Abstract
Placental development is essential for implantation and growth of foetus in the uterus of eutherian mammals. Numerous growth factors are responsible for placental development and cell lineage differentiation. Gene knockout mice have shown role of various genes in the placenta. Here using Wdr13 knockout mice, we show that this gene is important for proper placental development. Wdr13, a X-linked gene, expresses in multiple trophoblast cell types of placenta and the mutant placenta had reduced size after 17.5 dpc due to reduction of junctional zone (JZ) and labyrinth zone (LZ). We observed reduction in levels of angiopoietin-2 and cd44 mRNA in Wdr13 mutant placenta as compared to that in the wild type. Our findings show that Wdr13 is required for normal placental development and cell differentiation. Wdr13 heterozygous female placenta when the mutant allele was of maternal origin showed similar defects as those in case of Wdr13 null placenta. Using two types of heterozygous females carrying either maternally and paternally derived mutant Wdr13 allele we provide genetic evidence that development of placenta determines body weight of mice for the entire life.
Similar content being viewed by others
Introduction
Placenta is an essential organ for normal embryonic development in uterus. In the mouse uterus, soon after implantation at around 4.5 days post coitum (dpc) placental development starts. Trophoectoderm surrounding the blastocyst, differentiate into variety of trophoblast cell types. The trophoectoderm in direct contact with inner cell mass (ICM) is known as polar trophoectoderm and other which is not in contact with ICM is known as mural trophoectoderm1,2,3. The mural trophoectoderm differentiate into trophoblast giant cells (TGC), which are polyploid cells involved in separation of maternal decidua from placental layers and have function in early invasion, formation of yolk sack and endocrine signaling. The polar trophoectoderm produces two-cell type of early placenta, namely; the ectoplacental cone (EPC) and the chorion4,5. The EPC gives three trophoblast lineages: a second wave of TGCs adjacent to the maternal decidua, spongiotrophoblast (SpT) and glycogen trophoblast cells (GCs). Together these cells form the middle junctional zone of placenta. In contrast chorion gives syncytiotrophoblast and other trophoblast cell types of the labyrinth zone (LZ). In the LZ, the allantoic mesoderm gives rise to blood vessels and mesenchyme where as trophoblast cells gives rise to several epithelial derivatives. LZ is the site for foetal-maternal exchange6.
All the trophoblast giant cells (TGCs) are polyploid in nature with endocrine activity. However, they differ in their polyploidy, developmental origin, gene expression profiling and location4. Cell lineage tracing studies have shown that parietal TGCs (P-TGCs) and canals TGCs (C-TGCs) are originated from both Tpbpa positive and negative EPC progenitors. However spiral artery-associated TGCs (SpA-TGCs) are originated from Tpbpa positive and sinusoids TGCs (S-TGCs) from Tpbpa negative EPC progenitors4. However, despite all these differences all TGCs require Hand1 for proper differentiation7. The middle layer of placenta (JZ) contains spongiotrophoblast (SpT) and glycogen trophoblast cells (GCs). During the second half of pregnancy some GCs migrate into decidua and crowd around area of spiral arteries8. However, there is no evidence of movement of SpT and these remain in JZ. Tpbpa is a marker for both SpT and GCs. The origin of GCs is not understood properly. Some studies suggest that GCs originate from SpT while others suggest that GCs may originate directly from progenitors present in EPC8,9.
Wdr13 is a member of WD-repeat protein gene family and it expresses in various tissues in mouse and humans10,11. This gene is present on X- chromosome and is evolutionary conserved through out vertebrate evolution10,11. Our earlier work showed that Wdr13 mutant mice have slightly lower body weight at one month of age and become mildly obese with age as compared to Wild type littermates12,13. However, expression and role of this protein in placenta has not been documented. In the present study, we demonstrate that Wdr13 is expressed in placenta and has a role in placental development. Further, we took advantage of the preferential inactivation of paternally derived X-chromosome in the female placenta14 and show that Wdr13 heterozygous placenta- when the mutant allele is of maternal origin, have similar defects as in the case of Wdr13 null placenta (XWdr13Y, XWdr13XWdr13) and provide genetic evidence that placental development affects the lifetime body weight.
