Abstract
High on-treatment platelet reactivity (HTPR) is accompanied by an increased risk of adverse outcomes. Direct comparison of the antiplatelet effects between ticagrelor and high-dose clopidogrel has not yet been reported in acute myocardial infarction (AMI) or coronary artery in-stent restenosis (ISR) patients with HTPR. Consecutive patients with AMI or coronary artery ISR treated with standard-dose clopidogrel (75 mg/day) were screened with the VerifyNow assay, defining HTPR as P2Y12 reaction units (PRUs) >208. Of the 102 screened patients, 48 (47.06%) patients with HTPR were randomly assigned to either ticagrelor (180 mg/90 mg twice daily) or high-dose clopidogrel (150 mg/day) for 24 hours. Baseline characteristics and mean PRUs were similar in both groups. After 24 hours, ticagrelor was associated with a significantly lower platelet reactivity than high-dose clopidogrel (44.38 ± 40.26 vs. 212.58 ± 52.34 PRU, P < 0.05). No patient receiving ticagrelor exhibited HTPR, whereas 15 (62.50%) patients after treatment with high-dose clopidogrel remained HTPR (P < 0.05). During the follow-up (mean, 138.42 ± 53.59 days), no patient exhibited a major bleeding event in either treatment group. In conclusion, in patients with AMI or coronary artery ISR exhibiting HTPR after standard clopidogrel treatment, ticagrelor is significantly more effective compared with high-dose clopidogrel in overcoming HTPR.
Similar content being viewed by others
Introduction
Dual antiplatelet treatment with aspirin and clopidogrel is a cornerstone of therapy for patients with acute myocardial infarction (AMI) whether treated invasively or noninvasively1,2,3. Nevertheless, despite these antiplatelet agents, the frequency of recurrent thromboembolic events remains substantial (~10%)4. Recent data have reported inter-individual variability in response to clopidogrel5,6,7,8 and high on-treatment platelet reactivity (HTPR) has been associated with an increased risk of recurrent cardiovascular events in patients with AMI9,10. To overcome this problem, administration of a high daily dose of clopidogrel or adjunctive cilostazol to dual antiplatelet therapy is now occasionally used in clinical practice11,12. Despite this, an increased dose of clopidogrel or triple antiplatelet therapy did not reduce the incidence of cardiovascular death, accompanied with an increased risk of bleeding13,14,15.
Recently, new and more efficient alternative antiplatelet agents such as prasugrel and ticagrelor have been introduced into clinical application16,17. Unlike clopidogrel and prasugrel, ticagrelor is an orally active antagonist that reversibly binds to the P2Y12 platelet receptor, which yields faster, greater and more consistent inhibition of platelet aggregation18. The Response to Ticagrelor in Clopidogrel Non-responders and Responders and Effect of Switching Therapies Study (RESPOND) demonstrated that ticagrelor therapy overcame the problem of HTPR during clopidogrel therapy in patients with stable coronary disease19. However, the prevalence of HTPR in Chinese patients with AMI or coronary artery in-stent restenosis (ISR) and the beneficial impact of ticagrelor compared with high-dose clopidogrel in overcoming HTPR are still unknown.
Here, we report the first prospective, randomised trial to be conducted in China to determine the antiplatelet effects of ticagrelor versus high-dose clopidogrel (150 mg) in AMI or coronary artery ISR patients with HTPR.
