Abstract
To investigate the effects of high-altitude exposure on response inhibition, event-related potential (ERP) components N2 and P3 were measured in Go/NoGo task. The participants included an ‘immigrant’ high-altitude group (who had lived at high altitude for three years but born at low altitude) and a low-altitude group (living in low altitude only). Although the behavioural data showed no significant differences between the two groups, a delayed latency of NoGo-N2 was found in the high-altitude group compared to the low-altitude group. Moreover, larger N2 and smaller P3 amplitudes were found in the high-altitude group compared to the low-altitude group, for both the Go and NoGo conditions. These findings suggest that high-altitude exposure affects response inhibition with regard to processing speed during the conflict monitoring stage. In addition, high altitude generally increases the neural activity in the matching step of information processing and attentional resources. These results may provide some insights into the neurocognitive basis of the effects on high-altitude exposure on response inhibition.
Similar content being viewed by others
Introduction
As of 2006, approximately 12 million people resided in the Qinghai-Tibetan Plateau1. In recent years, more and more people who were born and raised in low-altitude areas are travelling or working in Tibet (average altitude over 4000 m). Living at such a high altitude, the largest and most important impact is hypoxia, which occurs because of a reduction of oxygen in the air and which affects cognition.
Long-term exposure to high altitude with hypoxia may lead to impairment on response inhibition. Previous studies have provided neuroimaging evidence of the impact of high-altitude exposure on the human brain. Evidence from functional magnetic resonance imaging (fMRI) studies has shown that activation of the prefrontal cortex (PFC) and the anterior cingulate cortex (ACC) are associated with response inhibition2,3. A meta-analysis of Go/NoGo tasks reported that a mainly right-lateralized network is associated with response inhibition, including the right middle/inferior frontal gyrus, right inferior parietal regions and medial frontal gyrus4. Moreover, structural modification of the inferior and middle frontal gyrus and ACC in a group of people born and raised at high altitude was found in chronic hypoxia research using magnetic resonance imaging (MRI)5,6. Based on the influence of altitude on the ACC and middle frontal gyrus, the response inhibition associated cortex could be affected, indicating that the response inhibition is influenced by high-altitude exposure. Response inhibition is an essential component of cognitive ability and an important aspect of executive function. However, there is no direct evidence on whether long-term exposure to high altitude will influence the response inhibition.
Compared with fMRI and MRI techniques, event-related potentials (ERPs) have high temporal resolution and can provide more insight into the time course of brain processes, making it possible to determine which stage of response inhibition processing is affected by high-altitude exposure7. Two ERP components correlate with response inhibition during performance of the Go/NoGo paradigm8,9. First, the frontocentral N2, related to response inhibition, was located in the right lateral orbitofrontal, right inferior frontal, middle frontal and anterior cingulate cortex10,11. It is a negative potential with a latency range of 200–300 ms, the NoGo-N2 amplitude increased in the NoGo compared to the Go trial, reflecting a conflict-monitoring process in the early stage of response inhibition8,12; the N2 latency would reflect the speed of this process13. A smaller and delayed NoGo-N2 component was found in the group of people with impaired inhibitory functioning14,15,16. Second, the central P3, a positive wave in the 300–600 ms time range, is larger in the NoGo than in the Go condition, which represents a later stage of the response inhibitory process17. The larger NoGo-P3 was thought to directly reflect conflict resolution through top-down inhibition processing18,19; its amplitude was related to cognitive demand and the attentional resources available for the task20. A smaller NoGo-P3 amplitude was found in the inhibition-impaired group21.
Additionally, the study of cognitive impairment due to high-altitude hypoxia in people who were born and raised in low-altitude areas and relocated in Tibet is increasingly important. Most prior research that has focused on local residents at high altitudes1,22 or individuals with acute exposure to high altitudes23,24 has found cognitive impairment caused by high altitude. However, the results from acute exposure or local residence at high altitude may not generalize to adult immigrants. This is because acute and chronic exposure to high altitudes affects cognition differently25 and local residents are different from low-altitude residents in terms of genetics and other physiological features1,26. The physiological and psychological changes in the immigrant group could reflect the effects of high altitude on cognition better than high-altitude residents or people with acute exposure to high altitudes. Study of cognitive changes in this population can provide a theoretical basis for the provision of cognitive training, prevention of cognitive impairment and other practical applications.