Results
The first set of experiments were sought to determine the effect of Wdr13 genotype on the weight of embryos. To generate Wdr13 null and wild type female and male mice, we crossed Wdr13 heterozygous female (XWdr13X, XXWdr13) with either hemizygous male (XWdr13Y) or wild type male (XY) [XWdr13designates an X chromosome carrying Wdr13 mutation and maternally derived X chromosome is designated first]. At 19.5 dpc the weight of Wdr13 null mice (XWdr13Y, XWdr13XWdr13) were significantly lower than wild type controls (XY, XX) (Fig. 1A).
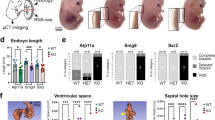
Wdr13-null foetus and placenta are smaller than wild type littermate control.
(A) Weight of Wdr13-null (XWdr13Y, XWdr13XWdr13) and wild type (XY, XX) foetus at various time points (n = 35–41). (B) Weight of maternally and paternally derived heterozygous females at 17.5 dpc (n = 9–11) (XWdr13designates an X chromosome carrying Wdr13 mutation and maternally derived X chromosome is designated first). (C) Weight of Wdr13-null (XWdr13Y, XWdr13XWdr13) and wild type (XY, XX) placenta at various time points (n = 35–41). (D) Weight of maternally and paternally derived heterozygous females placenta at 17.5 dpc (n = 9–11). (E) Weight of Wdr13-null and wild type placenta at 17.5 dpc from embryo transferred surrogate mother (n = 7–10).
The reduction in weight of 19.5 dpc Wdr13 null mice as compared to wild type mice may either be due to deficiency of Wdr13 gene felt in placenta or due to deficiency of this gene in embryo or both. To distinguish among these possibilities, weight of Wdr13 null and their wild type littermate’s embryo/placenta were measured at various time points. At 15.5 dpc there was no difference either in placenta or in the embryo weights between these two genotypes (Fig. 1A,C). However, from 17.5 dpc onward the weights of both Wdr13 null placenta and embryos were significantly lower (Fig. 1A,C). These results suggested the role of Wdr13 in placental development.
Wdr13 gene is present on X-chromosome10 and it is known that paternally derived X-chromosome gets preferentially inactivated in mouse placenta14,15. This presented us an opportunity to create conditional Wdr13 gene null mutation in placenta only. To achieve this we crossed Wdr13 heterozygous females with wild type males in order to generate maternally derived heterozygous (XWdr13X) placenta/ embryos and hemizygous males were crossed with wild type females to generate paternally derived heterozygous (XXWdr13) placenta/embryos. These crosses provided us two types of heterozygous female embryos: those developing in Wdr13 null placenta due to inactivation of wild type allele on paternal X chromosome and the maternal allele being the mutant and those developing in heterozygous placenta where mutant allele was present on inactivated paternal chromosome and wild type allele on maternal chromosome was intact. Indeed, qRT-PCR and western blot analysis of placenta and embryo confirmed this duality (Fig. 2). The expression of Wdr13 in placenta was analyzed by qRT-PCR, using Wdr13 specific primers (Supplemental Table S1). XWdr13X heterozygous placenta had only traces of expression (Fig. 2A) in contrast to XXWdr13 heterozygous and wild type placenta. It may be noted that there was no difference in the level of expression of Wdr13 gene in these two types of heterozygous, XWdr13X and XXWdr13 embryos (Fig. 2B). Consistent with qRT-PCR, western blot analysis showed expression of both 53 kDa and 43 kDa isoforms of WDR13 protein (Unpublished data) in wild type placenta (Fig. 2C) and as expected in Wdr13 null placenta both isoforms were absent, confirming authenticity of the antibody. XXWdr13 heterozygous placenta showed both isoforms of WDR13 protein as in wild type. However, in XWdr13X heterozygous placenta only traces of these isoforms were observed (Fig. 2C).