Method
Patient population and study design
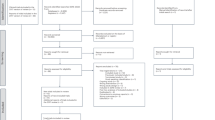
We performed a prospective, randomised, single-center, single-blind study to compare the antiplatelet effects of ticagrelor versus high-dose clopidogrel (150 mg) in AMI or coronary artery ISR patients with HTPR. From April 2014 to November 2014, patients aged between 20 and 80 years hospitalised for ST Segment Elevation Myocardial Infarction (STEMI), non-(NSTEMI), or coronary artery ISR who were on aspirin and clopidogrel therapy were prospectively monitored for platelet reactivity (PR). Restenosis was defined as at least 50% in-stent diameter stenosis at the follow-up angiogram. Patients meeting all the inclusion criteria were consecutively enrolled into the study and treated and followed per the protocol in order to minimize selection bias. Exclusion criteria were (1) contraindication to antiplatelet therapy; (2) haemoglobin <10 g/dL or platelet count <100 000/mm3; (3) active bleeding and bleeding diatheses; (4) stroke within 6 months; (5) aspartate aminotransferase or alanine aminotransferase level >2 times the upper normal limit; (6) chronic renal failure (creatinine clearance <30 mL/min); and (7) active peptic ulceration or gastrointestinal bleeding. The study was performed in accordance with ethical principles that have their origin in the Declaration of Helsinki and are consistent with ICH/Good Clinical Practice. Informed consents were signed and obtained by all the patients. All experimental protocol was approved by the Ethics Committee of Changhai Hospital, Second Military Medical University. The trial was registered at Chinese Clinical Trial Registry. Trial registration No. was ChiCTR-RCS-14004303 and the date of registration was January 2, 2014.
A flow chart diagram of the study is shown in Fig. 1. Patients received a loading dose of 600-mg of clopidogrel for at least 6 hours, 300-mg of clopidogrel at least 12 hours, or were receiving chronic clopidogrel therapy (75 mg daily for ≥7 days) before the PR measurement. Patients received maintenance dose of 100 mg, once a day of aspirin and 75 mg, once a day of clopidogrel. In patients undergoing percutaneous coronary intervention (PCI), PR measurement was carried out after PCI. If patients received tirofiban or heparin therapy during PCI, tirofiban had to be discontinued for at least 48 hours and heparin or low-molecular-weight heparin had to be discontinued for at least 12 hours before measurement of PR. Patients with HTPR (as defined subsequently) were randomized in a 1:1 ratio to receive either a high-dose clopidogrel of 150 mg daily or a loading-dose ticagrelor of 180 mg followed by 90 mg twice daily using a computer-generated randomisation table. A study nurse enrolled the participants and assigned participants to their groups. A second PR measurement was carried out 24 hours later in patients who received an alternate treatment. Patients who exhibited HTPR after being treated with high-dose clopidogrel were switched to 90 mg ticagrelor twice daily and a third PR measurement was performed 24 hours after administration of ticagrelor.
Clinical end points
Endpoints were pre-specified in the study protocol and statistical analysis plan. The primary end point of the study was PR after 24 hours of administration of ticagrelor versus high-dose clopidogrel, as determined by VerifyNow P2Y12 assay. The secondary end points included the rate of HTPR (defined as > 208 PRU). A clinical follow-up by telephone interviews was obtained at 1, 3 and 6 months and bleeding (major, minor, or minimal according to Thrombolysis in Myocardial Infarction [TIMI] criteria) and major adverse cardiovascular events (cardiovascular death, myocardial infarction, stent thrombosis and stroke) were evaluated during the follow-up term.
Blood sample collection
Blood samples were collected in 2 vacuum tubes (Becton-Dickinson, Franklin Lakes, NJ) containing 3.2% trisodium citrate, in which the first tube was discarded to avoid spontaneous platelet activation. The second tube was gently inverted 3 to 5 times to ensure complete mixing of the anticoagulant and the blood sample was analysed within 4 hours for rapid platelet-function assay.
Determination of HTPR
VerifyNow-P2Y12 (Accumetrics, San Diego, California) is a whole blood, rapid platelet function assay designed to measure the inhibition of clopidogrel on the P2Y12 receptor. Technical details of the platelet function assay were previously described20,21. Results were expressed as P2Y12 reaction units (PRUs), baseline value (BASE) and percent inhibition. The percent inhibition is calculated as follows: ([BASE-PRU]/BASE) × 100. A PRU of >208 was defined as HTPR or clopidogrel non-responder, linking the cut-off point to ischemic event occurrences22.
