 gathered data on measles in 19 island populations that varied in size. He found a correlation between population size and the presence of regular measles epidemics, but noted that other factors, in particular population density and connections to the outside world, were also important. Cliff and Haggett23 expanded and updated Black’s study and came to the same general conclusions. What these studies indicate is that populations such as the majority of indigenous Amazonian tribes, which are both isolated and small in size, tend to experience epidemics that are dispersed in time and that tend to affect high proportions of the population because the rarity of outbreaks means that most individuals lack prior exposure to the pathogen and have no pre-existing immunity.
Although the rarity of epidemics is understood, there is little consensus concerning the underlying mechanisms behind how such immense depopulation occurred with contact. One view is that native immune systems are inefficient because of high genetic homozygosity. New World indigenous populations and especially Amazonians are characterized by low underlying genetic variation24,25,26,27 as well as different frequencies of alleles in immune system genes28. It is possible that these genetic characteristics may facilitate destructive epidemics of genetically-similar hosts8,29. South America is at the end of a long chain of serial founder populations and indigenous populations have low genetic diversity due to the serial founder effect and loss of alleles as populations migrate out through time. Heterozygous alleles at loci in the major histocompatibility complex are known to give better disease resistance once exposed to a certain class of pathogens28,29. A related genetic hypothesis is that differential selective pressures are key, that natural selection has not favored alleles because New World populations did not go through the same selective filters that Old World populations have and hence do not have the alleles that are most resistant to certain classes of pathogens21. The relatively small sizes of traditional small-scale forager and horticultural populations, mostly isolated from one another except through brief hostile interactions, are not conducive to long-term perpetuation of explosive epidemic diseases and hence did not exert strong selective pressure on effective immune defense against diseases that arose in large Old World populations21. Genetic traits and alleles in Amazonian groups (both indigenous and urban) have been studied to see if their variation is related to susceptibility for a variety of infectious diseases and pathogens including malaria30,31,32, tuberculosis33,34,35,36, Helicobacter pylori37,38 and leprosy39.
Even though there is considerable evidence of genetic differences between New World indigenous populations and other groups, mechanistic evidence that those differences underlie the increased severity of diseases in indigenous groups has remained elusive, although susceptibility to tuberculosis for those with alleles that are common in the Ache (Paraguay) is suggestive evidence35,36. Some studies suggest that many native Amazonians appear to mount normal immune responses to measles and vaccinations20,40,41,42,43. Furthermore, aside from a few traits that clearly alter mechanisms related to pathogenicity, studies linking specific genes to infectious diseases have not been able to adequately account for observed differences in susceptibility. What is needed to make the case for many of these genes is to go beyond simple associations indicated through correlation analyses and identify mechanisms that can explain the observed differences among individuals. Examples of such mechanistic evidence include explanations for the high frequency of the CCR5-∆32 deletion in European populations, which changes the structure of the CCR5 cytokine receptor and prevents HIV from entering a cell44,45,46,47,48,49 and the evolution of certain alleles in the Duffy blood group system which code for altered blood cells that do not allow Plasmodium vivax to enter the cell50,51.
The difficulty of finding incontrovertible evidence to support genetic hypotheses has resulted in a variety of alternative perspectives which de-emphasize the genetic hypotheses as missing key points2,52. These environmental hypotheses focus instead on other factors of virgin soil epidemics that differentially affected indigenous populations, namely famine, overcrowding, warfare, psycho-social stress, social chaos, and, most importantly, the lack of childhood exposure to novel diseases. Numerous historical records document the severe impact of virgin soil epidemics and suggest that they are most lethal during first contacts when indigenous immune systems are mostly naïve (i.e., lacking immunity) to novel pathogens like measles, influenza and smallpox2,24. Exposed to completely new classes of pathogens for the first time, individuals are overwhelmed by new infections because no antibodies have previously formed during childhood. In virgin soil epidemics, typically people of all ages get sick, often from multiple afflictions, which leads to a general lack of food and necessary childcare52,53,54,55. Some behavioral responses may also have worsened epidemics, such as not burying corpses or participating in burial rituals that promote disease transmission, as was recently observed in the 2014 Ebola outbreak in Africa56.
