Abstract
Apoptosis of the splenic lymphocytes is often induced during the acute phase of Listeria infection in mice. However, the underlying mechanism remains incompletely understood. Here, we found that herpes virus entry mediator (HVEM) plays an important role for Listeria infection induced lymphocyte apoptosis. Mechanistically, HVEM is not directly involved in listeriolysin O (LLO) induced lymphocyte apoptosis or interferon beta induced T cell activation per se. Interestingly, HVEM is partially required for Listeria induced interferon (IFN)-I production in the spleen, particularly in macrophages. Consequently, the bystander activation of lymphocytes is significantly lower in HVEM deficient mice than that in wild-type (WT) mice upon Listeria infection. Thus, our results have revealed a novel role of HVEM on the regulation of IFN-I and immunopathology during Listeria infection.
Similar content being viewed by others
Introduction
Infection induced immunopathological injury is a common health risk for humans. The interplay between microbes and the host has been an intriguing topic in the field. L. monocytogens, a Gram-positive facultative intracellular bacterium, causes severe diseases in immunocompromised hosts1. Studies in murine models demonstrated acute and dramatic immunopathological injury of the lymphoid compartment in the spleen upon L. monocytogens infection2. The pathological lesion could occur as early as 24 h after infection and peaks around 48 h. Depending on the infection dose, the lesion is primarily found at T cell area and may extend to the B cell follicles3,4,5. Thus, L. monocytogens infection in mice represents an accessible and useful model to study the molecular and cellular mechanisms regulating the infection induced immunopathology.
Earlier studies have found that listeriolysin O (LLO), a member of the cholesterol-dependent cytolysin family of pore-forming proteins, is a major virulence factor of Listeria. Mainly known for its role in phagosome escape, LLO released extracellularly at infective foci can induce apoptosis of surrounding lymphocytes6. In addition, activated lymphocytes seem more susceptible to LLO induced apoptosis than resting cells5.
Induction of type I interferon (IFN-I) is a notable host innate response upon Listeria infection after their engulfment by phagocytic cells7,8. Production of IFN-I activates lymphocytes and sensitizes them to LLO induced apoptosis in a bystander way9. In line with this, deficiency of IFN-I receptor on lymphocytes rescues them from apoptosis and results in lower bacterial burden in the host10,11. Thus, Listeria seems to exploit host IFN-I response for its productive infection. How the host regulates Listeria induced IFN-I production remains largely unclear.
Herpes virus entry mediator (HVEM), a member of TNF receptor superfamily, was first identified as a mediator for entry of herpes simplex viruses (HSVs)12. Later work revealed that HVEM can act as both a receptor [for LIGHT (homologous to lymphotoxin, exhibits inducible expression and competes with the glycoprotein D for HVEM, a receptor expressed by lymphocytes)] and a ligand [for BTLA (B and T cell attenuator) and CD160], therefore delivers bidirectional signals in different types of cells13. Engagement of HVEM by LIGHT has been shown to transduce stimulatory pathways in T cells, NK cells and monocytes14,15,16,17. Ligation of HVEM to BTLA delivers inhibitory signals in T, B and dendritic cells (DCs)18,19,20. HVEM binding to CD160 may transduce either inhibitory or activating signals, depending on the scenarios21,22. Therefore, HVEM and its binding partners have been demonstrated diversified roles in regulating both innate and adaptive immune responses.
HVEM pathways have been found playing important roles in different types of cells during bacterial infection. HVEM was reported to enhance the bactericidal activity of human monocytes and neutrophils against Listeria and S. aureus17. In the mucosal epithelium, HVEM is required for signal transducer and activator of transcription 3 (STAT3) activation and host defense against pathological bacteria23. In the adaptive immunity, HVEM has been found to be important for the survival of the antigen activated CD8+ T cells during Listeria infection24. Interestingly, BTLA signaling was recently found promoting Listeria proliferation in CD8+ DCs, which also favors for long term T cell response25. While these studies have focused on the role of HVEM or BTLA for host defense, their impact on the host injury remains less clear. Understanding the mechanisms is necessary for better protection of the host when facing the infection.
