Abstract
Er3+ activated germanate glasses modified by La2O3 and Y2O3 with good thermal stability were prepared. 2.7 μm fluorescence was observed and corresponding radiative properties were investigated. A detailed discussion of J–O parameters has been carried out based on absorption spectra and Judd–Ofelt theory. The peak emission cross sections of La2O3 and Y2O3 modified germanate glass are (14.3 ± 0.10) × 10−21 cm2 and (15.4 ± 0.10) × 10−21 cm2, respectively. Non-radiative relaxation rate constants and energy transfer coefficients of 4I11/2 and 4I13/2 levels have been obtained and discussed to understand the 2.7 μm fluorescence behavior. Moreover, the energy transfer processes of 4I11/2 and 4I13/2 level were quantitatively analyzed according to Dexter’s theory and Inokuti–Hirayama model. The theoretical calculations are in good agreement with the observed 2.7 μm fluorescence phenomena. Results demonstrate that the Y2O3 modified germanate glass, which possesses more excellent spectroscopic properties than La2O3 modified germanate glass, might be an attractive candidate for mid-infrared laser.
Similar content being viewed by others
Introduction
In recent decades erbium doped materials have become a research hotspot due to 2.7 μm mid-infrared emission, which has wide applications in both civilian and military fields such as remote sensing, atmosphere pollution monitoring, eye-safe laser radar, medical surgery and precision guidance1,2,3,4. Er3+ is an ideal luminescent center for 2.7 μm emission corresponding to the 4I11/2 → 4I13/2 transition. However, for developing more efficient optical devices, besides the active ions, the host material must be considered as well. Thus, seraching for suitable host materials for mid-infrared lasers operating in this wavelength is essential.
Glass continues to attract considerable research interest owing to its feasibility of fabrication and ability to be used as high power solid state laser hosts. The host glasses for mid-infrared emission are expected to possess a minor absorption coefficient in the typical OH− absorption at ~3 μm, low nonradiative decay rates and high radiative emission rates5,6. Therefore, a lot of investigations have been focused on fluoride and chalcogenide glasses, especially fluoride glass, mainly owing to their low phonon energy and low OH− content. Although chalcogenide glass has quite low phonon energy and larger refractive index, its preparation process is fairly complex7. Compared to chalcogenide glass, fluoride glass is easily prepared and possesses low phonon energy, high rare earth solubility and extremely low OH− content8,9. Moreover, the fluorozirconate system (ZBLAN) has been made into fiber laser in 1999 and its output power can reach 1.7 W2. Nevertheless, the low mechanical strength and damage threshold limit its further applications in high power or energy fiber laser systems.
The thermal stability, chemical durability, mechanical strength of germanate glass are superior to fluoride glass8. Besides, germanate glass also possesses higher solubility of rare earth ions than chalcogenide glass7. Moreover, germanate glass has the advantages of low phonon energy and good infrared transmission in a wide wavelength range compared with other oxide glass10,11. Thus, from the viewpoint of technological application, germanate glass is quite suitable as host material for mid-infrared laser12. Particularly, barium gallo-germanate (BGG) glass has been investigated extensively as an exist window for high energy lasers operating in the infrared wavelength region13,14. However, BGG glass has the disadvantages of high melting temperature, high viscosity and a large number of hydroxyl groups15,16. A high concentration of hydroxyl groups might lead to a strong absorption band around 2.7 μm and it is harmful for corresponding mid-infrared emission. To address these questions, some fluorine could be added into germanate glass since fluoride ions (F−) proved to be capable of decreasing melt viscosity and minimizing the OH− content15,16,17. Additionally, germanate glass can be modified by adding or substituting other components such as La2O3 and Y2O3. The addition of La2O3 is expected to improve glass forming ability, while Y2O3 component is expected to improve thermal stability and further reduce OH− content in glass due to its collection of non-bridging oxygen of glass18.
Although the difference of thermal and physical properties of La2O3 and Y2O3 modified germanate glass has been investigated by Jewell et al.19, there are few reports on the spectroscopic properties and mid-infrared emissions of La2O3 and Y2O3 modified germanate glass. In this paper, the thermal stability and spectroscopic properties of GeO2-Ga2O3-BaO-R2O3-5NaF-Er2O3 system (R = La, Y) under the excitations of 808 nm LD were investigated. The research of 2.7 μm spectroscopic properties and corresponding energy transfer mechanism in both La2O3 and Y2O3 modified germanate glasses has been carried out. Besides, energy transfer microscopic parameters were calculated via Dexter’s theory and Inokuti–Hirayama model for better understanding of energy transfer processes of Er3+ ions.
Experimental processes. Er3+ doped germanate glasses were synthesized by conventional melting method, which has the following molar compositions: 65GeO2-15Ga2O3-5BaO-(10-x)La2O3-x
Y2O3-5NaF-0.5Er2O3, (x = 0, 10), denoted as GL, GY, respectively. Samples were synthesized by using high-purity of GeO2, Ga2O3, BaO, La2O3, Y2O3, NaF and Er2O3 powders. The stoichiometric chemicals were well-mixed and melted at 1400 °C for 30 min in a covered alumina crucible. The melts were poured onto a preheated steel plate and pressed by another plate for shaping. After annealing at around glass transition temperature, all samples were cut and polished into 10 × 10 × 1.5 mm3 for further measurement.