The genetic and environmental hypotheses are not mutually exclusive and together may help to explain the disease experiences of New World indigenous populations. Most authors acknowledge elements of both in their arguments. Indeed, genetic and environmental factors interact to form complex phenotypes and adaptive and innate immune defenses and epidemiological profiles are no exception57,58,59,60. In brief, multiple mechanisms often contribute to compromised immunity and newly contacted native Americans may be disadvantaged by a combination of nutritional stress and homozygous and naïve immune systems. Regardless of the specifics behind underlying mechanisms driving virgin soil epidemics and massive depopulation, the overall result from the last 500 years of European colonization in the Americas and elsewhere has been a natural experiment of repeated external perturbations to large samples of indigenous populations.
Here we analyze the ethnohistoric record of Greater Amazonia over the last century including many first sustained peaceful contacts between native Amazonians and the outside world. While most of the above cited literature on virgin soil epidemics emphasizes the immense calamity of European contacts and associated indigenous depopulation, we document temporal trends in mortality rates from contact-related epidemics and focus on improvements in survivorship through time to make recommendations for future first contacts. While our data do not speak directly to genetic hypotheses, the rapid improvements in survivorship through time do implicate a strong role for environmental factors, including increased immunity and better health care and conditions.
Results
(a) Descriptive data
Table 1 gives means and medians for the main variables in this study. The median epidemic in our sample occurred in 1965, afflicting indigenous populations of 180 people of which 18%, or 32 individuals, died within a year. The range of mortality rates across epidemics is immense, varying from less than 1% to 97%. We coded purported disease(s) involved in each epidemic (27 of 117 epidemics were missing a specific disease). Counting multiple diseases equally yields 126 total records (Fig. 1); of these the most common epidemics are 47 records of measles (37%), 32 of influenza (25%) and 17 of malaria (13%). Table 1 shows that the means and ranges of mortality rates are similar for epidemics of measles, influenza and malaria. There are not enough data to compare mortality rates for the other diseases and the lack of specific causes for many data points limits our ability to assess disease specific mortality comparisons. The estimated death count associated for each disease does appear to be approximately proportional to its occurrence because there are no statistically significant differences in mortality rates as a function of disease type when these are added as categorical variables to the linear mixed model.
We examined the spatial extent of particular epidemics by mapping the locations of outbreaks of the same disease and year. There are only 3 unequivocal examples of epidemics that spread to multiple societies and these are all in same region. Figure 2 shows the hotspot of 10 societies in the Upper Xingu who suffered from epidemics of influenza in 1948 and measles in both 1954 and 19656,61. All other epidemics in the dataset appear to have afflicted only single societies, or some diseases may have taken more than a year to spread to other locations that concealed the potential connection amongst different outbreaks.
Locations of 59 indigenous societies in this study.
White dots represent the hotspot of 10 societies conglomerated in the Upper Xingu region who suffered from epidemics of influenza in 1948 and measles in 1954 and 1965. Black dots represent other epidemics that appear to have only afflicted single societies. “U” is short for Uru-Eu-Wau-Wau. Map created in GenGIS software77.
(b) Temporal trends in mortality rates
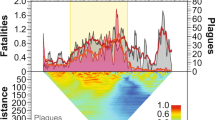
Mortality rates measured as the fraction of population dying per year from epidemics (Fig. 3) decrease exponentially as a function of both absolute year (y = ae−0.024x, n = 117, R2 = 0.19) and time since contact (y = 0.26 e−0.036x, n = 117, R2 = 0.19). The Kayapó are the only society with sufficient data to look at a longitudinal trend, albeit from various subgroups that made sustained peaceful contact at different times6,61,62. The epidemics afflicting the Kayapó have mortality rates that also show an exponential decline with time since contact (y = 0.77 e−0.123x, n = 11, R2 = 0.62) but at a steeper rate than the cross-sectional fit above.
Mortality rates (fraction of population dying per year) from 117 epidemics as a function of absolute year (left) and years since contact (right).