In this study, we have investigated the role of HVEM on Listeria induced immunopathology. At acute phase of Listeria infection, obvious protection against splenic lesions was found in Hvem-/- hosts. Mechanistically, production of IFN-I in the spleen was found at significantly lower level in Hvem-/- mice than in WT mice. Thus, our study reveals a novel role of HVEM in IFN-I response during Listeria infection induced immunopathology. HVEM may act as a new host factor tipping the balance of host defense and host injury during infection.
Results
HVEM deficiency alleviates the splenic lesion during acute phase of Listeria infection
To study the role of HVEM in Listeria induced splenic pathological change, WT and Hvem-/- mice were infected i.p. with 107 CFU of L. Monocytogenes. In WT mice, obvious splenic pathology was observed 24 h after infection and the lesion became severer at 48 h (Fig. 1A). In stark contrast, much less lesions were seen in the spleens from Hvem-/- mice throughout the observation period (Fig. 1A). Consistent with the pathological change, the total cellularity of the spleen was dramatically decreased in WT mice upon infection, but largely maintained in Hvem-/- mice (Fig. 1B). Flow cytometry analysis of different subsets of lymphocytes demonstrated significantly more loss of CD4+, CD8+ T cells and B cells in the spleens of WT mice compared to those in Hvem-/- mice (Fig. 1C–E). To determine whether the lymphocytes of Hvem-/- mice are protected from apoptosis, splenocytes were collected 24 h after infection and stained with apoptosis markers for flow cytometry analysis. Indeed, significantly lower rate of apoptosis of both CD4+ and CD8+ T cells and B cells were found in Hvem-/- mice compared to those in WT mice (Fig. 1F,G). Thus, HVEM seems a detrimental host factor leading to lymphocyte apoptosis during acute L. Monocytogenes infection.
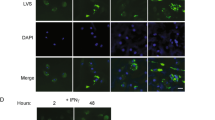
Alleviated spleen pathology in Hvem-/- mice during Listeria infection.
WT and Hvem-/- mice were uninfected or infected i.p. with 1 × 107 CFU of Listeria, 24 and 48 h after infection, spleens were examined by H&E (A), the total cellularity (B) and different subsets of lymphocytes (C–E) were counted. Original magnification is 20× for H&E staining. Error bar represents SEM. *P < 0.05; **P < 0.01; ***P < 0.001 (Student’s t-test). Data are representative from two experiments, n = 5 or 6 for each group in each experiment. (F,G) Mice were infected as described above, 24 h later, apoptosis of splenic lymphocytes was determined by Annexin V/7-AAD staining and FACS analysis. Data are pooled from two independent experiments, n = 5 or 6 for each group. Error bar represents SEM. *P < 0.05; **P < 0.01 (Student’s t-test).
HVEM is required for lymphocyte activation during Listeria infection
LLO can directly induce apoptosis of lymphocytes especially when lymphocytes are activated5. To determine whether HVEM expressed on lymphocytes intrinsically sensitizes them to LLO induced apoptosis, splenocytes from WT and Hvem-/- mice were harvested and treated with live L. Monocytogenes with different ratios of bacteria to splenocytes. 24 h after Listeria treatment, significant apoptosis of T cells was found by flow cytometry analysis. However, no difference was found for apoptosis rate between WT and Hvem-/- cells (Fig. S1). Therefore, it is unlikely that HVEM intrinsically regulates LLO induced lymphocyte apoptosis.
Since activated lymphocytes are more susceptible to LLO induced apoptosis than resting cells and HVEM has been well documented as a costimulatory molecule for T cell activation, we asked whether HVEM may promote T cell bystander activation upon Listeria infection. To this end, WT and Hvem-/- mice were infected with 107 CFU of L. Monocytogenes as before. 24 h later, activation status of lymphocytes was assayed by staining the activation marker CD69 and flow cytometry analysis (Fig. 2A,B). While nearly 40% of T cells and 60% of B cells were positive for CD69 surface expression in WT mice 24 h after Listeria infection, dramatically fewer CD69+ lymphocytes were found in Hvem-/- mice (Fig. 2C). The impaired lymphocyte activation found in Hvem-/- mice is well consistent with the reduced apoptosis of lymphocytes.