Refractive indexes of samples were measured by prism minimum deviation method at the wavelength of 1053 nm. The resolution of the instrument was ±0.5 × 10−4. The densities were tested by Archimedes principle using distilled water as an immersion liquid with error limit of ±0.05%. Differential scanning calorimeter (DSC) curve is measured using NETZSCH DTA 404 PC at the heating rate of 10 K/min with maximum error of ±5 °C. Absorption spectra were recorded with a Perkin-Elmer- Lambda 900UV/VIS/NIR spectrophotometer in the range of 350-1640 nm. Photoluminescence spectra in the ranges of 2600–2800 nm and 1400–1700 nm were determined via a combined fluorescence lifetime and steady state spectrometer (FLSP 920) (Edingburg Co., England), which was detected with a liquid-nitrogen-cooled PbS detector using an 808 nm laser diode (LD) as an excitation source. The 808 nm LD with the same power was also utilized to measure the lifetimes of Er3+:4I11/2 and 4I13/2 levels. The lifetimes were calculated by fitting a single exponential function to the measured data. The same experimental conditions for different samples were maintained so as to get comparable results. All the measurements were performed at ambient temperature.
Results and Discussion
Thermal stability and density
Figure 1 shows the differential DSC curves for the prepared glasses. The glass transition temperature Tg, onset crystallization temperature Tx and thermal stability ΔT( = Tx–Tg) in various glasses are displayed in Table 1. It is found that the ΔT of GL and GY samples are 190 °C and 175 °C, respectively. Compared with ∆T, the glass formation factor, Kgl = (Tx-Tg)/(Tm-Tg), where Tm is the glass melting temperature, is more suitable to estimate the glass thermal stability. It is clear that Kgl of GL and GY can reach 0.26 and 0.25, respectively. Both ΔT and Kgl of prepared samples are larger than those of tellurite20, bismuth21 and germanate glass22, while is comparable to BGG glass19. The result suggests that the prepared germanate glasses have good glass forming ability and thermal stability.
Tg is an important factor for laser glass, which gives glass good thermal stability to resist thermal damage at high pumping intensities. The values of Tg of both prepared samples are substantially larger than the other glasses in Table 1. It is interesting to find that the Tg of GY is larger than GL, which is in accordance with the work of John M. Jewell19. The importance of the density for describing the structure of a glass is evident. The density of glass is mainly influenced by the molecular weight of glass components, the integration and the compactness of the glass network. The density of GL (4.76) is larger than GY (4.47) and a most possible reason for the decrement in density is ascribed to the smaller molecular weight of Y2O3 compared to La2O3. Both of the prepared glasses have a smaller density than BGG glass (4.85)13.
Absorption spectra and J-O analysis
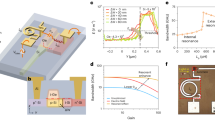
Figure 2 reveals the absorption spectra of Er3+ activated germanate glasses modified with La2O3 (GL) and Y2O3 (GY) at room temperature in wavelength region of 380–1600 nm. Absorption bands in this figure are labeled, which correspond to the transitions starting from the 4I15/2 ground state to higher 4I13/2, 4I11/2, 4I9/2, 4F9/2, 4S3/2, 2H11/2, 4F7/2, 4F5/2,3/2, 2H9/2 and 4G11/2 levels23. The shape and peak positions of each transition in present glasses are very similar to those of other Er3+ doped glasses24,25, except for some tiny divergences that originated from the different ligand field strength of host glasses. It is observed that two absorption peaks (4G11/2 → 4I15/2 and 2H11/2 → 4I15/2) are much stronger than other bands. They are sensitive to small changes of local environment around Er3+ ions, called hypersensitive transitions (HSTs)5. The inset of Fig. 2 displays the enlarged absorption spectrum in the range of 770–830 nm. Obvious absorption peaks around 808 nm manifested the prepared glasses can be pumped by low-cost 808 nm laser diodes (LDs).
Some important spectroscopic and laser parameters of rare earth doped glasses have been commonly analyzed by way of Judd-Ofelt (J-O) theory based on absorption data26,27,28. Details of the theory and method have been well described elsewhere29,30,31. Thus, only results will be presented here. The J-O intensity parameters Ωt (t = 2, 4, 6) of GL and GY glass were determined in Table 1. The root-mean-square deviation (δrms) in GL and GY glass is as low as 0.49 × 10−6, 0.29 × 10−6, respectively, proving the validity of the results and the reliable calculations.