In the present study, we aimed to investigate the time course of the impact of chronic high-altitude exposure on the response inhibition process using ERPs in a Go/NoGo paradigm. In our study, the high-altitude group was determined by the same considerations used in our previous experiment27. We considered the participants to be more representative of the influence of high altitude because they had immigrated to a high-altitude area in adulthood and had acclimated to the environment while living there for three years. We predicted that the effects of chronic exposure to high altitude would be found on behaviour and ERP results in our study. For the behaviour result, we did not find any long-term high-altitude exposure study using the Go/NoGo task to discuss the influence of high-altitude hypoxia on response inhibition. Based on the neuroimaging evidence of the impact of high-altitude exposure on the human brain, we only could speculate that the effects of high altitude would be found in behaviour results (miss rates, commission errors and reaction times). For the ERP results, the high-altitude effect on inhibition should be reflected in the N2 and P3 components; we predicted smaller and later NoGo-N2 components and smaller NoGo-P3 amplitudes would be found in the high-altitude group.
Results
Behavioural results
For the high-altitude and low-altitude groups, respectively, the average miss rate was 1.45 ± 3.76% (mean ± S.D.) and 0.60 ± 0.76% and the commission errors (false alarms) were 17.40 ± 13.06% and 16.56 ± 10.92%. The average time for correct responses was 304 ± 63 ms for the high-altitude group and 305 ± 36 ms for the low-altitude group (Table 1). No significant differences were found in behavioural performance between these two groups (ps > 0.05). In the signal detection analysis, the difference between the two groups was not significant for d’ [t (38) = −0.33, p = 0.75] and for β [t (38) = 0.98, p = 0.33].
ERPs
N2. With regard to the amplitude of the N2 component, the main effect of group was marginally significant [−2.22 ± 1.45 μV vs. 1.02 ± 1.15 μV; F (1,38) = 3.98, p = 0.053], with more negative N2 amplitude in the high-altitude group than in the low-altitude group (Fig. 1 and Fig. 2). The main effect of trial type was significant, with more negative N2 for NoGo stimuli than for Go stimuli in both groups [−3.47 ± 1.13 μV vs. 2.27 ± 0.72 μV; F (1,38) = 43.40, p < 0.001] (Fig. 1 and Fig. 2). The interaction between trial type and electrode site was significant [F (1,35) = 6.01, p = 0.005] and the trial type effect was significant at all three electrode sites [Fz: t (39) = 5.36, p < 0.001; FCz: t (39) = 6.21, p < 0.001; Cz: t (39) = 6.95, p < 0.001] (Table 2), with maximum effect at the Cz site. No other main effect or interaction was significant.
With regard to the latency of the N2 component, the interaction between trial type and group was significant [F (1,38) = 12.28, p < 0.001], with longer NoGo-N2 latency in the high-altitude group than in the low-altitude group [272.60 ± 4.47 ms vs. 232.67 ± 3.60 ms; F (1,38) = 48.41, p < 0.001]. The difference between the two groups was not significant in the Go trials [244.20 ± 6.64 ms vs. 229.33 ± 3.20 ms; F (1,38) = 0.71, p > 0.05] (Table 2).
P3. With regard to the amplitude of the P3 component, the main effect of trial type was significant, with a larger P3 amplitude for NoGo than for Go stimuli [16.23 ± 0.93 μV vs. 7.52 ± 0.63 μV; F (1,38) = 125.90, p < 0.001] (Fig. 1 and Fig. 2). The main effect of group was significant, with smaller P3 amplitude for the high-altitude group than for the low-altitude group [10.15 ± 1.50 μV vs. 13.60 ± 3.16 μV; F (1,38) = 7.01, p = 0.01] (Table 3). No other main effect or interaction was significant.
With regard to the P3 latency, the main effect of trial type was significant, with longer P3 latency for NoGo than for Go stimuli [383.64 ± 2.82 ms vs. 340.68 ± 2.01 ms; F (1,38) = 161.40, p < 0.001] (Table 3). No other main effect or interaction was significant.