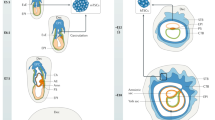
Expression of Wdr13 in placenta.
(A) Wdr13 gene expression in maternally and paternally derived heterozygous females placenta as compared to wild type females at 17.5 dpc. (n = 6) (B) Wdr13 gene expression in maternally and paternally derived heterozygous females embryo as compared to wild type females at 13.5 dpc. (n = 6) (C) Immunoblot for WDR13 protein expression in placenta from different genotype. Lower panel shows beta actin as loading control. *Shows non-specific band. Full-length immunoblots are included in Supplemental Fig. S2. (D) RNA in situ using Wdr13 cDNA antisense as probe shows expression in junctional and labyrinth zone of placenta. Sense probe was used as control. Lower panel shows higher magnification image. Filled arrow shows SpT, Closed arrow shows decidua layer.
In the background of above data we measured the weight of placenta/embryo of XWdr13X and XXWdr13 heterozygous genotype at 17.5 dpc. Interestingly weight of placenta and embryo of XWdr13X genotype were lower as compared to XXWdr13 genotype (Fig. 1B,D). These results show that Wdr13 has role in placental development and further, lower placental weight contributes to lower body weight of embryo.
To rule out any interaction between uterine genotype and embryo genotype on development of placenta, embryo transfer experiments were performed. 0.5-dpc embryos (XY, XX, XWdr13Y and XWdr13XWdr13) were transferred to wild type CD1 pseudo pregnant female. At 17.5 dpc pregnant mice were dissected and weight of placenta/embryo was measured. Consistent with our above-mentioned results embryo transferred Wdr13 null mice placenta showed less weight as compared to wild type littermates (Fig. 1E).
To further see the expression of Wdr13 gene in various cell types of placenta, RNA in situ was performed. RNA in situ with Wdr13 anti sense probe shows expression of Wdr13 mRNA most prominently in labyrinth zone (Fig. 2D lower panel). A lower level of expression of Wdr13 mRNA was observed in junctional zone SpT cells but no expression was observed in GCs (Fig. 2D upper panel). Some expression of Wdr13 mRNA was also observed in decidua layer of placenta (Fig. 2D upper panel).
After establishing that WDR13 expresses in placenta and Wdr13 mutant placenta have reduced weight as compared to wild type placenta, next we performed histological examination to understand the role of this gene in placental development. H&E staining showed overall reduction in placental size of Wdr13 mutants as compared to wild type placenta (Fig. 3A) due to reduction in junctional zone and labyrinth zone (Table 1). To further see the detailed structure of placental junctional zone, Periodic acid-Schiff (PAS) staining was performed for staining GCs of JZ at 17.5 dpc. PAS staining showed over all decrease in junctional zone SpT and GCs (Fig. 3B,C) in Wdr13 null placenta as compared to wild type placenta. For detailed structure of placental labyrinth zone, Masson’s trichrome (MT) staining was performed at 17.5 dpc. MT staining showed significant reduction in the number of S-TGCs in Wdr13 mutant placenta as compared to that in wild type placenta (Fig. 3D,E). Interestingly, we observed increased maternal blood space in Wdr13 mutant as compared to wild type placenta (Fig. 3D,F) probably as compensatory mechanism to increase the nutrient supply.
Wdr13 is important for junctional and labyrinth zone development.
(A) Gross histology of 17.5 dpc placenta (H&E staining) shows reduced placental size in Wdr13 mutant placenta as compared to wild type. (B) Periodic acid-Schiff (PAS) staining of 17.5 dpc placenta shows reduced junctional zone in Wdr13 mutant placenta as compared to wild type. (C) Magnified image of PAS staining. (D) Masson’s trichrome staining of 17.5 dpc placenta shows increased maternal blood space and reduced number of trophoblast giant cells in labyrinth zone of Wdr13 mutant placenta as compared to wild type. Filled arrowhead shows fetal endothelial cells and closed arrowhead show S-TGC. (E) Quantification of number of sinusoidal trophoblast giant cells in labyrinth zone of Wdr13 mutant and wild type placenta (n = 4). (F) Quantification of maternal blood space (MBS) in labyrinth zone of Wdr13 mutant and wild type placenta (n = 4). (G) Expression of various genes involved in glucose and amino acid transport, angiogenesis and differentiation (AP 1 target genes) in 17.5 dpc Wdr13 mutant and wild type placenta (n = 4–6).