It can be seen from Table 2 that the value of Ω2 in GY glass is higher than those of other Er3+ doped glasses. It is well known that Ω2 is strongly dependent on the RE3+ local environment and it is directly related to the symmetry or polarization of local structure and the covalence of chemical bonds formed by the RE3+ with its ligands. Based on this idea, the higher Ω2 in GY glass indicates the larger polarization of Y2O3 and asymmetry around Er3+ 32. Thus, the chemical bonds associated with the Er3+ ions is more covalent than those of silicate33, tellurite34, fluoride6 glasses as shown in Table 2. In addition, in this work, the Ω2 value of GL glass is lower than that of GY glass. According to the electronegativity theory, the covalency of the bond will become stronger with the decrease of the difference of electronegativity between cation and anion35. Since the values of electronegativity, for La, Y and O elements, are 1.1, 1.22, 3.5, respectively, the covalency of Y-O bond is stronger than that of La-O bond. This behavior will lead to the larger polarization of Y2O3 component than that of La2O3 and the asymmetry of the site occupied by Er3+ in GL glass is lower than that of GY glass. On the other hand, the Ω6 parameter is related to the overlap integrals of 4f and 5d orbit36. The Ω6 of GY is larger than those of GL, germanate37, tellurite34, silicate33 glasses, smaller than that of fluoride glass6.
Radiative properties
Since Smd is independent of ligand fields and Sed is a function of glass structure and composition38, in order to get flat emission spectrum, it can be effective to increase the relative contribution of the electric-dipole transition39. According to Judd-Ofelt theory, the line strength of the electric dipole components of 2.7 um emissions can be expressed as

According to Eq. (1), the Sed is mainly dominated by Ω6. From Table 2 it is noted that Ω6 in GY glass is higher than those of other various glasses except fluoride glass. Therefore, compared to GL glass, GY glass is more expected to be an appropriate host material that gets flat emission spectrum from the Er3+: 4I11/2 → 4I13/2 transitions.
Further calculation about spontaneous radiative transition probability (Arad), fluorescence lifetime (τrad) and branching ratios (β) of Er3+ various transition in prepared glasses are listed in Table 3. As is shown in Table 3, the GY glass possesses a larger Arad (36.21 s−1) than that of GL glass (35.03 s−1) for Er3+:4I11/2 → 4I13/2 respectively40. It is worth noting that both of prepared glasses possess evidently larger Arad than BGG glass (19 s−1)41. Furthermore, the values of β in both samples are comparable to those germanate glasses37,41.
Since multiphonon relaxation rate has a substantial impact on 2.7 μm emissions, a low nonradiative decay rate is required to achieve strong 2.7 μm fluorescence. The multiphonon relaxation rate constant (kmp) from a given excited state can be estimated from the energy-gap law42. The multiphonon relaxation rate constant (kmp) can be defined as,

where ΔE is the energy gap between the emitting level and the adjacent lower level. α and β are positive-definite constants depending on glasses. ћωmax is the highest phonon energy of the glass. Where  is the minimum number of phonons required to bridge the energy gap ΔE43. In this work, kmp is calculated using the parameters α = 4.6 × 10−3 cm, β = 6.1 × 107 s−1 and T = 300 K reported for germanate glass44. Via Eq. (2), the kmp values for Er3+:4I11/2 → 4I13/2 and 4I9/2 → 4I11/2 transitions of GL glass and GY glass are determined as shown in Table 3. The transition of 4I9/2 → 4I11/2 is proposed to be a multiphonon decay process compared to 4I11/2 → 4I13/2 transition. In addition, the kmp of 4I11/2 → 4I13/2 transition in both GL glass and GY glass are smaller than those of silicate glass (7.13 × 104 s−1)45 and other germanate glass (1.11 × 104 s−1)45, while comparable to fluorphosphate glass45.
is the minimum number of phonons required to bridge the energy gap ΔE43. In this work, kmp is calculated using the parameters α = 4.6 × 10−3 cm, β = 6.1 × 107 s−1 and T = 300 K reported for germanate glass44. Via Eq. (2), the kmp values for Er3+:4I11/2 → 4I13/2 and 4I9/2 → 4I11/2 transitions of GL glass and GY glass are determined as shown in Table 3. The transition of 4I9/2 → 4I11/2 is proposed to be a multiphonon decay process compared to 4I11/2 → 4I13/2 transition. In addition, the kmp of 4I11/2 → 4I13/2 transition in both GL glass and GY glass are smaller than those of silicate glass (7.13 × 104 s−1)45 and other germanate glass (1.11 × 104 s−1)45, while comparable to fluorphosphate glass45.
Fluorescence spectra at 2.7 μm
Figure 3 illustrates the mid-infraed emission spectra and cross sections of Er3+ doped GL and GY glasses pumped at 808 nm laser diode(LD). As shown in Fig. 3(a), the 2.7 μm emissions of both glasses can be observed. It is interesting to find that the 2.7 μm emission intensity of GY glass is stronger than GL glass. Some spectroscopic parameters have been calculated to better explain the interesting situation.