Source Localization
The sLORETA showed the strongest activation in the frontal lobe in both groups. Figure 3 shows the mean activation levels of the NoGo-N2 components in the brain regions of the two groups as plotted by standardized low resolution electromagnetic tomography analysis (sLORETA). The talairach coordinates were value = 4.67E-1, (X = 15 , Y = 35 , Z = 55) for the high-altitude group and value = 1.27E + 0, (X = 30 , Y = 40 , Z = 45) for the low-altitude group.
Source localization.
The source localization of the surface NoGo-N2 amplitude sLORETA images showing the standardized current density maxima for the high-altitude group (A) and low-altitude group (B), as seen from the horizontal, sagittal and coronal sections. Talairach coordinates (X,Y,Z) are indicated, the activity is colour-coded. Yellow colour indicates local maxima of the NoGo-N2 component is in the frontal Lobe (superior frontal gyrus and middle frontal gyrus) in the high and low altitude groups.
Discussion
Using a Go/NoGo task, our study investigated the neural mechanisms responsible for modulation of response inhibition in healthy young people after long-term exposure to high altitude. The results showed that a response inhibition effect was successfully elicited, as reflected by larger amplitudes of N2 and P3 in NoGo compared to Go trials in both groups. We focused our analyses on the differences between the high-altitude and low-altitude groups. The main results showed that a later NoGo-N2 was found in the high-altitude group compared to the low-altitude group and the amplitude of N2 was larger and that of P3 smaller in the high-altitude group compared to the low-altitude group in both the Go and NoGo trials.
Inconsistent with our hypothesis, larger N2 amplitude was found for the high-altitude group than for the low-altitude group for both NoGo and Go conditions. According to the model-based object recognition system28,29, if the given object matches the explicit model, then familiarity is achieved. In this study, the processing of the Go stimulus is a top-down process which provides a recent context for the subsequent visual target. When the Go stimulus appears, the object matches the Go model and when the NoGo stimulus appears, it mismatches with the model; the matching of the presented Go and/or NoGo stimuli with the internal representation of the Go responses was reflected in the N2 amplitude30,31. The result could be explained by generally increased neural activity in the matching step of information processing.
The amplitude of P3 was smaller for the high-altitude group than for the low-altitude group in both the NoGo and Go trials. According to the dissociable effects in more recent research, N2 and P3 are now thought to represent functionally separable processes in the Go/NoGo task32, NoGo-P3 amplitude was related to the late step of response inhibition10. However, we found that the P3 amplitude in both Go and NoGo trials were smaller in the high-altitude than in the low-altitude group. Beyond the field of response inhibition, the P3 component has generally been considered as a late stage of information processing, more specifically, as reflecting attentional resources being allocated to the task7,33. In our study, there was high cognitive demand in the high-altitude group, which would limit the attentional resources to resist the inhibitory control. The attentional resources declined in the high-altitude group compared to the low-altitude group, which produced smaller P3 in the high-altitude group than in the low-altitude group. Finally, in previous studies, decreased P3 amplitudes were found in aging adults (compared to young adults) and illness groups such as, Alzheimer’s disease patients, Parkinson’s disease patients and alcoholics with some degree of impaired inhibitory functioning15,16,34,35. In a high-altitude study that used an attention task, we also found decreased P3 amplitude in the high-altitude group compared to the low-altitude group27. Our previous results with regard to P3 amplitude suggest that long-term high-altitude exposure leads to diminished availability of attentional resources36.