Statistical analysis
For sample size calculation, we hypothesized that ticagrelor 90 mg, twice daily would result in a PR absolute difference of 50 PRU compared with clopidogrel 150 mg (with the assumption that the within-patient standard deviation of the response variable is 50 PRU), based on previously published data19,23. Choosing a power of 90% and a 2-sided alpha-level of 0.05, at least 46 patients in total (23 for each group) were required to reach statistical significance on the basis of the preceding assumptions. Continuous variables are reported as mean ± standard deviation (SD) and categorical variables are presented as frequencies and percentages. Continuous variables were compared by the unpaired Student t-test and categorical variables by the chi-square test or Fisher exact test. Analyses were performed using SPSS version 20.0 statistical software (SPSS Inc., Chicago, Illinois); P-value of < 0.05 were considered statistically significant.
Results
Baseline characteristics
Of the 102 (STEMI 63.73%, NSTEMI 16.67% and ISR 19.61%) consecutive patients who were on the standard dose of clopidogrel therapy, 48 (47.06%) were identified to have HTPR and randomly assigned to treatment with either high-dose clopidogrel (n = 24) or ticagrelor (n = 24). The demographics, clinical details and concomitant medications were well balanced across the 2 treatment groups (Table 1).
Laboratory characteristics
There were no significant differences between treatment groups with regard to laboratory characteristics (Table 2). After the standard dose of clopidogrel therapy, PR measured by the VerifyNow P2Y12 assay did not differ significantly in both groups (all P > 0.05).
Angiographic characteristics
Of the 48 randomised patients, 47 (97.92%) patients underwent PCI and no patient received any revascularization. There were no significant differences in the angiographic characteristics between the 2 groups (Table 3).
Platelet response to treatment modification
In the ticagrelor-treated group, the PRU level in patients with HTPR was significantly reduced from 269.04 ± 34.78 to 44.38 ± 40.26, 24 hours after administration of ticagrelor (P < 0.001; Fig. 2A), as determined by VerifyNow assay. In the high-dose clopidogrel group, the PRU values decreased from 252.71 ± 36.92 to 212.58 ± 52.34 after treatment with high-dose clopidogrel (P = 0.004; Fig. 2B). However, patients receiving ticagrelor achieved significantly lower PRU values compared with those who received high-dose clopidogrel (44.38 ± 40.26 vs. 212.58 ± 52.34, respectively; P < 0.001; Fig. 2C). Moreover, at the end of treatment periods, HTPR was still seen in 15 of 24 patients (62.50%) who received high-dose clopidogrel, but in no patients who received ticagrelor therapy (P < 0.001). In particular, for 15 patients with HTPR who were treated with high-dose clopidogrel, switching to ticagrelor led to further decrease in the PRU level (245.00 ± 28.57 vs. 30.91 ± 32.00, P < 0.05).
(A) Platelet reactivity (PR) in patients with high on-treatment platelet reactivity (HTPR) pre- and post-treatment with ticagrelor (180 mg/90 mg twice daily). (B) PR in patients with HTPR pre- and post-treatment with high-dose clopidogrel (150 mg/day). (C) PR in patients with HTPR post- treatment with high-dose clopidogrel (150 mg/day) or ticagrelor (180 mg/90 mg twice daily).
Clinical events during the follow-up term
During the follow-up period of mean 138.42 ± 53.59 days, no patient exhibited major adverse cardiovascular events or a major bleeding event. 3 patients (under ticagrelor treatment) reported a mild new-onset dyspnoea, 1 patient (under ticagrelor treatment) reported diarrhoea and 7 patients (4 under high-dose clopidogrel treatment and 3 under ticagrelor treatment) exhibited minimal bleeding.
Discussion
To our knowledge, this is the first study in China to compare the effects of ticagrelor versus high-dose clopidogrel on platelet inhibition in AMI or coronary artery ISR patients with HTPR. The major findings of the study are (1) HTPR while on clopidogrel treatment was a common phenomenon found in about one half (47.06%) in our population; (2) the antiplatelet effect of ticagrelor was obviously greater than high-dose clopidogrel therapy in patients with HTPR; and (3) all patients treated with ticagrelor had PR below 208 PRU cut-off point, whereas more than half of patients (62.50%) receiving high-dose clopidogrel remained non-responsive to clopidogrel.