According to the Fuchtbauer-Ladenburg theory the 2.7 μm emission cross section (σem) can be calculated by

where λ is the emission wavelength, Arad is the spontaneous radiative transition probability of Er3+: 4I11/2 → 4I13/2 transition, c is the velocity of light in vacuum, n is the refractive index of glass host (GL:1.76 and GY: 1.73), I(λ) is the 2.7 μm fluorescence intensity and ∫I(λ)dλ is the integrated fluorescence intensity. Based on emission cross section, σem, absorption cross section (σabs) can be obtained by[51],

where Zl and Zu are the partition functions for the lower and the upper levels involved in the considered optical transition, respectively. T is the temperature (here is 300 K), k is the Boltzmann constant and λZL is the wavelength for the transition between the lower Stark sublevels of the emitting multiplets and the lower Stark sublevels of the receiving multiplets.
As shown in Fig. 3(b), the absorption and emission cross sections can be calculated by Eq. (3) and (4). It can be seen that the peak absorption cross sections at 2.7 μm of GL and GY glass are (10.3 ± 0.10) × 10−21 cm2 and (10.1 ± 0.10) × 10−21 cm2, respectively, the peak emission cross section are (14.3 ± 0.10) × 10−21 cm2 and (15.4 ± 0.10) × 10−21 cm2, respectively. Higher emission cross section means that better laser gain can be achieved in glass. It is found that the obtained σem for both glasses are higher than those of fluoride (9.16 × 10−21 cm2)6, bismuthate (7.73 × 10−21 cm2)21 and tungsten- tellurite glass (6.05 × 10−21 cm2)46.
In addition, according to Eq. (5), the effective emission bandwidths (Δλeff) have been obtained.

where  is the peak emission cross section at 2.7 μm. Since the 2.7 μm emission band of Er3+ ions in glass is asymmetric, it is more reasonable to select effective emission bandwidth other than the full width at half maximum as presented in Fig. 3(b). For broadband amplifier, it is required that effective emission bandwidth is as wide as possible to provide multiple channels for signal transmission. It is calculated from Fig. 3(a) that the Δλeff of GL and GY glass can reach 58.4 and 59.3 nm, which is larger than that of chalcogenide glass (56 nm)5. High effective emission bandwidth means that the prepared glasses have potential applications in broadband amplifier operating at 2.7 μm.
is the peak emission cross section at 2.7 μm. Since the 2.7 μm emission band of Er3+ ions in glass is asymmetric, it is more reasonable to select effective emission bandwidth other than the full width at half maximum as presented in Fig. 3(b). For broadband amplifier, it is required that effective emission bandwidth is as wide as possible to provide multiple channels for signal transmission. It is calculated from Fig. 3(a) that the Δλeff of GL and GY glass can reach 58.4 and 59.3 nm, which is larger than that of chalcogenide glass (56 nm)5. High effective emission bandwidth means that the prepared glasses have potential applications in broadband amplifier operating at 2.7 μm.
According to the σem(λ) and σabs(λ), the gain spectra (G(λ)) at 2.7 μm can be calculated by the report18. Figure 4 indicates the gain spectra of 2.7 μm of prepared glasses. Evidently, both GL and GY, when the population inversion P > 0.4, the gain cross sections in range of 2683-2772 nm become positive. It is suggested that Er3+ activated GL and GY glass is an attractive candidate for mid-infrared laser with low pump threshold.
The product of  ×
×  , defined as gain bandwidth, is another important parameter to evaluate the gain performances of prepared samples47. Larger gain bandwidth means better gain property of the material. Due to higher emission cross section and larger emission bandwidth, the GY glass has higher gain performance (9.13 × 10−26 cm3) than GL glass (8.35 × 10−26 cm3). From the above comparsion, it is roundly expected that GY glass have a better gain properties than GL glass at 2.7 μm, which is also in good with the 2.7 fluorescence intensity as shown in Fig. 3(a).
, defined as gain bandwidth, is another important parameter to evaluate the gain performances of prepared samples47. Larger gain bandwidth means better gain property of the material. Due to higher emission cross section and larger emission bandwidth, the GY glass has higher gain performance (9.13 × 10−26 cm3) than GL glass (8.35 × 10−26 cm3). From the above comparsion, it is roundly expected that GY glass have a better gain properties than GL glass at 2.7 μm, which is also in good with the 2.7 fluorescence intensity as shown in Fig. 3(a).