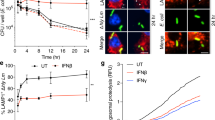
HVEM is required for lymphocyte activation during infection.
WT and Hvem-/- mice were infected as described above, 24 h later, splenic lymphocyte activation was determined by CD69 staining and FACS analysis with cells from uninfected mice as control. Representative FACS plots are shown in (A and B). Gate percentages indicate CD69+ cells. Statistical analysis is shown in (C). Data are pooled from two independent experiments, n = 7 or 8 for each group. Error bar represents SEM. *P < 0.05 (Student’s t-test).
HVEM does not intrinsically regulate lymphocyte activation upon Listeria infection
IFN-I has been found critical for Listeria infection induced lymphocyte activation26. We asked whether HVEM is directly involved in IFN-I induced lymphocyte activation. However, neither in vitro stimulation by IFN-β nor in vivo stimulation by poly(I:C) revealed difference on activation between WT and Hvem-/- lymphocytes (Fig. 3A–D). To further test whether HVEM intrinsically controls lymphocyte activation upon Listeria infection, bone marrow chimeric mice were generated with mixed bone marrow cells (with ratio of WT:HVEM = 1:1). 6–8 weeks after bone marrow transfer, chimeric mice were infected with Listeria and the lymphocyte activation was assayed as before (Fig. 3E). Comparable lymphocyte activation was found between WT and Hvem-/- compartments (Fig. 3F). These data suggest that HVEM does not intrinsically regulate T cell activation during Listeria infection.
HVEM does not intrinsically regulate lymphocyte activation.
(A,B) WT and Hvem-/- splenocytes were treated with IFN-β of different concentrations overnight in vitro, the expression of CD69 on CD4+ (A) and CD8+ (B) T cells was measured by FACS. Error bar represents SEM. No significance was found between WT and Hvem-/- groups (Student’s t-test). Data are representative from three experiments, n = 3 for each group in each experiment. (C) WT and Hvem-/- mice were treated with 100 μg poly(I:C) i.v. as depicted. 6 h later, splenocytes were harvested, CD69 expression on different subsets of lymphocytes was measured by FACS (D). Data are pooled from two independent experiments, n = 5 or 6 for each group. Error bar represents SEM. ns, nonsignificant (Student’s t-test). (E) Established chimeric mice with mixed bone marrows (WT : Hvem-/- = 1:1) were infected i.p. with 1 × 107 CFU Listeria as depicted. 24 h later, CD69 expression on different subsets of splenic lymphocytes was measured by FACS (F). Data are representative of two independent experiments, n = 3 for each group in each experiment. Error bar represents SEM. ns, nonsignificant (Student’s t-test). The drawing of the mouse is made by M.Z.
HVEM is partially required for IFN-I production in splenic macrophages for lymphocyte activation and apoptosis
The data described above indicate that HVEM may create an activating immune microenvironment for lymphocyte activation and/or apoptosis. IFN-I itself, as the most important activating factor for lymphocyte bystander activation during Listeria infection, was detected. IFN-I was rapidly induced upon Listeria infection in WT mice. Surprisingly, significantly lower IFN-I production was found in Hvem-/- mice as early as 24 h post infection as determined by RT-PCR for the total spleen and ELISA for the sera (Fig. 4A,B). Furthermore, significantly lower expression level of IFN-I was also found in purified splenic macrophages but not in DCs (Fig. 4C and data not shown). The impaired production of IFN-I is unlikely due to reduced bacterial burden in the spleen of Hvem-/- mice since comparable bacterial load was found between WT and Hvem-/- mice (Fig. S2A). It is also unlikely due to difference of bacterial load at cellular level in the macrophages (Fig. S2B). Therefore, it seems that HVEM controls the IFN-I expression specifically in macrophages on a per cell basis. Given the complicated molecular interactions between HVEM and its binding partners and their broad expression profiles, the cellular and molecular basis for HVEM to regulate IFN-I expression remains an interesting question for future study.