A number of studies have shown that the level of platelet inhibition achieved by clopidogrel varies considerably between individuals24. It has been estimated that nearly 4% to 30% of the patients exhibit low or no response to clopidogrel loading and maintenance therapy6,8,25,26. The antiplatelet effect of clopidogrel has been shown to be dose dependent and has been examined to improve responses by increasing the dose11,27. However, persistent presence of HPPR was apparent despite high-dose of clopidogrel and the clinical benefit is less clear. Compared with administration of clopidogrel 75 mg in patients with high-risk type 2 diabetes mellitus, clopidogrel 150 mg is associated with enhanced antiplatelet effects, but enhanced PR continues to persist in 60% of the patients on the 150-mg regimen28. In addition, the Gauging Responsiveness with A VerifyNow assay-Impact on Thrombosis And Safety (GRAVITAS) trial did not show any reduction in the incidence of death from cardiovascular causes, nonfatal MI, or ST with high-dose clopidogrel (600 mg LD, 150 mg/day MD) compared with standard-dose clopidogrel (no additional LD, 75 mg/day MD) in 2214 patients with HTPR after PCI with drug-eluting stents29.
Inter-individual variability in response to clopidogrel has prompted the development of novel P2Y12 inhibitors such as ticagrelor. Ticagrelor is the first oral P2Y12 receptor antagonist that blocks ADP-induced platelet aggregation in a reversible manner18. Unlike clopidogrel, it does not require activation via CYP450 enzymes because it is an active drug. In recent studies, ticagrelor has resulted in more potent inhibition of ADP-induced platelet aggregation than clopidogrel18,30. Bliden reported that compared with clopidogrel, ticagrelor was rapidly and consistently associated with a very low (0%–8%) prevalence of HTPR assessed with the VerifyNow assay30. In the PLATO trial, in patients with acute coronary syndrome (ACS), ticagrelor compared with clopidogrel significantly reduced the adverse event rate, without an increase in bleeding risk31. Notably, ticagrelor has been shown to be superior to high loading dose of clopidogrel in terms of reversing non-responsiveness to clopidogrel in patients with stable coronary artery disease19.
In the PLATO trial, the antiplatelet effects of ticagrelor in ACS patients being nonresponsive to clopidogrel are unknown. Also, the present study population differs from the RESPOND study that enrolled patients with AMI or coronary artery ISR; the clinical setting in which hyper-reactive platelets may be more considerable and the risk for ischemic complications is higher compared with stable coronary artery disease. Indeed, in the present study, 48 (47.06%) patients with HTPR were identified according to the VerifyNow system, a finding obviously significant than the previous reports in which the prevalence of clopidogrel to non-responsiveness was about 28%8,19. Other factors contributing to the high prevalence of HTPR in our population may include the definitions of PR cut-offs and the amount of clopidogrel loading dose. Nevertheless, whether the high rate of HTPR is related to the differences between races of human beings, large clinical trials might be needed to further proven.
High PR in patients with AMI has been shown predict the PCI angiographic success and recurrent atherothrombotic events32. Thus, the early and strong platelet inhibition seems to be of outmost importance, particularly in a population with high risk of ischemic events. Our study provides the first laboratory evidence that ticagrelor, compared with a high-dose clopidogrel of 150 mg/day may significantly eliminate HTPR in patients with AMI or coronary artery ISR. In the study, baseline rates of HTPR were 47.06%, which was reduced to 0 by switching from clopidogrel to ticagrelor compared with high-dose clopidogrel (31.25%). For the patients who were still resistant to high-dose clopidogrel, the level of PR was also decreased to below 208 PRU after switching to ticagrelor (P < 0.05). The results from this study suggest that in the presence of HTPR, switching to ticagrelor is a more reliably effective option for inhibiting platelet function compared with increasing the dose of clopidogrel. These findings have important clinical implications for guiding antiplatelet therapy in patients with high risk of cardiovascular events who are non-responsiveness to clopidogrel.