Energy transfer mechanism and microparameters
Figure 5 reveals the energy transfer process of Er3+ pumped by 808 nm LD. Under 808 nm pumping, the ions in Er3+: 4I15/2 level are excited to the 4I9/2 state by ground state absorption process (GSA). Then the ions in 4I9/2 level non-radiatively decay to 4I11/2 state by multiphonon relaxation process due to small energy gap between 4I9/2 and 4I11/2 level. The ions in 4I11/2 state are populated to 4F7/2 level owing to excited state absorption (ESA: 4I11/2 + a photon → 4F7/2) or energy transfer upconversion (ETU1: 4I11/2 + 4I11/2 → 4I15/2 + 4F7/2). Afterwards, ions in 4F7/2 level relax non-radiatively to 2H11/2 state due to multiphonon relaxation process. Due to small energy gaps among 2H11/2, 4S3/2 and 4F9/2 levels, ions in 2H11/2 state decay nonradiatively to 4S3/2 and 4F9/2 level. On the other hand, ions in 4I11/2 level can decay to lower 4I13/2 level by radiative or nonradiative process and radiative process generates 2.7 μm fluorescence. Finally, ions in 4I13/2 state relax radiatively to the ground state and 1.53 μm fluorescence occurs. Besides, ions in 4I13/2 level can also undergo ETU2 process (4I13/2 + 4I13/2 → 4I15/2 + 4I9/2), thus resulting in the further population accumulations of 4I9/2 level. The nonradiative rate of 4I9/2 → 4I11/2 transition is so large that ions in 4I9/2 level decay quickly to 4I11/2 level, which is beneficial to populations of the 4I11/2 level and 2.7 μm emissions. Moreover, as is discussed in above, for Er3+: 4I11/2 → 4I15/2 transition, GY has a higher multiphonon relaxation rate constant than GL, which makes it have a more active energy transfer process mentioned above. It is worth mentioning that the residual OH− of glass is to the disadvantage of mid-infrared emission. It can quench the 2.7 μm fluorescence via the following processes (4I11/2 + 0 → 4I13/2 + 1) in prepared glasses as depicted in Fig. 5. These energy transfer processes are harmful for mid-infrared emissions. Hence, it is necessary to minimize OH− content and weaken unwanted energy transfer from Er3+ to OH−.
To make clear of mid-infrared emission mechanism, a quantitative understanding of energy transfer process about Er3+: 4I11/2 level in present glasses is required. According to FÖster48 and Dexter49, the probability rate of energy transfer between donor and acceptor can be estimated as50,51

Where  is the matrix element of the perturbation Hamiltonian between initial and final states in energy transfer process, N is the total phonons in the transfer process m + k = N,
is the matrix element of the perturbation Hamiltonian between initial and final states in energy transfer process, N is the total phonons in the transfer process m + k = N,  is the integral overlap between the m-phonon emission sideband of donor ions and k-phonon absorption line shapes of acceptor ions. In our case, both donor and acceptor are Er3+ ions on 4I11/2 level. In the case of weak electron-phonon coupling, it is suitable for rare earth ions.
is the integral overlap between the m-phonon emission sideband of donor ions and k-phonon absorption line shapes of acceptor ions. In our case, both donor and acceptor are Er3+ ions on 4I11/2 level. In the case of weak electron-phonon coupling, it is suitable for rare earth ions.  can be approximated by
can be approximated by

Where  and
and  are the Huang-Rhys factors, SDA (0, 0, E) is the overlap between the zero phonon line shape of emission and absorption. Then the integral overlap in the case of m-phonon emission by the donor and no phonon involvement by the acceptor can be expressed as
are the Huang-Rhys factors, SDA (0, 0, E) is the overlap between the zero phonon line shape of emission and absorption. Then the integral overlap in the case of m-phonon emission by the donor and no phonon involvement by the acceptor can be expressed as

Where ΔE = mhω0. Since the measurements are carried out at some finite temperature T, the multi-phonon probability must be included and the emission cross section (σemis) with m phonon emission and absorption cross section (σabs) with k phonon absorption can be proposed as


Where E1 = mhω0, E2 = khω0 and ΔE = E1 + E2,  denotes the transiation of emission cross section wavelength by m-phonon emission,
denotes the transiation of emission cross section wavelength by m-phonon emission,  = 1/(1/λ- mhω0) and
= 1/(1/λ- mhω0) and  represents the translation of absorption cross section spectra wavelength due to k-phonon absorption
represents the translation of absorption cross section spectra wavelength due to k-phonon absorption  = 1/(1/λ + mhω0).
= 1/(1/λ + mhω0).
The energy transfer coefficient is then expressed by

In this work, both the donor and acceptor are Er3+ ions. Energy transfer properties of 4I11/2 and 4I13/2 level in GL and GY have been calculated using Eqs (6)–(11), and listed in Table 4. The results show that the energy transfer processes of Er3+: 4I11/2 and 4I13/2 level are scarcely phonon dependent. The energy transfer coefficient CD-A in GL and GY of Er3+: 4I13/2 level are as high as 50 × 10−40 and 52 × 10−40 cm6/s, respectively, but 4I11/2 level is 4.61 × 10−40 and 4.63 × 10−40 cm6/s, respectively. This suggests the energy of 4I13/2 level in present glasses can more efficiently transfer to the same level nearby compared with 4I11/2 level, which is helpful to deplete the populations of 4I13/2 level and promote population inversion between 4I11/2 and 4I13/2 level.