At least three possibilities exist for the reduction in the number of various trophoblast cells in Wdr13 mutant placenta: 1) reduced cell proliferation, 2) increased apoptosis and 3) affected EPC, chorion differentiation. So we performed cell proliferation assay in placenta by injecting BrdU in pregnant female mice. At 17.5 dpc there was no difference in number of BrdU positive in Wdr13 mutant and wild type placenta (Supplemental Fig. S1A). Next we performed apoptosis using TUNEL assay on placenta sections. At 15.5 dpc number of apoptotic cells were similar in both Wdr13 mutant and wild type placenta (Supplemental Fig. S1B). It appears that reduction in the number of various trophoblast cells in Wdr13 mutant placenta may be due to differentiation defects in EPC, chorion and their progenitors. So we performed gene expression analysis for gene(s) involved in solute transport, angiogenesis and differentiation. There was no difference in glucose and amino acid transporter in Wdr13 mutant and wild type placenta at 17.5 dpc (Fig. 3G). Interestingly, angiogenic gene angiopoietin-2 was significantly down regulated in Wdr13 mutant as compared to wild type placenta (Fig. 3G) where as there was no change in angiopoietin-1 and vascular endothelial growth factor (VEGF). Parallel study in our lab showed that Wdr13 activate AP1 target genes in presence of JNK signaling (Unpublished data). So we analyzed some AP1 target genes having role in placental trophoblast cell differentiation. We observed that cd44 was down regulated in Wdr13 null placenta as compared to wild type placenta whereas there was no change in axin2, igr5 and c-Jun levels.
The next set of experiments were sought to determine the effect of Wdr13 null placenta phenotype on postnatal growth of mice. As shown above that XWdr13X heterozygous placenta was deficient in WDR13 protein and XXWdr13 heterozygous placenta showed WDR13 protein levels as comparable to wild type while the two heterozygous female embryos (XWdr13X and XXWdr13) had comparable levels of Wdr13 expression (Fig. 2B). We measured the body weight of XX, XWdr13X and XXWdr13 females on normal chow as well as on high fat diet and found that on both diets regimes XX and XXWdr13 females showed higher body weight as compared to XWdr13X females from birth till termination of experiments (Fig. 4A,B). These results indicate that due to the impaired placental development in XWdr13X females their body weight is lower throughout their life suggesting that gene expression set during early development influenced their body weights during adulthood. This is a strong genetic evidence to support that during initial development placenta determines body weight for entire life.
Discussion
Numerous growth factors and genes have been implicated in placental growth, function and development16,17,18. Role of various WD repeat protein such as Fbxw8 and RACK1 have been shown in placental development19,20. In the present study, we show for the first time that Wdr13 gene, which is a member of WD repeat protein, expresses in placenta and the lack of this protein causes reduction in size of placenta. The reduction in placental size is mainly due to reduction in JZ and LZ (Table 1). Consequently, this reduction in size of placenta causes reduced body weight of embryo after birth.
Wdr13 gene expresses in placenta in decidua, SpT of JZ and in LZ. The reduction in the number of various trophoblast cell type indicates role of Wdr13 gene during the development of placenta. These trophoblast cells are derived from EPC and chorion4. P-TGCs, S-TGCs and syncytiotrophoblast cells originate from Tpbpa− cells whereas SpT originates from Tpbpa+ cells of EPC and chorion4. So it is unlikely that Wdr13 has specific role in particular cell type. At present we do not know the expression of Wdr13 in EPC, chorion; and Wdr13 mutant placenta shows weight difference only after 15.5 dpc. So it is likely that Wdr13 has role in these cell types only in late stages of development. To further understand the cell type specific expression of Wdr13 in placenta requires experimentations using cell type specific markers.