Both relationships are best fit with a negative exponential. High mortality outliers in the absolute year graph are from first contacts that occurred between 1950 and 1980.
Table 2 shows results from a mixed effects model predicting mortality rates (natural logged) as a function of absolute year and years since contact. The parameter estimates for these variables from the mixed effects model are similar to those obtained in the bivariate relationships in Fig. 3. Moreover, there is no interaction effect between absolute year and years since contact. Therefore, we conclude that the time variables, both absolute time and relative time since contact, have mostly independent effects on mortality rates.
We show that the intensity of epidemics declines through time; further analysis shows how the frequency declines as well. Using the longitudinal record of those societies with multiple records of epidemics gives the time between successive outbreaks within a particular society. The inter-epidemic period averages around 7 years and positively correlates with years since contact (r = 0.50, n = 58, p < 0.001), a pattern also visible in Fig. 3 where most epidemics occur soon after contact. Hence, contact epidemics become less frequent within societies as time progresses.
Discussion
Our results highlight the suffering that indigenous populations in Greater Amazonia experienced from an onslaught of infectious diseases introduced by colonization. An average mortality rate of 25% per year across epidemics speaks to the severity of these disease outbreaks. Similarly, our mixed effect model indicates that contacts occurring in the average year of 1963 led to mortality rates of 24% per year but are predicted to drop to 10% for a hypothetical contact in the year 2015. The 3 biggest killers in our dataset—measles, influenza and malaria—combined for 75% of epidemics in our data. These diseases all likely arrived in the Americas with Europeans, but many of the indigenous populations were so isolated that they may have been spared the ravages of European-introduced diseases at the time of colonization. As Black’s22 and Cliff and Haggett’s23 work on island epidemics suggests, because of the small size and isolation of these populations, the epidemic patterns for most of the European-introduced diseases would tend to follow Bartlett’s19 Type III pattern—they would occur only rarely and spread quickly, infecting nearly everyone and causing high mortality, but they would likely be short-lived and would be followed by long periods of time where the disease was absent from the population. These time lags between epidemics would often be long enough that most or all of the people who survived and developed immunity to a disease during a previous epidemic would have completed their natural life span, leaving a population almost entirely without immunity to that disease. As a consequence, in many cases, each time a disease such as measles or influenza entered the population, the population level of immunity was so low that it would essentially have been a virgin soil outbreak, setting the stage for high morbidity and mortality.
This type of disease pattern does not require that affected indigenous populations experience direct exposure to non-indigenous groups. Instead the contact could be along a chain of neighboring populations, beginning with acculturated groups and extending eventually to isolated indigenous populations that interact with their nearest neighbors and contact with outsiders. As Fig. 2 shows, however, outbreaks are generally isolated to individual societies or regionally localized within remote areas as in the case of the 3 waves of epidemics in the Upper Xingu. That this hotspot of epidemics was recorded may in part be due to the fact that the Upper Xingu was particularly well studied and of high visibility. Alternatively, this region is also somewhat unusual in that first peaceful contacts made by the Villas-Bôas brothers and others occurred in many groups in a short time span and in several cases groups of people were moved into Xingu Park’s boundaries and into closer proximity with one another6,61,63. Regardless, it is likely that the generally localized pattern of outbreaks observed in Fig. 2 is a consequence of small populations and their relative isolation from each other, which makes the chances of widespread transmission between groups relatively rare before epidemics die out.
The health and contact situation has improved rapidly through time across Greater Amazonia, fortunately and the few epidemics since the year 2000 all have mortality rates under 5%. Concurrently, survivorship also improves with time since contact and the best fit line predicts 1% mortality after 100 years post contact (Fig. 3). The secular trend of improving survivorship bodes well for future contacts. We did not, however, find an interaction effect between absolute time and relative time since contact which would have indicated that improvements with contact have progressively increased through time.