HVEM regulates IFN-I expression in splenic macrophages to enhance lymphocyte activation and apoptosis.
(A,B) WT and Hvem-/- mice were infected as described above, 24 and/or 48 h later, splenic expression of IFN-β was determined by qRT-PCR (A), IFN-β protein level in the sera was determined by ELISA (B). Samples from uninfected mice were also determined. Error bar represents SEM. *P < 0.05 (Student’s t-test). Data are pooled from three experiments for qRT-PCR assay, n = 6 to 8 for each group. For ELISA assay, data are representative from two independent experiments, n = 4 for each group in each experiment. (C) WT and Hvem-/- mice were infected as above, 24 h later, splenocytes from mice of each group were pooled and the macrophages were sorted by flow cytometry as a sample. Expression of IFN-β was determined by qRT-PCR. Data are pooled from five independent experiments, n = 5 for each group. *P < 0.05 (paired Student’s t-test). (D) WT and Hvem-/- mice were treated with 500 μg poly(I:C) i.v. and then infected with 1 × 107 Listeria i.p. as depicted. (E,F) 24 h later, activation and apoptosis of splenic lymphocytes were determined as in Fig. 1. Representative FACS plots are shown in (E and G); statistical analysis is shown in (F and H). Data are representative of two independent experiments, n = 3 for each group in each experiment. Error bar represents SEM. ns, nonsignificant (Student’s t-test). The drawing of the mouse is made by M.Z.
To further test the causal relationship between the lower expression level of IFN-I and the alleviated lymphocyte activation and apoptosis in Hvem-/- mice, mice were treated with poly(I:C) before Listeria infection (Fig. 4D). Poly(I:C) treatment induced comparable levels of IFN-I in both WT and Hvem-/- mice (Fig. S3). Indeed, both lymphocyte activation (Fig. 4E,F) and apoptosis rate (Fig. 4G,H) were elevated in Hvem-/- mice upon poly(I:C) treatment and reached to the level comparable to those in WT mice. Thus, HVEM deficiency does not confer lymphocytes inherent inability for activation upon IFN-I. IFN-I is downstream or independent of HVEM for the regulation of lymphocyte activation and apoptosis during Listeria infection.
HVEM deficiency results in diminished inhibitory effect of Listeria clearance
IFN-I is a detrimental factor to the host during Listeria infection. IFN-I inhibits interferon gamma receptor (IFNGR) expression on innate cells thus limiting their activation induced by IFN-γ27. In addition, IFN-I sensitizes Listeria induced lymphocyte apoptosis, which upregulates anti-inflammatory cytokine IL-10 therefore dampening Listeria control11. We asked whether impaired IFN-I production in Hvem-/- mice would result in rescued innate response, thus contribute to the Listeria resistance. We then examined IFNGR and IL-10 expression. Indeed, significantly higher level of IFNGR expression was found in Hvem-/- mice on both DCs and macrophages compared to that in WT mice upon Listeria infection (Fig. 5A,B). In addition, IL-10 expression level was also significantly lower in the spleens of Hvem-/- mice than that in WT mice upon Listeria infection (Fig. 5C). Together, these results provide new information how HVEM regulates innate immunity during Listeria infection.
HVEM deficiency results in diminished inhibitory effect of Listeria clearance.
(A,B) WT and Hvem-/- mice were infected as above, 24 h later, splenocytes from mice of each group were pooled, DCs and macrophages were sorted by flow cytometry. Expression of IFNGR in DCs (A) and macrophages (B) were determined by qRT-PCR. Cells from uninfected mice were also determined. Data are pooled from three independent experiments, n = 3 or 4 for each group. *P < 0.05 (Student’s t-test). (C) WT and Hvem-/- mice were infected as described above, 24 and 48 h later, mRNA level of IL-10 in spleen was determined by qRT-PCR. Data are representative of two experiments, n = 3 to 5 for each group in each experiment. Error bar represents SEM. **P < 0.01 (Student’s t-test).