In recent years, an increasing number of studies have used VerifyNow P2Y12 assay to assess the antiplatelet effect of clopidogrel and its relation to ischemic event occurrence during clopidogrel therapy has been demonstrated33. In our study, the change in PR was measured using VerifyNow P2Y12 assay as a point-of care test, with non-responder status predefined at >208 PRU and <85 PRU as thresholds to bleeding outcome34,35. In the present study, ticagrelor suppressed PR to a very low level (44.38 ± 40.26 PRU), which is far below the previous thresholds associated with bleeding risk, but without observing a major bleeding event. The low incidence of bleeding might be contributed to the sample size of the present study that is too small to assess the relation of PR with a bleeding event. However, the rate of bleeding in our study is in line with that was observed in the PLATO substudy, showing a relative low incidence of the bleeding event in Chinese patients16. Moreover, the data showed that overall bleeding events were similar in either treatment group, indicating that the benefits of ticagrelor were not associated with the increased rates of bleeding events compared with high-dose clopidogrel. Ticagrelor was more frequently accompanied by other mild side effects such as dyspnea and diarrhoea. This study had small sample size, therefore, large-scale randomised clinical trials and sufficient duration are required to confirm our findings and to fully evaluate clinical outcomes, such as the incidence of stent thrombosis and major bleeding. Because of poor correlation between different tests of platelet function36, only single test of platelet function have been used in our study. However, the VerifyNow assay is most widely accepted test to assess the antiplatelet effects of P2Y12 receptor inhibitors and has been shown to be useful in assessing the antiplatelet effects for identifying HTPR after clopidogrel or ticagrelor therapy24,33,37.
Conclusions
In conclusion, the present results provide clinical evidence that, in patients with AMI or coronary artery ISR exhibiting HTPR after standard clopidogrel treatment, ticagrelor therapy is more effective than high-dose clopidogrel (150 mg) in reducing the PR and HTPR rate. The possible clinical benefits of this regimen require further validation.
Additional Information
How to cite this article: Li, P. et al. Ticagrelor overcomes high platelet reactivity in patients with acute myocardial infarction or coronary artery in-stent restenosis: a randomized controlled trial. Sci. Rep. 5, 13789; doi: 10.1038/srep13789 (2015).
References
Beinart, S. C. et al. Long-term cost effectiveness of early and sustained dual oral antiplatelet therapy with clopidogrel given for up to one year after percutaneous coronary intervention. Results from the clopidogrel for the reduction of events during observation (CREDO) trial. J. Am. Coll. Cardiol. 46, 761–769 (2005).
Steinhubl, S. R. et al. Clopidogrel for the reduction of events during observation: early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention. J. A. M. A. 288, 2411–2420 (2002).
Hamm, C. W. et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The task force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation. Eur. Heart J. 32, 2999–3054 (2011).
Gurbel, P. A. & Tantry, U. S. Clopidogrel response variability and the advent of personalized antiplatelet therapy. A bench to bedside journey. Thromb Haemost. 106, 265–271 (2011).
Gurbel, P. A., Antonino, M. J. & Tantry, U. S. Recent developments in clopidogrel pharmacology and their relation to clinical outcomes. Expert Opin. Drug Metab. Toxicol. 5, 989–1004 (2009).
Serebruany, V. L. et al. Variability in platelet responsiveness to clopidogrel among 544 individuals. J. Am. Coll. Cardiol. 45, 246–251 (2005).
Hochholzer, W. et al. Impact of the degree of peri-interventional platelet inhibition after loading with clopidogrel on early clinical outcome of elective coronary stent placement. J. Am. Coll. Cardiol. 48, 1742–1750 (2006).
Gurbel, P. A., Bliden, K. P., Hiatt, B. L. & O’Connor, C. M. Clopidogrel for coronary stenting: response variability, drug resistance and the effect of pretreatment platelet reactivity. Circulation. 107, 2908–2913 (2003).