There are a number of limitations to our analysis. One of which is the sparsity of data for latter years, namely only 4 epidemics since the year 2000 that include 2 outbreaks of hepatitis (likely type A) with case fatality rates that are known to be low. However, this result is unlikely a bias in reporting because more recent outbreaks should be better documented than in the past. If real, this result corroborates the improved health situation where high-mortality epidemics from measles and other severe diseases are (hopefully) no longer occurring. A decline in mortality over time from vaccine preventable diseases may be due to these diseases no longer being introduced with the same probability as they were in the past, since new visitors are now much more likely to be vaccinated. Our study cannot directly test genetic hypotheses to explain the apparent susceptibility of native Amazonians to devastating epidemics, but rapid improvements in survivorship through time do suggest a strong role for environmental factors of better immunity and health care and conditions. We cannot determine the relative contribution of these two important factors given the general lack of genetic and serologic evidence and the many limitations of any ethnographic accounts of healthcare. We do have the impression that healthcare is generally becoming more available to most indigenous Amazonians through time, with more access to distant hospitals, small clinics in more villages and periodic vaccination campaigns by governments and others. However, efforts are still quite underfunded and meager in the many remote areas11.
One of us (KRH) was on site within weeks of first peaceful contact with Ache (Paraguay 1978), Yora Nahua (Peru 1986) and Mashco-Piro (3 women in Peru 1983) and in a Yomiwato Matsiguenga community (Peru 1986) when they were extremely isolated and suffering from new contact related epidemics even though intermittent contact had occurred for 25 years. From these personal experiences, the most important lesson learned was that mortality can be reduced to near zero levels if the contact team is prepared to provide around-the-clock medical treatment on site for a sustained period of time and complement it with food supplementation. A well-designed contact implies minimal rather than catastrophic mortality and can be quite safe, compared to the disastrous outcomes from accidental contacts. But safe contact events require a qualified team committed to staying on site for years or even decades. For example, foreign missionaries provided great care with the Yora for up to 6 months, but when they decided to take a furlough dozens of people died within a few weeks. A similar situation occurred with Catholic missionaries at an Ache community in 1975. Care had been provided for a year but when the missionaries took a vacation, many people died. In contrast, there have been several success stories such as when a band of Northern Ache was contacted in 1978 (only 1 contact-related death out of 25 people). Missionaries and anthropologists injected antibiotics when primary respiratory infections progressed to pneumonia and provided food to the sick. Likewise, the Puerto Barra Ache were contacted by a family of missionaries in 1979 who have provided continual care to this day with excellent outcomes (0 contact-related deaths out of 35 people).
A previous study14 of population dynamics of most of these same indigenous Amazonian societies shows rapid depopulation before and after contact but with fast population rebound within a decade of contact at which point annual population growth rates average nearly 4% because the surviving population is composed of mostly reproductive adults (i.e., most epidemics disproportionately kill infants and elders). Despite the catastrophic mortality of indigenous Amazonians over the last 500 years, surviving populations are remarkably resilient and remain demographically viable in large part because the disease situation ameliorates quickly as a function of time since contact. A population viability analysis of recently contacted populations suggests that even groups as small as 50 people have reasonable survival prospects14. Taken together these results have positive implications for the long-term survival of currently isolated populations, provided they are immediately attended to by modern health care professionals and are allowed to maintain access to protected habitats large enough to support their subsistence needs.
We are now in a position to largely remove most of the environmental factors that were responsible for many deaths and social chaos of past contacts. The Type III disease pattern also has the advantage that epidemics are relatively easy to contain. The ethnographic sources we consulted corroborate our personal experiences and are clear on a number of points. Forced migration, frightened refugees, bad hygiene, food shortage and lack of medicine were recipes for disastrous contacts, whereas airlifting patients to hospitals, missionary assistance and even meager health care and support saved many lives6,24,61,62,64,65,66,67,68,69,70,71,72,73,74. Provisioning food, shelter, security, antibiotics, vaccines and antiviral drugs, as well as encouraging isolation of sick and exposed individuals are just some of the basic logical first steps to mostly eliminate deaths, confusion and despair that characterized previous contacts. Unfortunately, many intermittent contacts today are made by loggers, miners, narcotraffickers and others without the best interests of isolated people in mind6,10,12. Some missionary contacts have led to many deaths (e.g., Zuruahã in 1978, Zo’é in 1982, Ayoreo in 2008), although not well documented and hence not covered in our dataset. It is clear that dedicated professionals are indispensable components of well-planned contact teams. They should be willing to provide long-term and continuous health care to recently contacted communities and be able to quickly mitigate against all potential threats75.