Figure 6 displays the decay curves of 4I11/2 and 4I13/2 level in both glasses pumped by 808 LD. It is found that the decay tendency of GY is slower than GL at both 975 and 1530 nm. To shed new light on the population behavior of 4I11/2 level and 4I13/2 level, the energy transfer processes of these energy levels were analyzed quantitatively on the basis of Inokuti–Hirayama (I-H) model. I-H model can also be used to estimate the energy transfer process among Er3+ ions, which is expressed as52,53

where s is 6, 8 or 10 depending on whether the dominant mechanism of interaction is dipole-dipole, dipole-quadrupole or quadrupole-quadrupole, respectively. τ0 is the intrinsic lifetime. The energy transfer parameter (Q) is defined as

where Γ(1-3/s) is equal to 1.77 for dipole-dipole interactions (s = 6), 1.43 for dipole- quadrupole interactions (s = 8) and 1.3 in the case of quadrupole-quadrupole interactions (s = 10). NEr is the concentration of Er3+ ions (in ions cm−3) and Rc is the critical transfer distance defined as the donor-acceptor separation for which the energy transfer rate is equal to the rate of intrinsic decay of the donors.
The decay curves of present samples have been well fitted by I-H model for s = 6 and the results are listed in Table 5. This indicates that the energy transfer among Er3+ ions takes place due to dipole-dipole interactions. From Table 5, it can be found that the energy transfer parameter Q for GY sample is lower than that of GL sample in 4I11/2 level while the Q value of GY sample is higher than that of GL sample in 4I13/2 level. The higher the value Q is, the stronger the energy transfer process becomes. It is indicated that higher Q of 4I13/2 level and lower Q of 4I11/2 level for GY sample are more beneficial for the population inversion between them and enhancing 2.7 μm emissions. It is in accordance with the result of Fig. 3(a).
Conclusions
Er3+ doped germanate glasses modified by La2O3 and Y2O3 with good thermal stability were prepared. 2.7 μm fluorescence was observed and corresponding radiative properties were investigated. J–O parameters have been discussed in detail based on absorption spectra and Judd–Ofelt theory. The peak emission cross sections of La2O3 and Y2O3 modified germanate glass are (14.3 ± 0.10) × 10−21 cm2 and (15.4 ± 0.10) × 10−21 cm2, respectively. To understand the 2.7 μm fluorescence, non-radiative relaxation rate constants and energy transfer coefficients of 4I11/2 and 4I13/2 levels have been obtained and discussed. Moreover, the energy transfer processes of 4I11/2 and 4I13/2 level were quantitatively analyzed according to Dexter’s theory and Inokuti–Hirayama model. The theoretical calculations are in good agreement with the observed 2.7 μm fluorescence phenomena. Results demonstrate that the Y2O3 modified germanate glass possesses more excellent spectroscopic properties than La2O3 modified germanate glass and might be an attractive candidate for mid-infrared laser materials.
Additional Information
How to cite this article: Cai, M. et al. R2O3 (R =La, Y) modified erbium activated germanate glasses for mid-infrared 2.7 µm laser materials. Sci. Rep. 5, 13056; doi: 10.1038/srep13056 (2015).
References
Yao, Y., Hoffman, A. J. & Gmachl, C. F. Mid-infrared quantum cascade lasers. Nat Photon 6, 432–439 (2012).
Jackson, S. D., King, T. A. & Pollnau, M. Diode-pumped 1.7-W erbium 3-μm fiber laser. Opt. Lett. 24, 1133–1135 (1999).
Pierce, M. C., Jackson, S. D., Dickinson, M. R., King, T. A. & Sloan, P. Laser-tissue interaction with a continuous wave 3-μm fibre laser: Preliminary studies with soft tissue. Laser Surg Med 26, 491–495 (2000).
Godard, A. Infrared (2–12 μm) solid-state laser sources: a review. C.R. Phys. 8, 1100–1128 (2007).
Tian, Y., Xu, R., Hu, L. & Zhang, J. 2.7 μm fluorescence radiative dynamics and energy transfer between Er3+ and Tm3+ ions in fluoride glass under 800nm and 980nm excitation. J. Quant. Spectrosc. Radiat. Transfer 113, 87–95 (2012).
Tian, Y., Xu, R., Hu, L. & Zhang, J. Spectroscopic properties and energy transfer process in Er3+ doped ZrF4-based fluoride glass for 2.7 μm laser materials. Opt. Mater. 34, 308–312 (2011).
Coleman, D. J. et al. in Advanced Solid State Lasers. MB10 (Optical Society of America).
Huang, F. et al. Sensitizing effect of Ho3+ on the Er3+: 2.7 μm-emission in fluoride glass. Opt. Mater. 36, 921–925 (2014).
Faucher, D., Bernier, M., Caron, N. & Vallée, R. Erbium-doped all-fiber laser at 2.94 μm. Opt. Lett. 34, 3313–3315 (2009).
Wei, T. et al. Optical spectroscopy and population behavior between 4I11/2 and 4I13/2 levels of erbium doped germanate glass. Opt. Mater.express 4, 2150–2165 (2014).
Xu, R., Tian, Y., Hu, L. & Zhang, J. Efficient ~2 μm emission and energy transfer mechanism of Ho3+ doped barium gallium germanate glass sensitized by Tm3+ ions. Appl. Phys. B 108, 597–602 (2012).
Zhang, Q. et al. Quantum Cutting in Tm3+/Yb3+ -Codoped Lanthanum Aluminum Germanate Glasses. J. Am. Ceram. Soc. 93, 654–657 (2010).