Matetzky, S. et al. Clopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation. 109, 3171–3175 (2004).
Parodi, G. et al. High residual platelet reactivity after clopidogrel loading and long-term cardiovascular events among patients with acute coronary syndromes undergoing PCI. J. A. M. A. 306, 1215–1223 (2011).
von Beckerath, N. et al. Absorption, metabolization and antiplatelet effects of 300-, 600- and 900-mg loading doses of clopidogrel: results of the ISAR-CHOICE (intracoronary stenting and antithrombotic rgimen: choose between 3 high oral doses for immediate clopidogrel effect) trial. Circulation. 112, 2946–2950 (2005).
Shim, C. Y. et al. The clopidogrel resistance can be attenuated with triple antiplatelet therapy in patients undergoing drug-eluting stents implantation. Int. J. Cardiol. 29, 351–355 (2009).
The CURRENT-OASIS 7 investigators. et al. Dose comparisons of clopidogrel and aspirin in acute coronary syndromes. N. Engl. J. Med. 363, 930–942 (2010).
Suh, J. W. et al. Multicenter randomized trial evaluating the efficacy of cilostazol on ischemic vascular complications after drug-eluting stent implantation for coronary heart disease: results of the CILON-T (influence of CILostazol-based triple antiplatelet therapy ON ischemic complication after drug-eluting stenT implantation) trial. J. Am. Coll. Cardiol. 57, 280–289 (2011).
Roberts, D. I. & Nawarskas, J. J. Treatment options for patients with poor clopidogrel response. Cardiol. Rev. 21, 309–317 (2013).
Wallentin, L. et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 361, 1045–1057 (2009).
Wiviott, S. D. et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 357, 2001–2015 (2007).
Husted, S. et al. Pharmacodynamics, pharmacokinetics and safety of the oral reversible P2Y12 antagonist AZD6140 with aspirin in patients with atherosclerosis: a double-blind comparison to clopidogrel with aspirin. Eur. Heart J. 27, 1038–1047 (2006).
Gurbel, P. A. et al. Response to ticagrelor in clopidogrel nonresponders and responders and effect of switching therapies. Circulation. 121, 1188–1199 (2010).
Malinin, A. et al. Monitoring platelet inhibition after clopidogrel with the VerifyNow-P2Y12 rapid analyzer: the Verify Thrombosis Risk Assessment (VERITAS) study. Thromb. Res. 119, 277–284 (2007).
Gremmel, T. et al. Comparison of methods to evaluate clopidogrel-mediated platelet inhibition after percutaneous intervention with stent implantation. Thromb. Haemost. 101, 333–339 (2009).
Stone, G. W. et al. Platelet reactivity and clinical outcomes after coronary artery implantation of drug-eluting stents (ADAPT-DES): a prospective multicentre registry study. Lancet. 382, 614–623 (2013).
Angiolillo, D. J. et al. Variability in individual responsiveness to clopidogrel: clinical implications, management and future perspectives. J. Am. Coll. Cardiol. 49, 1505–1516 (2007).
Siller-Matula, J. M. et al. Response variability to P2Y12 receptor inhibitors: expectations and reality. J. A. C. C. Cardiovasc. Interv. 6, 1111–1128 (2013).
Müller, I. et al. Prevalence of clopidogrel nonresponders among patients with stable angina pectoris scheduled for elective coronary stent placement. Thromb. Haemost. 89, 783–787 (2003).
Lev, E. I. et al. Aspirin and clopidogrel drug response in patients undergoing percutaneous coronary intervention: the role of dual drug resistance. J. Am. Coll. Cardiol. 47, 27–233 (2006).
Bonello, L. et al. Adjusted clopidogrel loading doses according to vasodilator-stimulated phosphoprotein phosphorylation index decrease rate of major adverse cardiovascular events in patients with clopidogrel resistance: a multicenter randomized prospective study. J. Am. Coll. Cardiol. 51, 1404–1411 (2008).