Methodology
We compiled information on 117 epidemics that affected 59 different indigenous societies in Greater Amazonia (Fig. 2) and caused over 11,000 deaths between 1875 and 2008. Much information comes from Ricardo and Ricardo13 and the accompanying website of the Instituto Socioambiental (http://pib.socioambiental.org). The website includes ethnographic entries for all indigenous societies in Brazil along with news stories from the 1960s to the present that often feature disease outbreaks. These website entries and other original ethnographic sources (Supplementary Information) were searched for the following index items: “epidemics”, “disease” and “contact”.
Each record in our data includes ethnolinguistic name, year of contact (defined as first sustained peaceful contact with outsiders), estimated population size before the reported epidemic, year of epidemic, number of deaths or fraction of population dying, specific disease(s) if available and time interval over which the epidemic (or string of epidemics) occurred. With this information we calculated years since contact, years between successive epidemics within ethnolinguistic populations and the mortality rate of the epidemic (defined as the fraction of the population dying from an epidemic per year). Common ethnohistoric accounts are of the form: “In 1965, 32 of 180 people died from measles”. Therefore, we assume a one-year time interval for most epidemics. Only when mortality was measured over multiple years does this adjustment go into the denominator of mortality rate. Unfortunately, the majority of records are silent on the age distribution of records; the few exceptions only serve to highlight variation from “most deaths were children” to “most deaths were elderly”.
Mortality rates decline exponentially with both absolute year and years since contact. Therefore, we first natural-log transformed mortality rate and fit it with a linear mixed effects model in R (lme4 package76). Years since contact and absolute year (centered on mean year of epidemics of 1963) are covariates; ethnolinguistic name is included as a random intercept because each society has anywhere from 1 to 11 separate records with an average of 2.
Additional Information
How to cite this article: Walker, R. S. et al. Mortality from contact-related epidemics among indigenous populations in Greater Amazonia. Sci. Rep. 5, 14032; doi: 10.1038/srep14032 (2015).
References
Bodard, L. Green Hell: Massacre of the Brazilian Indians. (Dutton, New York, 1974).
Crosby, A. W. Virgin soil epidemics as a factor in the aboriginal depopulation in America. William Mary Q. 33, 289–299 (1976).
McNeill, W. H. Plagues and Peoples. (Anchor Books, New York, 1977).
Hemming, J. Red Gold: The Conquest of the Brazilian Indians. (Harvard University Press, Cambridge, 1978).
Hemming, J. Amazon Frontier: The Defeat of the Brazilian Indians. (Harvard University Press, Cambridge, 1987).
Hemming, J. Die If You Must: Brazilian Indians in the Twentieth Century. (Pan Macmillan, London, 2003).
Joralemon, D. New World depopulation and the case of disease. J. Anthropol. Res. 38, 108–127 (1982).
Black, F. L. Why did they die? Science 258, 1739–1740 (1992).
Dobyns, H. F. Disease transfer at contact. Ann. Rev. Anthropol. 22, 273–291 (1993).
Castillo, B. H. Indigenous peoples in isolation in the Peruvian Amazon. (International Work Group for Indigenous Affairs, Copenhagen, 2004).
Hurtado, A. M. et al. Human rights, biomedical science and infectious diseases among South American indigenous groups. Annu. Rev. Anthropol. 34, 639–665 (2005).
Vaz, A. Isolados no Brasil. Política de Estado: Da Tutela para a Política de Direitos – Uma Questão Resolvida? (Estação Gráfica, Brasília, 2011).
Ricardo, B. & Ricardo, F. Povos Indígenas no Brasil 2006/2010. (Instituto Socioambiental, São Paulo, 2011).
Hamilton, M. J., Walker, R. S. & Kesler, D. C. Crash and rebound of indigenous populations in lowland South America. Sci. Rep. 4, 4541 (2014).