Placental angiogenesis is regulated by vascular endothelial growth factor (VEGF) and its high affinity receptor tyrosine kinases VEGFR-1, VEGFR-2 along with angiopoietin-1 (Ang-1), angiopoietin-2 (Ang-2) and its receptor Tunicainterna endothelial cell kinase-2 (Tie-2)21,22,23. VEGF, Ang-1 and Ang-2 are expressed in cytotrophoblast, syncytiotrophoblast and endothelial cells with in placenta24,25. VEGF and Ang-1 promote angiogenesis whereas Ang-2 antagonize Ang-1 function in endothelial cells26. In trophoblast cells Ang-2 and to a lesser extent Ang-1 promote DNA synthesis and cell growth21. In Wdr13 knockout placenta we observed a reduction in Ang-2 mRNA levels as compared to that in wild type placenta, while the levels of VEGF and Ang-1 were comparable between these two genotypes (Fig. 3G). Reduced level of Ang-2 mRNA may be one of the contributing factors in reduction of placental growth of Wdr13 mutant mice. In the case of human placenta, down regulation of Ang-2 is associated with intrauterine growth restriction of placenta21. However, at this time we do not know the reduction of Ang-2 mRNA is in endothelial cells or trophoblast cells, which requires further experimentation.
Ang-2 promoter contains AP-1 sites27 and parallel studies in our lab have shown that WDR13 activates AP1 target genes in presence of JNK signaling by interacting with c-Jun (Unpublished data). Thus, it is possible that down regulation of Ang-2 gene is directly regulated by WDR13. In support of these results analysis of gene expression of various AP1 target genes showed reduced expression of cd44 in Wdr13 knockout placenta as compared to wild type placenta (Fig. 3G). cd44 gene expresses in placenta and is important for cell migration and differentiation28,29. Various AP1 components like JunB and Fra1 are essential for placenta formation particularly in LZ30,31. So it appears that reduced expression of AP1 target genes may responsible for reduced placenta weight in Wdr13 mutant mice.
The relation between placental size and body weight of young one is well documented and lower birth weight is associated with metabolic syndrome including type 2 diabetes, insulin resistance, dyslipidemia and non-alcoholic fatty liver disease32,33. Due to preferential inactivation of paternal X-chromosome XWdr13X placenta shows characteristics Wdr13 null placenta where as XXWdr13 placenta shows characteristics of wild type placenta. Due to different characteristics of these two types of placenta, XWdr13X heterozygous female shows less body weight as compared to XXWdr13 females. Interestingly, both XWdr13X and XXWdr13 heterozygous females have same genetic contents and similar levels of Wdr13 expression (Fig. 2B) but they differ only in placental environment. What is the impact of reduced body weight in later stages of life and how will these two heterozygous females develop in wild type placental environment is not understood at present? Detailed metabolic parameter analysis and tetraploid complementation experiments in order to maintain a wild type placental environment for these two types of heterozygote females will be needed to understand these questions.
In summary, we provide evidence for role of Wdr13 gene in mouse placenta. Wdr13 gene expresses in placenta and Wdr13 mutant placenta shows reduced size and reduced number of S-TGC in LZ. The reduction in placental size results in reduced embryo weight. We provide genetic evidence to support that initial placental development determines the body weight for the entire life.