More importantly, the present study found that the high-altitude group presented delayed NoGo-N2 latency compared to that of the low-altitude group. The peak latency of ERP component represents processing speed13,37. In our test, the processing of the Go stimulus provides a mental template for the subsequent visual target, the N2 component for NoGo stimuli reflects a mismatch between the current stimulus and a mental template38 and the NoGo-N2 latency results may reflect the mismatch processing speed. As in the mismatch condition, later NoGo-N2 latency was found in the high-altitude group compared to the low-altitude group, which suggested prolonged mismatch processing in the high-altitude group. According to previous studies39,40, the terms conflict and mismatch share some similarity and both implicate a process of comparison. When the information from a stimulus is transmitted into the brain, the information of the previous stimulus is retrieved and compared with the latter one. The information difference between the two stimuli indicates some kind of information mismatch or conflict41. According to a previous study42, the mismatch between the input stimulus and the current goal engendered cognitive conflict in the goal direct test. More specifically, in the Go/NoGo task, when the NoGo stimulus was compared with the Go stimulus, the comparison process led to a mismatch and the mismatch between the NoGo stimuli and the current goal (intention to Go simuli) may have engendered conflict monitoring that is reflected in the NoGo-N2. In other words, the NoGo-N2 reflects a conflict monitoring process whenever it detects a mismatch. The longer latency of NoGo-N2 for the high-altitude group suggests that high-altitude subjects are slower in the timing of conflict monitoring, consistent with the hypothesis of cognitive deficits after long-term exposure to high altitudes22,23,24.
On the basis of the previous studies, the N2 component was located in the middle frontal, right inferior frontal and ACC10,11 which correlated with conflict monitoring in the response inhibition process30,43. Using sLORETA, we localized the NoGo-N2 component in the superior frontal and middle frontal gyrus; maximal activation was found at the right side of the frontal area in our experiment (Fig. 3)44. The middle frontal cortex has been reported to be influenced by chronic hypoxia5,6, which may explain why delayed NoGo-N2 latency was found in the high-altitude group. Although the response inhibition effect was reflected in the amplitude of NoGo-N2 in most of the previous studies10,45, the latency was also a very important indicator of the ERP component. It is a sensitive index of the timing of information processing during visual perception and the N2 latency reflected the slowdown in the processing speed of response inhibition in the conflict-monitoring stage.
The group differences were not significant in terms of behaviour results. It is an interesting question as to how the high-altitude group managed to preserve task performance. Previous acute exposure studies using different cognitive tasks have found increased reaction time in high-altitude areas46,47,48, but the opposite result was found with prolonged exposure to high altitude22,49. This finding may have occurred because acute and chronic exposure to high altitudes affect cognition differently25. The disappearance of the effects on behaviour after prolonged exposure may result from adaptation supported by a compensatory mechanism, which was also found in our previous study27. After the stimulus appeared, delayed NoGo-N2 latency was found in the high-altitude group than the low-altitude group, which reflected the group difference on the processing time in the early stage of response inhibition. However, N2 amplitude in the high-altitude group was enhanced in both the Go and NoGo trials. As the amplitude of ERP component reflects the neural activity level, the increased N2 amplitude in high-altitude group suggests that the high-altitude group, in comparison, engaged a higher level of neural activity to finish the same task. Therefore, the slower processing at the early stage did not slacken the late processing stage or affect the behavioral results. The group differences were not significant on P3 latency and the behavioural result. With a more difficult task, the behavioural effects may be more obvious. From another aspect, the disappearance of effect at the behavioural level may also be due to lower sensitivity of the behavioural measure used.
The primary limitation of the present research was that the climatic or cultural effects of high altitude should also be considered. Although complete physical adaptation to 3,600 m occurs after 40 days25 and three years’ exposure to high altitude is sufficient for acclimation, there may still be climatic or cultural effects; thus, the results should be interpreted cautiously.
In conclusion, the present findings reveal that chronic high-altitude exposure affected response inhibition and specifically, processing speed at the conflict-monitoring stage, as indicated by the later NoGo-N2 latency found in the high-altitude group. A general high-altitude effect was also found in terms of matching step of information processing and attentional resources for both the Go and NoGo conditions, as indicated by the larger N2 and smaller P3 components elicited in the high-altitude group compared to the low-altitude group. Because there is no previous ERP study on response inhibition after long-term exposure to high altitude, these findings make a valuable contribution to the basic science of altitude effects on cognition.