Kesler, D. C. & Walker, R. S. Geographic distribution of isolated indigenous societies in Amazonia and the efficacy of indigenous territories. PLoS ONE 10, e0125113 (2015).
Walker, R. S., Hamilton, M. J. & Groth, A. A. Remote sensing and conservation of isolated indigenous villages in Amazonia. Roy. Soc. Open Sci. 1, 140246 (2014).
Walker, R. S. & Hamilton, M. J. Amazonian societies on the brink of extinction. Am. J. Hum. Biol. 26, 570–572 (2014).
Diamond, J. Guns, Germs and Steel: The Fates of Human Societies. (Norton, New York, 1997).
Bartlett, M. S. Measles periodicity and community size. J R Statist Soc A 120, 48–70 (1957).
Neel, J. V., Centerwall, W. R., Chagnon, N. A. & Casey, H. L. Notes on the effect of measles and measles vaccine in a virgin-soil population of South American Indians. Am. J. Epidemiol. 91, 418–429 (1970).
Black, F. L. Infectious diseases in primitive societies. Science 187, 515–518 (1975).
Black, F. L. Measles endemicity in insular populations: critical community size and its evolutionary implication. J. Theoret. Biol. 11, 207–211 (1966).
Cliff, A. D. & Haggett, P. The epidemiological significance of islands. Health & Place 1, 199–209 (1995).
Nutels, N. Medical problems of newly contacted Indian groups. Biomedical challenges presented by the American Indian 165, 68–76 (1968).
Salzano, F. M. & Callegari-Jacques, S. M. South American Indians: A Case Study in Evolution. (Oxford University Press, Oxford, 1988).
Wang, S. et al. Genetic variation and population structure in Native Americans. PLoS Genet. 3, e185 (2007).
Lewis, C. M. Hierarchical modeling of genome-wide short tandem repeat (STR) markers infers Native American prehistory. Am. J. Phys. Anthropol. 141, 281–289 (2010).
Lindenau, J. D. et al. Distribution patterns of variability for 18 immune system genes in Amerindians – relationship with history and epidemiology. Tissue Antigens 82, 177–185 (2013).
Black, F. L. An explanation of high death rates among New World peoples when in contact with Old World diseases. Perspect. Biol. Med. 37, 292–303 (1994).
Beiguelman, B., et al. The association of genetic markers and malaria infection in the Brazilian Western Amazon region. Mem. Inst. Oswaldo Cruz 98, 455–460 (2003).
Cavasini, C. E. et al. Duffy blood group gene polymorphisms among malaria vivax patients in four areas of the Brazilian Amazon region. Malar. J. 6, 167 (2007).
Pereira, V. A., et al. IL10A genotypic association with decreased IL-10 circulating levels in malaria infected individuals from endemic area of the Brazilian Amazon. Malar. J. 14, 30 (2015).
Araújo, M. S. et al. No evidence of association between MBL2A/O polymorphisms and Mycobacterium tuberculosis infection in populations from the Brazilian Amazon region. Hum. Immunol. 74, 82–84 (2013).
Boechat, A. L., Ogusku, M. M., Sadahiro, A. & dos Santos, M. C. Association between the PTPN22 1858C/T gene polymorphism and tuberculosis resistance. Infect. Genet. Evol. 16, 310–313 (2013).
Lindenau, J. D. et al. Cytokine gene polymorphisms are associated with susceptibility to tuberculosis in an Amerindian population. Int. J. Tuberc. Lung Dis. 18, 952–957 (2014a).
Lindenau, J. D. et al. Association between HLA-DR4 haplotypes and tuberculosis skin test response in the Aché population. Tissue Antigens 84, 479–483 (2014b).
Contreras, M. et al. Helicobacter pylori seroprevalence in Amerindians from isolated locations. Am. J. Trop. Med. Hyg. 78, 574–576 (2008).
Melo, B. H. et al. Interleukin-1 and TNF-α polymorphisms and Helicobacter pylori in a Brazilian Amazon population. World J. Gastroenterol. 15, 1465–1471 (2009).