Methods
Animal experimentation
All the mice experiments were approved by and performed under guidelines of the institutional animal ethics committee of CSIR-Centre for Cellular and Molecular Biology, Hyderabad, India. All methods were carried out in accordance with the approved guidelines. Wdr13 mutant mice on CD1 genetic background were genotyped by PCR as described previously using primers mentioned in Supplemental Table S112. Wdr13 heterozygous females were crossed with wild type male to generate wild type, knockout and maternally derived heterozygous (XWdr13X) placenta and embryos. Hemizygous males were crossed with wild type females to generate paternally derived heterozygous (XXWdr13) placenta and embryos. At given time point of pregnancy female mice were dissected and weight of individual placenta and embryos were recorded. Genotyping of individual placenta and embryo was confirmed by isolating DNA from embryo tail as described previously12. The gender of individual placenta and embryo was confirmed by using Sry primers (Supplemental Table S1). Maternally derived heterozygous (XWdr13X), paternally derived heterozygous (XXWdr13) and wild type females body weight was measured fortnightly on normal diet and on high fat diet.
Uterine transfer of mouse embryos
Isolation and transfer of mouse embryo in oviduct were performed as described previously34. Briefly, fertilized eggs were isolated from oviduct of pregnant mice at 0.5 dpc and incubated in M16 medium. Same day embryos were transferred to oviduct of pseudo-pregnant female (CD1) after mating with vasectomized male.
Histochemistry, in situ hybridization, cell proliferation and apoptotic assay
Placentas were collected in PBS, fixed in 4% paraformaldehyde overnight and processed as described previously12. Briefly, 4 μm thick paraffin embedded sections were made and mounted on positively charged slides (Fischer scientific). Slides were stained with Hematoxylin-Eosin (Sigma) for visualizing tissue morphology by light microscopy. Periodic acid-Schiff (Sigma) and Masson’s trichrome (Sigma) staining were performed using standard procedure. For RNA in situ of Wdr13 gene in placenta, primer pairs 5′-aacgcctaccgtacaccaac-3′ and 5′-acatggtacatgcctgcaaa-3′ were used to generate the anti sense and sense probe. Paraffin embedded placenta sections were used to perform RNA in situ using DIG RNA labeling kit from Roche applied sciences (cat no-11175025910) as per manufacturers instructions. To assay the cell proliferation in placenta pregnant female mice were injected BrdU (100 mg/kg body weight), sacrificed 1.5h after injection and fixed and processed as described above. BrdU staining was performed using Anti-BrdU Antibody (Sigma) followed by staining with anti mouse HRP conjugated secondary antibody and developed with DAB substrate (Cat no-11718096001, Roche). The number of positive cells were counted manually using Axioskop (Axivision software). TUNEL assays were performed using Dead END Fluorometric TUNEL System (Promega). Images were recorded in confocal microscope and TUNEL-positive cells were counted manually from the images from total of 4 mice per genotype.
Data quantification and statistical analysis
Cavalieri technique (Simple grid method) was used to measure area of placenta, JZ, LZ and MBS2,35. Total placenta area and area of various placental layer were quantified using ImageJ software (NIH; http://rsb.info.nih.gov/ij/) on H&E stained samples. A minimum of four placenta for each genotype and at least three sections from each placenta was used to quantify placental area. For counting S-TGCs in labyrinth layer Masson’s trichrome stained placenta were imaged with 1000X magnification. At least four placentas from each genotype were analyzed with three to four field from each placenta. For MBS Masson’s trichrome stained placenta were imaged and ImageJ software was used to calculate MBS per unit area. MBS was calculated from minimum of four placentas per genotype with three to four field per placenta. For determining statistical significance an unpaired two-tailed student’s t-test with unequal variance was used to compare genotypes.
RNA isolation, real time PCR and western blot analysis
Total RNA from 17.5 dpc placenta was isolated using RNeasy Mini Kit (Qiagen) and reverse transcription was performed using ImProm-IITM kit (Promega) after DNase (Promega) treatment of RNA samples. Real time PCR was performed for various genes using Syber Green master mix (Invitrogen) and list of primers sequences is provided in Supplemental Table S1. For western blot analysis, 17.5-dpc placentas were lysed in RIPA buffer, quantified and blotted on PVDF membrane. Anti-WDR13 purified antibody (HPA000913) from Sigma and beta actin (sc-47778) from Santacruz were used for visualization of the protein.