Methods
Participants
Forty healthy young college students from the Han ethnic group, aged 21–24 years old, took part in this experiment. All participants signed an informed consent form before the experiment. The experiment was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the Institute of Psychology, Chinese Academy of Sciences. All participants were right-handed and had normal or corrected-to-normal vision. All participants had been born and raised in a low-altitude location (<1000 m). The twenty participants in the high-altitude group (10 male, 21.78 ± 1.41 years) had lived at high altitude (3650 m) for three years and the twenty participants in the low-altitude group (10 male, 22.75 ± 1.08 years) had never been to a high-altitude area.
Procedure
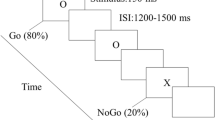
The visual stimuli for the Go/NoGo task were the capital letters ‘O’ and ‘X’. White visual stimuli were presented against a black background in the centre of a computer screen (AOC 17-in. LCD monitor), with a visual angle of approximately 2.6° vertically and 1.8° horizontally. One of the two letters was presented in a single trial and either a response (Go) or the with holding of a response (NoGo) was required of the participant. The association between letters and trial type was counter balanced between the two blocks, with the letter ‘O’ serving as a Go stimulus in one block and as a NoGo stimulus in the other block. Each trial began with a Go or a NoGo stimulus lasting for 150 ms, followed by a black screen. The inter-stimulus interval (ISI) range was 1200–1500 ms (Fig. 4).
Participants were tested individually in a dimly lit, sound-attenuated room. After 20 practice trials, two experimental blocks of 240 trials in each block were completed, with 192 Go (80%) and 48 NoGo (20%) trials per block. Most of the trials were Go trials; thus, when the NoGo stimulus appeared, participants had to control their impulse to respond, inducing the impulse inhibition process. Behavioural data were collected and the stimuli were presentated using the E-prime software system (Version 1.1, Psychology Software Tools, Inc., Pittsburgh, PA). Participants were instructed to press ‘M’ with the right hand on a computer keyboard when a Go stimulus occurred and to give no response to a NoGo stimulus. Speed and accuracy were equally emphasized.
EEG recording
Electroencephalography (EEG) data were recorded from 64 scalp sites (10/20 system), using Ag/AgCl electrodes mounted in an elastic cap (Neuroscan Inc.). The physical reference electrode was approximately 2 cm posterior to CZ. Electrode impedances were kept below 5 kΩ. Vertical and horizontal electrooculogram (EOG) data were recorded from above and below the left eye and from the outer canthi of both eyes, respectively. EEG and EOG were continuously recorded at a sampling rate of 500 Hz, applying a filter bandwidth of 0.05–100 Hz.
Data analysis
Data were analyzed with SPSS (SPSS, Inc., Chicago) for Windows. P < 0.05 were considered statistically significant. The reaction time, the miss rate and the commission errors (false alarm) from both groups were subjected to independent t-tests comparing the high-altitude and low-altitude groups. Signal detection analysis was also used to analyze the behavioural data. The sensitivity index (d’) and response bias index (β) according to the signal detection theory were calculated50. The hit and false alarm rates were transformed into z scores. Trial type was a within subjects factor and group was a between subjects factor. Independent-samples t-test was used to compare the high-altitude and low-altitude groups.
The EEG data were re-referenced to the average of left and right mastoid (M1 and M2). Ocular artifacts were removed from the EEG signal using a regression procedure implemented with Neuroscan software51. The ERP data were digitally filtered with a 40 Hz low pass. Continuous EEG data was segmented into stimulus-locked (−200 ms to 1000 ms) ERP segments, including a 200 ms pre-stimulus baseline. Trials with various artifacts were rejected, with a criterion of ±75 μV. Waveforms averages for each individual subject within each condition were calculated.