Discussion
IFN-I production is a common innate response during infections of numerous pathogens ranging from viruses, bacteria, parasites to fungi. Both protective and detrimental roles of IFN-I to the host have been reported7. Understanding how IFN-I production is regulated is an important topic in this field. Recent years have witnessed discoveries of increasing number of intracellular signaling molecules regulating IFN-I expression28. However, relatively little is known about its extracellular and intercellular regulation. Lymphotoxin beta receptor (LTβR), a member of TNF receptor superfamily has been reported to control IFN-I expression from fibroblasts during human or murine cytomegalovirus infection29,30. In addition, TNF has been also found to upregulate IFN-I from fibroblasts31. Whether other members of TNF receptor superfamily control IFN-I expression is unclear. In addition, whether IFN-I production from the immune cells could be regulated by members of TNF receptor superfamily remains elusive. Here, we have reported that HVEM is partially required for IFN-I expression in splenic macrophages upon Listeria infection.
Impaired expression of IFN-I in Hvem-/- mice is likely a major contributing factor resulting in the lower level of lymphocyte activation and alleviated lymphocyte apoptosis. In line with this, increasing IFN-I production by poly(I:C) treatment enhanced lymphocyte activation and apoptosis rate in Hvem-/- mice to levels comparable to those in WT mice. Furthermore, impaired IFN-I expression in Hvem-/- mice leads to diminished IL-10 expression and higher IFNGR expression, which provide further information how HVEM deficiency confers the host resistant to Listeria infection.
Several types of innate cells including macrophages and TNF-iNOS producing dendritic cells (Tip-DCs) have been reported for IFN-I production upon Listeria infection32,33,34. In our study, IFN-β RNA expression was found in both macrophages (defined as CD11b+ F4/80+) and Tip-DCs (defined as ly6ChiCD11b+ CD11c+ MHC-II+), with about 10 folds higher level in the latter cell population. However, given the larger amount of macrophages than Tip-DCs (40 folds more at 24 h after infection), macrophages may be still the major contributor of IFN-β. In our study, we interestingly found that it is the macrophage but not TipDCs that showed impaired IFN-I production in the HVEM deficiency. The mechanism for the cell type selective regulation by HVEM remains to be determined.
The underlying cellular and molecular mechanisms for HVEM to control IFN-I expression during Listeria infection remain elusive. Hvem-/- bone marrow derived macrophages seem to respond normally to Listeria stimulation compared with WT cells (data not shown). This suggests that HVEM does not intrinsically alter the capability of macrophage for IFN-I production induced by Listeria. More interestingly, our preliminary data indicate that HVEM may not function as a receptor for IFN-I regulation, since an agonistic anti-HVEM (14C1.1) does not promote Listeria induced IFN-I expression in macrophages (data not shown), although other stimuli or primary macrophages should be tested. Considering the complicated cellular interactions mediated by HVEM and its interacting partners, it would be intriguing to dissect which molecule (BTLA or CD160) interacts with HVEM, which cell interacts with macrophages and how the interaction works. Systemic comparison of the gene expression profile in macrophages from WT and Hvem-/- mice may provide cues for the underlying intracellular mechanisms. Conditional knockout of HVEM, BTLA, or CD160 would be valuable tools to address the cellular interactions in vivo.
In conclusion, our study has demonstrated a novel important role of HVEM in the regulation of Listeria induced IFN-I production and immunopathology. This may open a new avenue to understand and control the IFN-I response not only for Listeria infection, but also for other pathogens.
Methods
Mice
Hvem-/- mice were described previously35. WT C57BL/6 mice were purchased from Vital River laboratory Animal Technology Co. Beijing, China. All mice were housed under specific pathogen-free conditions in the animal care facilities at the Institute of Biophysics, Chinese Academy of Sciences. All animal experiments were performed in accordance with the guidelines of the Institute of Biophysics, Chinese Academy of Sciences, using protocols approved by the Institutional Laboratory Animal Care and Use Committee.