Lázaro, F. P. et al. A major gene controls leprosy susceptibility in a hyperendemic isolated population from north of Brazil. J. Infect. Dis. 201, 1598–1606 (2010).
Kaplan, J. E. et al. Infectious disease patterns in the Waorani, an isolated Amerindian population. Am. J. Trop. Med. Hyg. 29, 298–312 (1980).
van Mazijk, J., Pinheiro, F. P. & Black, F. L. Measles and measles vaccine in isolated Amerindian tribes I. The 1971 Trio (Tiriyo) epidemic. Trop. Geogr. Med. 34, 3–6 (1982).
Baruzzi, R. G., Abdala, N. & Black, F. L. Measles and measles vaccination in isolated Amerindian tribes. II. The 1978/79 Xingu epidemic. Trop. Geogr. Med. 34, 7–12 (1982).
Black, F. L., Schiffman, G. & Pandey, J. P. HLA, Gm and Km polymorphisms and immunity to infectious diseases in South Amerinds. Exp. Clin. Immunogenet. 12, 206–216 (1995).
Alkhatib, G. et al. CC CKR5: a RANTES, MIP-1α, MIP-1ß receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 272, 1955–1958 (1996).
Choe, H. et al. The ß-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell 85, 1135–1148 (1996).
Deng, H. et al. Identification of a major co-receptor for primary isolates of HIV-1. Nature 381, 661–666 (1996).
Doranz, B. J. et al. A dual-tropic primary HIV-1 isolate that uses fusin and the ß-chemokine receptors CKR-5, CKR-3 and CKR-2b as fusion cofactors. Cell 85, 1149–1158 (1996).
Dragic, T. et al. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR5. Nature 381, 667–673 (1996).
Stephens, J. C. et al. Dating the origin of the CCR5-∆32 AIDS-resistance allele by the coalescence of haplotypes. Am. J. Hum. Genet. 62, 1507–1515 (1998).
Chaudhuri, A. & Pogo, A. O. The Duffy blood group system and malaria. In Blood cell biochemistry. Molecular basis of human blood group antigens [ Cartron, J.-P. & Rouger, R. (eds.)] [243–265] (Plenum, New York, 1995).
Pogo, A. O. & Chaudhuri, A. The Duffy blood group system and its extension in nonhuman primates. In Molecular biology and evolution of blood group and MHC antigens in primates [ Blancher, A., Klein, J. & Socha, W. W. (eds.)] [219–235] (Springer-Verlag, Berlin, 1997).
Jones, D. S. Virgin soils revisited. William Mary Q. 60, 703–742 (2003).
Inhorn, M. C. & Brown, P. J. The anthropology of infectious diseases. Ann. Rev. Anthropol. 19, 89–117 (1990).
Herring, D. A. “There were young people and old people and babies dying every week”: the 1918-1919 influenza pandemic at Norway House. Ethnohistory 41, 73–105 (1994).
Herring, D. A. & Sattenspiel, L. Death in winter: the Spanish flu in the Canadian Subarctic. In The Spanish Influenza Pandemic of 1918-19 [ Phillips, H. & Killingray, D. (eds.)] [156–172] (Routledge, London, 2003).
Omonzejele, P. F. Ethical challenges posed by the Ebola virus epidemic in West Africa. J Bioeth. Inq. 11, 417–420 (2014).
Hurtado, A. M., Hill, K. R., Kaplan, H. & Lancaster, J. The epidemiology of infectious diseases among South American Indians: a call for guidelines for ethical research. Curr. Anthropol. 42, 425–432 (2001).
Hill, A. V. S. The immunogenetics of human infectious diseases. Ann. Rev. Immunol. 16, 593–617 (1998).
Delves, P. J. & Roitt, I. M. The immune system: first of two parts. N. Eng. J. Medicine 343, 37–49 (2000).
Brodin, P. et al. Variation in the human immune system is largely driven by non-heritable influences. Cell 160, 37–47 (2015).
Heckenberger, M. Epidemias, índios bravos e brancos: contato cultural e etnogênese do Alto Xingu. In Os Povos do Alto Xingu: História e Cultura [ Franchetto, B. & Heckenberger, M. (eds.)] [77–110] (Editora UFRJ, Rio de Janeiro, 2000).