Bayya, S. S., Chin, G. D., Sanghera, J. S. & Aggarwal, I. D. Germanate glass as a window for high energy laser systems. Opt. Express 14, 11687–11693 (2006).
Jiang, X. P., Yang, Z. M., Liu, T. & Xu, S. H. Energy transfer between Yb3+ and Er3+ in barium gallogermanate glass. J. Appl. Phys. 105, 103113 (2009).
Bayya, S. S., Chin, G. D., Sanghera, J. S. & Aggarwal, I. D. Germanate glass as a window for high energy laser systems. Opt. Express 14, 11687–11693 (2006).
Cao, G., Lin, F., Hu, H. & Gan, F. A new fluorogermanate glass. J. Non-Cryst. Solids 326-327, 170–176 (2003).
Bai, G., Tao, L., Li, K., Hu, L. & Tsang, Y. H. Enhanced light emission near 2.7 μm from Er–Nd co-doped germanate glass. Opt. Mater. 35, 1247–1250 (2013).
Wei, T. et al. Mid-infrared fluorescence, energy transfer process and rate equation analysis in Er3+ doped germanate glass. Sci. Rep. 4, 10 (2014).
Jewell, J. M., Higby, P. L. & Aggarwal, I. D. Properties of BaO–R2O3–Ga2O3–GeO2 (R = Y, Al, La and Gd) Glasses. J. Am. Ceram. Soc. 77, 697–700 (1994).
Zheng, S. et al. Improvement of 1.53 μm band fluorescence and energy transfer in Er3+/Ce3+ codoped tellurite glasses. J. Alloys Compd. 566, 90–97 (2013).
Zhao, G., Wang, S., Fan, H. & Hu, L. Mid-infrared spectroscopic properties and energy transfer of Er3+/Yb3+ co-doped bismuth germanate glass. Spectrochim. Acta, Part A 101, 49–53 (2013).
Xu, R., Tian, Y., Hu, L. & Zhang, J. Enhanced emission of 2.7 μm pumped by laser diode from Er3+/Pr3+ -codoped germanate glasses. Opt. Lett. 36, 1173–1175 (2011).
Guo, Y., Gao, G., Li, M., Hu, L. & Zhang, J. Er3+-doped fluoro-tellurite glass: A new choice for 2.7 μm lasers. Mater. Lett. 80, 56–58 (2012).
Wei, T. et al. 2.7 μm fluorescence and energy transfer in Er3+ doped germanosilicate glasses. Mater. Res. Bull. 54, 20–23 (2014).
Wei, T., Chen, F., Tian, Y. & Xu, S. 1.53 μm emission properties in Er3+ doped Y2O3 and Nb2O5 modified germanate glasses for an optical amplifier. J. Lumin. 154, 41–45 (2014).
Guo, Y., Li, M., Hu, L. & Zhang, J. Intense 2.7 μm emission and structural origin in Er3+-doped bismuthate (Bi2O3-GeO2-Ga2O3-Na2O) glass. Opt. Lett. 37, 268 (2012).
Zhao, G., Tian, Y., Fan, H., Zhang, J. & Hu, L. Efficient 2.7-um emission in Er3+-doped bismuth germanate glass pumped by 980-nm laser diode. Chin Opt. Lett. 10, 091601–091603 (2012).
Wang, P. et al. Concentration effect of Nd3+ ion on the spectroscopic properties of Er3+/Nd3+ co-doped LiYF4 single crystal. Mater. Chem. Phys. 144, 349–354 (2014).
Nachimuthu, P. & Jagannathan, R. Judd-Ofelt Parameters, Hypersensitivity and Emission Characteristics of Ln3+ (Nd3+, Ho3+ and Er3+) Ions Doped in PbO-PbF2 Glasses. J. Am. Ceram. Soc. 82, 387–392 (1999).
Quimby, R. S. & Miniscalco, W. J. Modified Judd-Ofelt technique and application to optical transitions in Pr3+-doped glass. J. Appl. Phys. 75, 613–615 (1994).
Qiao, X., Fan, X., Wang, J. & Wang, M. Judd-Ofelt analysis and luminescence behavior of Er3+ ions in glass ceramics containing SrF2 nanocrystals. J. Appl. Phys. 99, 074302 (2006).
Tong, F., Risk, W. P., MacFarlane, R. M. & Lenth, W. 551 nm diode-laser-pumped upconversion laser. Electron. Lett 25, 1389–1391 (1989).
Xu, S. et al. Optical transitions and upconversion mechanisms in Er3+-doped heavy metal oxyfluoride germanate glass. J. Alloys Compd. 377, 253–258 (2004).
Reddy, A. A., Babu, S. S., Pradeesh, K., Otton, C. J. & Vijaya Prakash, G. Optical properties of highly Er3+-doped sodium–aluminium–phosphate glasses for broadband 1.5 μm emission. J. Alloys Compd. 509, 4047–4052 (2011).