Listeria infection, treatment and determination of CFU
The recombinant Listeria strain Listeria-OVA was described previously36 and provided by Dr. Honglin Xu (National Vaccine and Serum Institute, Beijing, China). Listeria was grown in brain-heart infusion broth with 5 μg/ml erythromycin. For determination of bacterial load in tissues, WT and Hvem-/- mice were infected with 1 × 107 Listeria by i.p. injection. At indicated time points after infection, organs were homogenized and lysed in sterile water with 0.5% Triton-100. Serial dilutions were plated on brain-heart infusion agar plates. Colonies were counted after incubation at 37 °C for 2 days. For poly(I:C) treatment, mice were injected i.v. with 100 or 500 μg poly(I:C) (Sigma) dissolved in sterile saline.
Bone marrow transfer
Bone marrow chimeras were generated with 5 × 106 bone marrow cells from donor mice transplanted i.v. into lethally irradiated congenic C57BL/6 host mice. To generate mixed bone marrow chimaeras, 2.5 × 106 bone marrow cells were obtained from WT and Hvem-/- mice and mixed at a ratio of 1:1. Chimeras were given prophylactic water containing antibiotics and were analyzed 6–8 weeks after transplantation.
Histology
Spleens were fixed in 4% paraformaldehyde and embedded in paraffin. Sections were stained with hemotoxylin eosin (H&E). Slides were scanned on Leica SCN400 F slide scanner.
Flow Cytometry
The single cell suspension of spleen was prepared. The following antibodies were used for immunofluorescence staining: 7-AAD (BD Biosciences), F4/80(BM8), CD4(RM4-5), CD8(53-6.7), CD19(6D5), CD69(H1.2F3) all from eBioscience; Annexin V, CD11b(M1/70), CD11c(N418), Ly6C(HK1.4), MHC II(M5/114.15.2) all from Biolegend. Samples were acquired on BD LSRFortessa instrument and analyzed with FlowJo software. CD11b+ F4/80+ macrophages and Ly6C+ CD11b+ CD11c+ MHC II+ Tip-DCs were sorted on BD FACSAria III.
ELISA
Mice were infected i.p. with Listeria or injected i.v. with 100 μg poly(I:C). Sera were collected 24 h after infection or 6 h after poly(I:C) treatment. The levels of IFN-β were determined using Mouse IFN-β ELISA kit with pre-coated plates (Biolegend). Plates were read at 450 nm using a SpectraMax Plus (Molecular Devices, Sunnyvale, CA, USA).
Quantitative PCR
Total RNA from spleens was isolated with Trizol (Invitrogen). RNA from sorted cells was extracted by RNeasy Plus Mini Kit (Qiagen). RNA was digested with DNaseI and reverse transcribed into cDNA for real-time PCR. The levels of gene expression were normalized to β-actin. The following primers were used: β-actin, forward primer: 5′-ACACCCGCCACCAGTTCGC, reverse primer: 5′- ATGGGGTACTTCAGGGTCAGGGTCAGGATA; IFN-β, forward primer: 5′-CCATCCAAGAGATGCTCCAG, reverse primer: 5′-GTGGAGAGCAGTTGAGGACA; IFNGR, forward primer: 5′-CCTGTCGTATGCTGGGAATA, reverse primer: 5′-AATGTTGGTGCAGGAATCAG; IL-10, forward primer: 5′-CGCTGTCATCGATTTCTCC, reverse primer: 5′-ACACCTTGGTCTTGGAGCTT.
Statistical Analysis
All data were analyzed using unpaired two-tail Student’s t-test, except the mRNA levels of sorted cells was analyzed by paired two-tail Student’s t-test. Analyses were performed using GraphPad Prism software (GraphPad Software Inc., San Diego, CA, USA). A value of P < 0.05 was considered statistically significant (*P < 0.05; **P < 0.01; and ***P < 0.001).
Additional Information
How to cite this article: Lv, M. et al. Herpes virus entry mediator licenses Listeria infection induced immunopathology through control of type I interferon. Sci. Rep. 5, 12954; doi: 10.1038/srep12954 (2015).
References
Schlech, W. F., 3rd . Foodborne listeriosis. Clin Infect Dis 31, 770–775, 10.1086/314008 (2000).
Pamer, E. G. Immune responses to Listeria monocytogenes. Nat Rev Immunol 4, 812–823, 10.1038/nri1461 (2004).