Angiolillo, D. J. et al. Randomized comparison of a high clopidogrel maintenance dose in patients with diabetes mellitus and coronary artery disease: results of the Optimizing Antiplatelet Therapy in Diabetes Mellitus (OPTIMUS) study. Circulation. 115, 708–716 (2007).
Price, M. J. et al. Standard vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. J. A. M. A. 305, 1097–1105 (2011).
Bliden, K. P. et al. The effect of ticagrelor versus clopidogrel on high on-treatment platelet reactivity: Combined analysis of the ONSET/OFFSET and RESPOND studies. Am. Heart. J. 162, 160–165 (2011).
Wallentin, L. et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 361, 1045–1057 (2009).
Campo, G. et al. Value of platelet reactivity in predicting response to treatment and clinical outcome in patients undergoing primary coronary intervention: insights into the STRATEGY Study. J. Am. Coll. Cardiol. 48, 2178–2185 (2006).
Jeong, Y. H. et al. Usefulness of the VerifyNow P2Y12 assay to evaluate the antiplatelet effects of ticagrelor and clopidogrel therapies. Am. Heart J. 164, 35–42 (2012).
Price, M. J. et al. Platelet reactivity and cardiovascular outcomes after percutaneous coronary intervention: a time-dependent analysis of the Gauging Responsiveness with a VerifyNow P2Y12 assay: Impact on Thrombosis and Safety (GRAVITAS) trial. Circulation. 124, 1132–1137 (2011).
Campo, G. et al. Prospective evaluation of onclopidogrel platelet reactivity over time in patients treated with percutaneous coronary intervention relationship with gene polymorphisms and clinical outcome. J. Am. Coll. Cardiol. 57, 2474–2483 (2011).
Lordkipanidzé, M. et al. Comparison of four tests to assess inhibition of platelet function by clopidogrel in stable coronary artery disease patients. Eur. Heart J. 29, 2877–2885 (2008).
Breet, N. J. et al. Comparison of platelet function tests in predicting clinical outcome in patients undergoing coronary stent implantation. J. A. M. A. 303, 754–762 (2010).
Acknowledgements
This work was funded by the grants from platform construction of clinical evaluation and technical research on new drugs of cardiovascular diseases (2011ZX09302-002-02) and “1255” subject promotion plan of Changhai hospital (CH125520400). The authors acknowledge Dr Amit Bhat (Indegene Lifesystems Pvt Ltd, Bangalore), for providing manuscript editing support and technical assistance.
Author information
Authors and Affiliations
Contributions
P.L. wrote the manuscript. Y.Y. and J.L. performed the clinical test on subjects. Z.W. and L.M. designed the clinical test. T.C., Y.L. and A.C. analyzed and interpreted the data. X.Z. and Y.Q. revised the manuscript. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Li, P., Yang, Y., Chen, T. et al. Ticagrelor overcomes high platelet reactivity in patients with acute myocardial infarction or coronary artery in-stent restenosis: a randomized controlled trial. Sci Rep 5, 13789 (2015). https://doi.org/10.1038/srep13789
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep13789
This article is cited by
-
Serum Free Triiodothyronine and the Responsiveness to Clopidogrel in Patients Undergoing Elective Percutaneous Coronary Intervention
Advances in Therapy (2021)
-
The risk of dyspnea in patients treated with third-generation P2Y12 inhibitors compared with clopidogrel: a meta-analysis of randomized controlled trials
BMC Cardiovascular Disorders (2020)
-
Half-dose ticagrelor versus high-dose clopidogrel in reducing platelet reactivity in acute coronary syndrome patients with high on-clopidogrel platelet reactivity (divide study)
European Journal of Clinical Pharmacology (2019)
-
Contemporary Antiplatelet Pharmacotherapy in the Management of Acute Coronary Syndromes
Current Treatment Options in Cardiovascular Medicine (2018)
-
Low-dose ticagrelor yields an antiplatelet efficacy similar to that of standard-dose ticagrelor in healthy subjects: an open-label randomized controlled trial
Scientific Reports (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.