Zhao, C., Yang, G. F., Zhang, Q. Y. & Jiang, Z. H. Spectroscopic properties of GeO2- and Nb2O5-modified tellurite glasses doped with Er3+. J. Alloys Compd. 461, 617–622 (2008).
Peng, Y.-P., Yuan, X., Zhang, J. & Zhang, L. The effect of La2O3 in Tm3+-doped germanate-tellurite glasses for ~2 μm emission. Sci. Rep. 4, (2014).
Wei, T. et al. Mid-infrared fluorescence of Y2O3 and Nb2O5 modified germanate glasses doped with Er3+ pumped by 808nm LD. Opt. Mater. 36, 1350–1356 (2014).
Tanabe, S. Optical transitions of rare earth ions for amplifiers: how the local structure works in glass. J. Non-Cryst. Solids 259, 1–9 (1999).
Tanabe, S., Ohyagi, T., Todoroki, S., Hanada, T. & Soga, N. Relation between the Ω6 intensity parameter of Er3+ ions and the 151 Eu isomer shift in oxide glasses. J. Appl. Phys. 73, 8451–8454 (1993).
Judd, B. Optical Absorption Intensities of Rare-Earth Ions. Phys. Rev. 127, 750–761 (1962).
Xiao, K. & Yang, Z. Thermal stability and optical transitions of Er3+/Yb3+-codoped barium gallogermanate glass. Opt. Mater. 29, 1475–1480 (2007).
van Dijk, J. M. F. On the nonradiative and radiative decay rates and a modified exponential energy gap law for 4f–4f transitions in rare-earth ions. J Chem Phys 78, 5317 (1983).
Markus, P., Hehlen, N. J. C. & Gosnell, T. R. Spectroscopic properties of Er3+ - and Yb3+-doped soda-lime silicate and aluminosilicate glasses. Phys. Rev. B 56, 9302–9318 (1997).
Payne, S. A., Chase, L., Smith, L. K., Kway, W. L. & Krupke, W. F. Infrared cross-section measurements for crystals doped with Er3+, Tm3+ and Ho3+. IEEE J. Quantum Electron. 28, 2619–2630 (1992).
Zou, X. & Izumitani, T. Spectroscopic properties and mechanisms of excited state absorption and energy transfer upconversion for Er3+-doped glasses. J. Non-Cryst. Solids 162, 68–80 (1993).
Guo, Y. et al. 2.7 mum emission properties of Er3+ doped tungsten-tellurite glass sensitized by Yb3+ ions. Spectrochim. Acta, Part A 111, 150–153 (2013).
Yi, L. X. et al. Emissions properties of Ho3+: 5I7→5I8 transition sensitized by Er3+ and Yb3+ in fluorophosphate glasses. Opt. Mater. 31, 1586–1590 (2009).
Förster, T. Zwischenmolekulare energiewanderung und fluoreszenz. Ann. Phys. 437, 55–75 (1948).
Dexter, D. L. A Theory of Sensitized Luminescence in Solids. J Chem Phys 21, 836 (1953).
Miyakawa, T. & Dexter, D. Phonon sidebands, multiphonon relaxation of excited states and phonon-assisted energy transfer between ions in solids. Physical Review B 1, 2961 (1970).
Tarelho, L., Gomes, L. & Ranieri, I. Determination of microscopic parameters for nonresonant energy-transfer processes in rare-earth-doped crystals. Phys. Rev. B 56, 14344 (1997).
Shanmugavelu, B., Venkatramu, V. & Ravi Kanth Kumar, V. V. Optical properties of Nd3+ doped bismuth zinc borate glasses. Spectrochim. Acta, Part A 122, 422–427 (2014).
Yamauchi, H., Senthil Murugan, G. & Ohishi, Y. Optical properties of Er3+ and Tm3+ ions in a tellurite glass. J. Appl. Phys. 97, 043505 (2005).
Acknowledgements
This research was financially supported by the Chinese National Natural Science Foundation (No. 51172252, 51372235, 61308090 and 51272243), Zhejiang Provincial Natural Science Foundation of China (No. LR14E020003, LY13F050003 and LY14E020007).
Author information
Authors and Affiliations
Contributions
M.Z.C. wrote the main manuscript text. B.E.Z., F.C.W. and T.W. checked up. J.J. Zhang. and S.Q.X. are responsible for the experiment. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Cai, M., Zhou, B., Wang, F. et al. R2O3 (R = La, Y) modified erbium activated germanate glasses for mid-infrared 2.7 μm laser materials. Sci Rep 5, 13056 (2015). https://doi.org/10.1038/srep13056
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep13056
This article is cited by
-
Controllable Synthesis of Monodisperse Er3+-Doped Lanthanide Oxyfluorides Nanocrystals with Intense Mid-Infrared Emission
Scientific Reports (2016)
-
Numerical analysis of 2.7 μm lasing in Er3+-doped tellurite fiber lasers
Scientific Reports (2016)
-
Er3+-doped transparent glass ceramics containing micron-sized SrF2 crystals for 2.7 μm emissions
Scientific Reports (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.