Conlan, J. W. Early pathogenesis of Listeria monocytogenes infection in the mouse spleen. J Med Microbiol 44, 295–302 (1996).
Merrick, J. C., Edelson, B. T., Bhardwaj, V., Swanson, P. E. & Unanue, E. R. Lymphocyte apoptosis during early phase of Listeria infection in mice. Am J Pathol 151, 785–792 (1997).
Carrero, J. A., Calderon, B. & Unanue, E. R. Listeriolysin O from Listeria monocytogenes is a lymphocyte apoptogenic molecule. J Immunol 172, 4866–4874 (2004).
Portnoy, D. A., Auerbuch, V. & Glomski, I. J. The cell biology of Listeria monocytogenes infection: the intersection of bacterial pathogenesis and cell-mediated immunity. J Cell Biol 158, 409–414, 10.1083/jcb.200205009 (2002).
McNab, F., Mayer-Barber, K., Sher, A., Wack, A. & O’Garra, A. Type I interferons in infectious disease. Nat Rev Immunol 15, 87–103, 10.1038/nri3787 (2015).
Woodward, J. J., Iavarone, A. T. & Portnoy, D. A. c-di-AMP secreted by intracellular Listeria monocytogenes activates a host type I interferon response. Science 328, 1703–1705, 10.1126/science.1189801 (2010).
Carrero, J. A., Calderon, B. & Unanue, E. R. Type I interferon sensitizes lymphocytes to apoptosis and reduces resistance to Listeria infection. J Exp Med 200, 535–540, 10.1084/jem.20040769 (2004).
O’Connell, R. M. et al. Type I interferon production enhances susceptibility to Listeria monocytogenes infection. J Exp Med 200, 437–445, 10.1084/jem.20040712 (2004).
Carrero, J. A., Calderon, B. & Unanue, E. R. Lymphocytes are detrimental during the early innate immune response against Listeria monocytogenes. J Exp Med 203, 933–940, 10.1084/jem.20060045 (2006).
Montgomery, R. I., Warner, M. S., Lum, B. J. & Spear, P. G. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell 87, 427–436 (1996).
Steinberg, M. W., Cheung, T. C. & Ware, C. F. The signaling networks of the herpesvirus entry mediator (TNFRSF14) in immune regulation. Immunol Rev 244, 169–187, 10.1111/j.1600-065X.2011.01064.x (2011).
Watts, T. H. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol 23, 23–68, 10.1146/annurev.immunol.23.021704.115839 (2005).
Fan, Z. et al. NK-cell activation by LIGHT triggers tumor-specific CD8+ T-cell immunity to reject established tumors. Blood 107, 1342–1351, 10.1182/blood-2005-08-3485 (2006).
Croft, M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol 3, 609–620, 10.1038/nri1148 (2003).
Heo, S. K. et al. LIGHT enhances the bactericidal activity of human monocytes and neutrophils via HVEM. J Leukoc Biol 79, 330–338, 10.1189/jlb.1104694 (2006).
Murphy, T. L. & Murphy, K. M. Slow down and survive: Enigmatic immunoregulation by BTLA and HVEM. Annu Rev Immunol 28, 389–411, 10.1146/annurev-immunol-030409-101202 (2010).
Sun, Y. et al. B and T lymphocyte attenuator tempers early infection immunity. J Immunol 183, 1946–1951, 10.4049/jimmunol.0801866 (2009).
Murphy, K. M., Nelson, C. A. & Sedy, J. R. Balancing co-stimulation and inhibition with BTLA and HVEM. Nat Rev Immunol 6, 671–681, 10.1038/nri1917 (2006).
Cai, G. & Freeman, G. J. The CD160, BTLA, LIGHT/HVEM pathway: a bidirectional switch regulating T-cell activation. Immunol Rev 229, 244–258, 10.1111/j.1600-065X.2009.00783.x (2009).
Sedy, J. R. et al. CD160 activation by herpesvirus entry mediator augments inflammatory cytokine production and cytolytic function by NK cells. J Immunol 191, 828–836, 10.4049/jimmunol.1300894 (2013).