Peak amplitudes and peak latencies were used for statistical analyses of the N2 and P3 components. The time windows for the peak detection of N2 and P3 components were 200–300 ms and 250–450 ms, respectively. Electrode sites for analysis were chosen based on the scalp distributions of the current data and previous research demonstrating that the N2 is focal over the fronto-medial locations and P3 is over the midline electrodes7,30. Fz, FCz and Cz were selected for N2; Fz, FCz, Cz, CPz and Pz were selected for the P3 data analysis30,52,53. The amplitude and latency of N2 were subjected to a mixed-model ANOVA, respectively. The ANOVA factors for the N2 component included trial type (two levels: Go and NoGo), electrode sides (three levels: Fz, FCz, Cz) as within-subject factors and altitude group (two levels: high altitude and low altitude) as between-subject factor. The amplitude and latency of P3 were also subjected to a mixed-model ANOVA, respectively. The ANOVA factors included trial type (two levels: Go and NoGo), electrode sides (five levels: Fz, FCz, Cz, CPz, Pz) as within-subject factors and altitude group (two levels: high altitude and low altitude) as between-subject factor. The Greenhouse–Geisser correction was used to compensate for sphericity violations. Simple effect analyses were conducted to explore interaction effects.
Source localization of NoGo-N2 amplitude values was plotted using the sLORETA44. The sLORETA is an efficient tool for functional mapping and source localizing; it is consistent with physiology localization. Fifty-eight electrodes (the M1, M2, two VEOG electrodes and two HEOG electrodes were excluded from the 64 electrodes) were used for analysis. A transformation matrix was created using the electrode coordinates. The averaged waveforms in the NoGo-N2 time range were converted and saved into ASCII values for both the high-altitude and low-altitude groups. sLORETA images were constructed based on the high-altitude and low-altitude group data, respectively. The version of sLORETA employed in our study was made available at http://www.unizh.ch/keyinst/NewLORETA/LORETA01.htm.
Additional Information
How to cite this article: Ma, H. et al. Long-Term Exposure to High Altitude Affects Response Inhibition in the Conflict-monitoring Stage. Sci. Rep. 5, 13701; doi: 10.1038/srep13701 (2015).
References
Wu, T. & Kayser, B. High altitude adaptation in Tibetans. High Alt Med & Biol 7, 193–208 (2006).
Liddle, P. F., Kiehl, K. A. & Smith, A. M. Event-related fMRI study of response inhibition. Hum Brain Mapp 12, 100–109 (2001).
Simmonds, D. J., Pekar, J. J. & Mostofsky, S. H. Meta-analysis of Go/No-go tasks, demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia 46, 224–232 (2008).
Buchsbaum, B. R., Greer, S., Chang, W. L. & Berman, K. F. Meta-analysis of neuroimaging studies of the Wisconsin card-sorting task and component processes. Hum brain mapp 25, 35–45 (2005).
Yan, X., Zhang, J., Shi, J., Gong, Q. & Weng, X. Cerebral and functional adaptation with chronic hypoxia exposure: A multi-modal MRI study. Brain Res 1348, 21–29 (2010).
Yan, X., Zhang, J., Gong, Q. & Weng, X. Prolonged high-altitude residence impacts verbal working memory: an fMRI study. Exp Brain Res 208, 437–445 (2011).
Luck, S. J. & Kappenman, E. S. The Oxford Handbook of Event-Related Potential Components, 3–51 (OUP USA, 2012).
Eimer, M. Effects of attention and stimulus probability on ERPs in a Go/Nogo task. Biol Psychol 35, 123–138 (1993).
Falkenstein, M., Hoormann, J. & Hohnsbein, J. ERP components in Go Nogo tasks and their relation to inhibition. Acta Psychologica 101, 267–291 (1999).
Bokura, H., Yamaguchi, S. & Kobayashi, S. Electrophysiological correlates for response inhibition in a Go/NoGo task. Clin Neurophysiol 112, 2224–2232 (2001).
Karch, S. et al. Separating distinct aspects of the voluntary selection between response alternatives: N2-and P3-related BOLD responses. Neuroimage 51, 356–364 (2010).
Yu, F., Yuan, J. & Luo, Y. J. Auditory-induced emotion modulates processes of response inhibition: an event-related potential study. Neuroreport 20, 25–30 (2009).
Gajewski, P. D., Stoerig, P. & Falkenstein, M. ERP-correlates of response selection in a response conflict paradigm. Brain Res 1189, 127–134 (2008).
Rentrop, M. et al. Temporal variability and spatial diffusion of the N2 event-related potential in high-functioning patients with schizophrenia. Schizophr Res 131, 206–213 (2011).