Verswijver, G. The Club-fighters of the Amazon: Warfare among the Kaiapo Indians of Central Brazil. (Rijksuniversiteit Te Gent, Gent, 1992).
Franchetto, B. & Heckenberger, M. (eds.) Os Povos do Alto Xingu: História e Cultura. (Editora UFRJ, Rio de Janeiro, 2000).
Smole, W. J. The Yanoama Indians: A Cultural Geography. (University of Texas Press, Austin, 1976).
Wagley, C. Welcome of Tears: The Tapirapé Indians of Central Brazil. (Waveland Press, Prospect Heights, 1977).
Larrick, J., Yost, J., Kaplan, J., King, G. & Mayhall, J. Patterns of health and disease among the Waorani Indians of Eastern Ecuador. Med. Anthropol. 3, 147–189 (1979).
Hill, K. R. & Kaplan, H. Population and dry-season subsistence strategies of the recently contacted Yora of Peru. Nat. Geo. Res. 5, 317–334 (1989).
Conklin, B. A. Images of Health, Illness and Death among the Wari’ (Pakaas Novos) of Rondônia, Brazil, PhD thesis, University of California, San Francisco (1989).
Chagnon, N. A. Yanomamö: The Last Days of Eden. (Harcourt, Orlando, 1992).
Balée, W. Footprints of the Forest: Ka’apor Ethnobotany—The Historical Ecology of Plant Utilization by an Amazonian People. (Columbia University Press, New York, 1994).
Ramos, A. R. Sanumá Memories: Yanomami Ethnography in Times of Crisis. (University of Wisconsin Press, Madison, 1995).
Hill, K. R. & Hurtado, A. M. Ache Life History: The Ecology and Demography of a Foraging People. (Aldine de Gruyter, New York, 1996).
Cormier, L. A. Kinship With Monkeys. (Columbia University Press, New York, 2003).
Silva, M. Romance de Primas e Primos: Uma Etnografia do Parentesco Waimiri-Atroari. (Valer Editora, Manaus, 2009).
Walker, R. S. & Hill, K. R. Protecting isolated tribes. Science 348, 1061 (2015).
Bates, D., Maechler, M., Bolker, B. M., & Walker, S. lme4: Linear mixed-effects models using Eigen and S4. ArXiv e-print, R package version 1.1-8. (2014) Available at: http://CRAN.R-project.org/package=lme4. (Accessed: 10th June 2015).
Parks, D. H. et al. GenGIS 2: Geospatial analysis of traditional and genetic biodiversity, with new gradient algorithms and an extensible plugin framework. PLoS One 8, e69885 (2013).
Acknowledgements
We thank Dylan Kesler, Marcus Hamilton and Magdalena Hurtado for their help on this project. Financial support was provided by a National Geographic Society Research and Exploration grant (#9165-12), University of Missouri Research Board grant, Research Council grant and Richard Wallace faculty incentive grant.
Author information
Authors and Affiliations
Contributions
R.S.W., L.S. and K.R.H. wrote and reviewed the paper. Figures and tables generated by R.S.W. Data set compiled by R.S.W.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Walker, R., Sattenspiel, L. & Hill, K. Mortality from contact-related epidemics among indigenous populations in Greater Amazonia. Sci Rep 5, 14032 (2015). https://doi.org/10.1038/srep14032
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep14032
This article is cited by
-
Vaccine coverage and effectiveness against laboratory-confirmed symptomatic and severe Covid-19 in indigenous people in Brazil: a cohort study
BMC Public Health (2023)
-
Remote sensing evidence for population growth of isolated indigenous societies in Amazonia
Scientific Reports (2023)
-
Brazil’s planned exploitation of Amazonian indigenous lands for commercial agriculture increases risk of new pandemics
Regional Environmental Change (2021)
-
Sperm-dependent asexual hybrids determine competition among sexual species
Scientific Reports (2019)
-
Salmonella enterica genomes from victims of a major sixteenth-century epidemic in Mexico
Nature Ecology & Evolution (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.