Shui, J. W. et al. HVEM signalling at mucosal barriers provides host defence against pathogenic bacteria. Nature 488, 222–225, 10.1038/nature11242 (2012).
Steinberg, M. W. et al. BTLA interaction with HVEM expressed on CD8(+) T cells promotes survival and memory generation in response to a bacterial infection. PLoS One 8, e77992, 10.1371/journal.pone.0077992 (2013).
Yang, X. et al. A BTLA-mediated bait and switch strategy permits Listeria expansion in CD8alpha(+) DCs to promote long-term T cell responses. Cell Host Microbe 16, 68–80, 10.1016/j.chom.2014.05.021 (2014).
Feng, H. et al. Listeria-infected myeloid dendritic cells produce IFN-beta, priming T cell activation. J Immunol 175, 421–432 (2005).
Rayamajhi, M., Humann, J., Penheiter, K., Andreasen, K. & Lenz, L. L. Induction of IFN-alphabeta enables Listeria monocytogenes to suppress macrophage activation by IFN-gamma. J Exp Med 207, 327–337, 10.1084/jem.20091746 (2010).
Porritt, R. A. & Hertzog, P. J. Dynamic control of type I IFN signalling by an integrated network of negative regulators. Trends Immunol 36, 150–160, 10.1016/j.it.2015.02.002 (2015).
Benedict, C. A. et al. Lymphotoxins and cytomegalovirus cooperatively induce interferon-beta, establishing host-virus detente. Immunity 15, 617–626 (2001).
Banks, T. A. et al. A lymphotoxin-IFN-beta axis essential for lymphocyte survival revealed during cytomegalovirus infection. J Immunol 174, 7217–7225 (2005).
Reis, L. F., Ho Lee, T. & Vilcek, J. Tumor necrosis factor acts synergistically with autocrine interferon-beta and increases interferon-beta mRNA levels in human fibroblasts. J Biol Chem 264, 16351–16354 (1989).
Stockinger, S. et al. Characterization of the interferon-producing cell in mice infected with Listeria monocytogenes. PLoS Pathog 5, e1000355, 10.1371/journal.ppat.1000355 (2009).
Dresing, P., Borkens, S., Kocur, M., Kropp, S. & Scheu, S. A fluorescence reporter model defines “Tip-DCs” as the cellular source of interferon beta in murine listeriosis. PLoS One 5, e15567, 10.1371/journal.pone.0015567 (2010).
Solodova, E., Jablonska, J., Weiss, S. & Lienenklaus, S. Production of IFN-beta during Listeria monocytogenes infection is restricted to monocyte/macrophage lineage. PLoS One 6, e18543, 10.1371/journal.pone.0018543 (2011).
Wang, Y. et al. The role of herpesvirus entry mediator as a negative regulator of T cell-mediated responses. J Clin Invest 115, 711–717, 10.1172/JCI22982 (2005).
Pope, C. et al. Organ-specific regulation of the CD8 T cell response to Listeria monocytogenes infection. J Immunol 166, 3402–3409 (2001).
Acknowledgements
We thank Dr. Yang-Xin Fu (The Department of Pathology and Committee on Immunology, The University of Chicago,) for providing some materials and helpful suggestions; Dr. Honglin Xu (Department of Virology, National Vaccine and Serum Institute, Beijing, China) for the recombinant Listeria stain Listeria-OVA. We are grateful for technical support from the Core Facilities of Institute of Biophysics, Chinese Academy of Sciences. This work was supported by grants from the Ministry of Science and Technology (2012CB518900 to M.Z.) and National Natural Science Foundation of China (81261130022 to M.Z.).
Author information
Authors and Affiliations
Contributions
M.L. and M.Z. designed the experiments and analyzed the data; M.L. conducted the experiments with some assistance of W.W.; Y.Z. contributed to preparation of some materials; M.L. and M.Z. wrote the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Lv, M., Wu, W., Zhang, Y. et al. Herpes virus entry mediator licenses Listeria infection induced immunopathology through control of type I interferon. Sci Rep 5, 12954 (2015). https://doi.org/10.1038/srep12954
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep12954
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.