Porjesz, B., Begleiter, H., Bihari, B. & Kissin, B. Event-related brain potentials to high incentive stimuli in abstinent alcoholics. Alcohol 4, 283–287 (1987).
Bokura, H., Yamaguchi, S. & Kobayashi, S. Event-related potentials for response inhibition in Parkinson’s disease. Neuropsychologia 43, 967–975 (2005).
Bokura, H., Yamaguchi, S. & Kobayashi, S. Electrophysiological correlates for response inhibition in a Go/NoGo task. Clin neurophysiol 112, 2224–2232 (2001).
Bruin, K., Wijers, A. & Van Staveren, A. Response priming in a go/nogo task: do we have to explain the go/nogo N2 effect in terms of response activation instead of inhibition? Clinl Neurophysiol 112, 1660–1671 (2001).
Smith, J. L., Johnstone, S. J. & Barry, R. J. Response priming in the Go/NoGo task: The N2 reflects neither inhibition nor conflict. Clinl Neurophysiol 118, 343–355 (2007).
Polich, J. Updating p300: An integrative theory of P3a and P3b. Clin Neurophysiol 118, 2128–2148 (2007).
Tye, C. et al. Attention and inhibition in children with ASD, ADHD and co-morbid ASD plus ADHD: an event-related potential study. Psychol Med 44, 1101–1116 (2014).
Richardson, C. et al. Neurophysiological evidence for cognitive and brain functional adaptation in adolescents living at high altitude. Clinl Neurophysiol 122, 1726–1734 (2011).
Hayashi, R., Matsuzawa, Y., Kubo, K. & Kobayashi, T. Effects of simulated high altitude on event-related potential (P300) and auditory brain-stem responses. Clin Neurophysiol 116, 1471–1476 (2005).
Thakur, L., Ray, K., Anand, J. & Panjwani, U. Event related potential (ERP) P300 after 6 months residence at 4115 meter. Indian J Med Res 134, 113–117 (2011).
Zubieta-Calleja, G. R. Human adaptation to high altitude and sea level: acid-base equilibrium, ventilation and circulation in chronic hypoxia, 38–47 (VDM Verlag, 2010).
Wang, B. et al. On the origin of Tibetans and their genetic basis in adapting high-altitude environments. PLoS One 6, e17002 (2011).
Wang, Y. et al. Long-Term Exposure to High Altitude Affects Voluntary Spatial Attention at Early and Late Processing Stages. Sci Rep 4, 4443 (2014).
Grimson, W. E. L. & Huttenlocher, D. P. On the verification of hypothesized matches in model-based recognition. IEEE T Pattern Anal 13, 1201–1213 (1991).
Lamdan, Y. & Wolfson, H. Geometric hashing: A general and efficient model-based recognition scheme. IEEE 2nd ICCV, Tampa, Florida, 238–249 (1988).
Nieuwenhuis, S., Yeung, N., van den Wildenberg, W. & Ridderinkhof, K. R. Electrophysiological correlates of anterior cingulate function in a go/no-go task: effects of response conflict and trial type frequency. Cogn, Affect & Behav Neurosci 3, 17–26 (2003).
Folstein, J. R. & Van Petten, C. Influence of cognitive control and mismatch on the N2 component of the ERP: a review. Psychophysiology 45, 152–170 (2008).
Randall, W. M. & Smith, J. L. Conflict and inhibition in the cued-Go/NoGo task. Clin Neurophysiol 122, 2400–2407 (2011).
Kramer, A. F., Wickens, C. D. & Donchin, E. Processing of stimulus properties: evidence for dual-task integrality. J Expl Psychol Human 11, 393–408 (1985).
Jeong, J. EEG dynamics in patients with Alzheimer’s disease. Clin Neurophysiol 115, 1490–1505 (2004).
Pfefferbaum, A. & Ford, J. M. ERPs to stimuli requiring response production and inhibition: effects of age, probability and visual noise. Electroencephalogr Clin Neurophysiol 71, 55–63 (1988).
Nieuwenhuis, S., Aston-Jones, G. & Cohen, J. D. Decision making, the P3 and the locus coeruleus-norepinephrine system. Psychol Bull 131, 510–532 (2005).