Additional Information
How to cite this article: Singh, V. P. et al. Role of mouse Wdr13 in placental growth; a genetic evidence for lifetime body weight determination by placenta during development. Sci. Rep. 5, 13371; doi: 10.1038/srep13371 (2015).
References
Cross, J. C. How to make a placenta: mechanisms of trophoblast cell differentiation in mice—a review. Placenta 26 Suppl A, S3–9 (2005).
Georgiades, P., Ferguson-Smith, A. C. & Burton, G. J. Comparative developmental anatomy of the murine and human definitive placentae. Placenta 23, 3–19 (2002).
Watson, E. D. & Cross, J. C. Development of structures and transport functions in the mouse placenta. Physiology (Bethesda) 20, 180–193 (2005).
Simmons, D. G., Fortier, A. L. & Cross, J. C. Diverse subtypes and developmental origins of trophoblast giant cells in the mouse placenta. Dev Biol 304, 567–578 (2007).
Cross, J. C., Werb, Z. & Fisher, S. J. Implantation and the placenta: key pieces of the development puzzle. Science 266, 1508–1518 (1994).
Haggarty, P., Allstaff, S., Hoad, G., Ashton, J. & Abramovich, D. R. Placental nutrient transfer capacity and fetal growth. Placenta 23, 86–92 (2002).
Riley, P., Anson-Cartwright, L. & Cross, J. C. The Hand1 bHLH transcription factor is essential for placentation and cardiac morphogenesis. Nat Genet 18, 271–275 (1998).
Coan, P. M., Conroy, N., Burton, G. J. & Ferguson-Smith, A. C. Origin and characteristics of glycogen cells in the developing murine placenta. Dev Dyn 235, 3280–3294 (2006).
Bouillot, S., Rampon, C., Tillet, E. & Huber, P. Tracing the glycogen cells with protocadherin 12 during mouse placenta development. Placenta 27, 882–888 (2006).
Suresh, A. et al. A mouse gene encoding a novel member of the WD family of proteins is highly conserved and predominantly expressed in the testis (Wdr13). Mol Reprod Dev 72, 299–310 (2005).
Singh, B. N. et al. A highly conserved human gene encoding a novel member of WD-repeat family of proteins (WDR13). Genomics 81, 315–328 (2003).
Singh, V. P. et al. Lack of Wdr13 gene in mice leads to enhanced pancreatic beta cell proliferation, hyperinsulinemia and mild obesity. PLoS One 7, e38685 (2012).
Singh, V. P. et al. Genetic deletion of Wdr13 improves the metabolic phenotype of Lepr (db/db) mice by modulating AP1 and PPARgamma target genes. Diabetologia 58, 384–392 (2015).
Reik, W. & Walter, J. Genomic imprinting: parental influence on the genome. Nat Rev Genet 2, 21–32 (2001).
Reik, W. & Lewis, A. Co-evolution of X-chromosome inactivation and imprinting in mammals. Nat Rev Genet 6, 403–410 (2005).
Jones, R. L., Stoikos, C., Findlay, J. K. & Salamonsen, L. A. TGF-beta superfamily expression and actions in the endometrium and placenta. Reproduction 132, 217–232 (2006).
Reynolds, L. P. et al. Animal models of placental angiogenesis. Placenta 26, 689–708 (2005).
Cross, J. C. et al. Genes, development and evolution of the placenta. Placenta 24, 123–130 (2003).
Wang, C. C., Lo, H. F., Lin, S. Y. & Chen, H. RACK1 (receptor for activated C-kinase 1) interacts with FBW2 (F-box and WD-repeat domain-containing 2) to up-regulate GCM1 (glial cell missing 1) stability and placental cell migration and invasion. Biochem J 453, 201–208 (2013).
Tsunematsu, R. et al. Fbxw8 is essential for Cul1-Cul7 complex formation and for placental development. Mol Cell Biol 26, 6157–6169 (2006).
Dunk, C. et al. Angiopoietin-1 and angiopoietin-2 activate trophoblast Tie-2 to promote growth and migration during placental development. Am J Pathol 156, 2185–2199 (2000).