Schmitt, B. M., Munte, T. F. & Kutas, M. Electrophysiological estimates of the time course of semantic and phonological encoding during implicit picture naming. Psychophysiology 37, 473–484 (2000).
Ferrari, V., Bradley, M. M., Codispoti, M. & Lang, P. J. Detecting novelty and significance. J Cogn Neurosci 22, 404–411 (2010).
Mager, R. et al. Mismatch and conflict: neurophysiological and behavioral evidence for conflict priming. J Cogn Neurosci 21, 2185–2194 (2009).
Yeung, N., Botvinick, M. M. & Cohen, J. D. The neural basis of error detection: conflict monitoring and the error-related negativity. Psychol Rev 111, 931–959 (2004).
Wang, Y., Cui, L., Wang, H., Tian, S. & Zhang, X. The sequential processing of visual feature conjunction mismatches in the human brain. Psychophysiology 41, 21–29 (2004).
Fink, G. R. et al. The neural consequences of conflict between intention and the senses. Brain 122, 497–512 (1999).
Botvinick, M. M., Cohen, J. D. & Carter, C. S. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci 8, 539–546 (2004).
Pascual-Marqui, R. D., Esslen, M., Kochi, K. & Lehmann, D. Functional imaging with low-resolution brain electromagnetic tomography (LORETA): a review. Method Find Exp Clin 24, 91–95 (2002).
Oddy, B. W. & Barry, R. J. The relationship of N2 and P3 to inhibitory processing of social drinkers in a Go/NoGo task. Int J Psychophysiol 72, 323–330 (2009).
Bolmont, B., Bouquet, C. & Thullier, F. Relationships of personality traits with performance in reaction time, psychomotor ability and mental efficiency during a 31-day simulated climb of Mount Everest in a hypobaric chamber’. Percept Mot Skills 92, 1022–1030 (2001).
Kramer, A. F., Coyne, J. T. & Strayer, D. L. Cognitive Function at High-Altitude. Hum Factors 35, 329–344 (1993).
Wesensten, N. J. et al. Effects of simulated high altitude exposure on long-latency event-related brain potentials and performance. Avia Space Environ Med 64, 30–36 (1993).
West, J. B. Human Physiology at Extreme Altitudes on Mount Everest. Science 223, 784–788 (1984).
Macmillan, N. A. & Creelman, C. D. Detection theory: A user’s guide. (CUP UK, 1991).
Semlitsch, H. V., Anderer, P., Schuster, P. & Presslich, O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology 23, 695–703 (1986).
Smith, J. L. To Go or not to Go, that is the question: Do the N2 and P3 reflect stimulus- or response-related conflict? Int J Psychophysiol 82, 143–152 (2011).
Wu, J. H. et al. Response inhibition in adolescent earthquake survivors with and without posttraumatic stress disorder A combined behavioral and ERP study. Neurosci Lett 486, 117–121 (2010).
Acknowledgements
This research was supported by the National Natural Science Foundation of China (Y2JJ081004) and Key Laboratory of Mental Health, Institute of Psychology, Chinese Academy of Sciences.
Author information
Authors and Affiliations
Contributions
Y.W., H.M. and J.W. designed research; H.M., Y.W. and P.L. performed research; H.M. and Y.W. analyzed data; and H.M., Y.W. and B.H. wrote the paper.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Ma, H., Wang, Y., Wu, J. et al. Long-Term Exposure to High Altitude Affects Response Inhibition in the Conflict-monitoring Stage. Sci Rep 5, 13701 (2015). https://doi.org/10.1038/srep13701
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep13701
This article is cited by
-
Dynamic brain functional states associated with inhibition control under different altitudes
Cognitive Neurodynamics (2024)
-
Long-term exposure to high altitude reduces alpha and beta bands event-related desynchronization in a Go/NoGo task
Scientific Reports (2023)
-
Consistent differences in brain structure and functional connectivity in high-altitude native Tibetans and immigrants
Brain Imaging and Behavior (2023)
-
Competition among the attentional networks due to resource reduction in Tibetan indigenous residents: evidence from event-related potentials
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.