Albrecht, E. D., Babischkin, J. S. & Pepe, G. J. Regulation of placental villous angiopoietin-1 and -2 expression by estrogen during baboon pregnancy. Mol Reprod Dev 75, 504–511 (2008).
Ferrara, N. & Davis-Smyth, T. The biology of vascular endothelial growth factor. Endocr Rev 18, 4–25 (1997).
Clark, D. E., Smith, S. K., Sharkey, A. M. & Charnock-Jones, D. S. Localization of VEGF and expression of its receptors flt and KDR in human placenta throughout pregnancy. Hum Reprod 11, 1090–1098 (1996).
Goldman-Wohl, D. S., Ariel, I., Greenfield, C., Lavy, Y. & Yagel, S. Tie-2 and angiopoietin-2 expression at the fetal-maternal interface: a receptor ligand model for vascular remodelling. Mol Hum Reprod 6, 81–87 (2000).
Maisonpierre, P. C. et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 277, 55–60 (1997).
Hegen, A. et al. Expression of angiopoietin-2 in endothelial cells is controlled by positive and negative regulatory promoter elements. Arterioscler Thromb Vasc Biol 24, 1803–1809 (2004).
St Jacques, S., Dadi, H. K. & Letarte, M. CD44 in human placenta: localization and binding to hyaluronic acid. Placenta 14, 25–39 (1993).
Marzioni, D. et al. Hyaluronate and CD44 expression patterns in the human placenta throughout pregnancy. Eur J Histochem 45, 131–140 (2001).
Schorpp-Kistner, M., Wang, Z. Q., Angel, P. & Wagner, E. F. JunB is essential for mammalian placentation. EMBO J 18, 934–948 (1999).
Schreiber, M. et al. Placental vascularisation requires the AP-1 component fra1. Development 127, 4937–4948 (2000).
Lindsay, R. S. et al. Type 2 diabetes and low birth weight: the role of paternal inheritance in the association of low birth weight and diabetes. Diabetes 49, 445–449 (2000).
Nobili, V., Alisi, A., Panera, N. & Agostoni, C. Low birth weight and catch-up-growth associated with metabolic syndrome: a ten year systematic review. Pediatr Endocrinol Rev 6, 241–247 (2008).
Hogan, B., Beddington, R., Constantini, F. & Lacy, E. Manipulating the Mouse Embryo: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY (1994).
Coan, P. M., Ferguson-Smith, A. C. & Burton, G. J. Developmental dynamics of the definitive mouse placenta assessed by stereology. Biol Reprod 70, 1806–1813 (2004).
Acknowledgements
We are grateful to Prof. Graham J Burton (Centre for Trophoblast Research, University of Cambridge) and Prof. Claudia Kappen (Pennington Biomedical Research Center) for their guidance and suggestions. We thank Chandrashekaran and Archana B. Siva for reviewing the manuscript. CSIR-Centre for Cellular and Molecular Biology, Hyderabad and Department of Biotechnology, India financially supported VPS through a research fellowship.
Author information
Authors and Affiliations
Contributions
V.P.S. researched data, wrote manuscript J.L.A. researched data, J.L. researched data, P.S. researched data, T.A.R. researched data, S.K. contributed to discussion, reviewed/edited manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Singh, V., Alex, J., Lakshmi, B. et al. Role of mouse Wdr13 in placental growth; a genetic evidence for lifetime body weight determination by placenta during development. Sci Rep 5, 13371 (2015). https://doi.org/10.1038/srep13371
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep13371
This article is cited by
-
Molecular characterization of Wdr13 knockout female mice uteri: a model for human endometrial hyperplasia
Scientific Reports (2020)
-
WD-repeat protein WDR13 is a novel transcriptional regulator of c-Jun and modulates intestinal homeostasis in mice
BMC Cancer (2017)
-
Phosphoinositide 3-Kinase (PI3K) Subunit p110δ Is Essential for Trophoblast Cell Differentiation and Placental Development in Mouse
Scientific Reports (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.