Abstract
Despite some slight differences in symptomatology, differential diagnosis of methamphetamine-induced psychosis (MAP) versus schizophrenia can be challenging because both disorders present a large overlap in their clinical symptoms. However, a recent study has shown that near-infrared spectroscopy (NIRS) performed during a cognitive task can be a powerful tool to differentiate between these two disorders. Here, we evaluated verbal fluency task performance during NIRS in 15 patients diagnosed with MAP and 19 with schizophrenia matched for age and sex. We used prefrontal probes and a 24-channel NIRS machine to measure the relative concentrations of oxyhaemoglobin every 0.1 s during the task. For each patient, the neurocognitive function and clinical psychopathology were evaluated using the Positive and Negative Symptom Scale (PANSS) and the Brief Assessment of Cognition in Schizophrenia (BACS). Oxyhaemoglobin changes in the prefrontal cortex were significantly higher in the MAP group compared to those in the schizophrenia group, particularly in the right dorsolateral prefrontal cortex. In contrast, we found no significant difference in PANSS and BACS scores. Our findings suggest that NIRS measurement could be applied to differentiate patients with MAP from those with schizophrenia, even in cases where clinical symptoms are similar.
Similar content being viewed by others
Introduction
Methamphetamine (MA) intake causes psychotic symptoms such as paranoid delusions and hallucinations in some subjects that do not have pre-existing psychotic manifestations1,2. Methamphetamine-induced psychosis (MAP), which occurs in 10 to 60% of MA abusers3,4,5, is likely due to repeated administration or the use of high doses of MA6,7. MAP results in a lasting enhancement of dopamine release in the striatum and nucleus accumbens8 and therefore is usually well-treated with dopamine antagonists. On the other hand, schizophrenia is a psychiatric disorder that begins during adolescence and is typically characterized by hallucinations and paranoid delusions. The dopamine hypothesis of schizophrenia is attributable to the principal descriptive model of antipsychotic drug action. Because of this, there are similarities between MAP and schizophrenia, which range from clinical symptomatology to pharmacotherapeutical characteristics9.
Schizophrenia patients present both positive and negative symptoms; however, even though MAP patients obviously suffer from positive symptoms similar to schizophrenia10, the existence of negative symptoms in these patients is controversial. Previous reports showed that the severity of psychotic symptoms including negative symptoms observed in MAP and schizophrenia are almost identical11,12, whereas the negative symptoms evaluated by the Scale for the Assessment of Negative Symptoms (SANS) differ13. MAP and schizophrenia are usually diagnosed based on their symptoms and it can be difficult to distinguish schizophrenia from MAP or other drug use-associated disorders in case that the psychotic symptoms are clearly identical.
Patients with schizophrenia show a wide variety of neurocognitive deficits in verbal, working and implicit memory and social cognition14,15, which are related to aberrant gamma-aminobutyric acid signalling in the prefrontal cortex16,17. In a similar manner, patients with MAP also show similar cognitive dysfunctions in working memory and other executive functions18,19; which makes it challenging to distinguish MAP and schizophrenia based on clinical symptoms20,21. Furthermore, both diagnostic groups also share other psychiatric symptoms, such as impaired inhibitory control and impulsivity22,23. Interestingly, clinical investigations have suggested that exposure to MA may cause a persistent schizophrenia-like psychosis, which stirs debate as to whether persistent MAP is distinct from schizophrenia24,25. In spite of this, few studies have investigated similarities and differences in brain dysfunction between MAP and schizophrenia. Recently, Okada et al. reported that using near-infrared spectroscopy (NIRS), only patients with MAP showed reduced activation in the frontopolar prefrontal cortex during the stop-signal inhibitory task26. It has been previously reported that patients with schizophrenia have significant cognitive dysfunctions27,28. Therefore, in this study, we monitored verbal fluency task (VFT) performance during NIRS and evaluated the potential application of this technique for the differential diagnosis of MAP and schizophrenia.
Results
Demographic and clinical data
The demographic characteristics of the study participants are presented in Table 1. There were no significant differences in age, sex, JART IQ, duration of illness, neuroleptics, number and duration of hospitalization between patients with MAP and schizophrenia. Moreover, there were no significant differences between both groups in PANSS or BACS subscales (Table 2).
NIRS data from participants performing verbal fluency tasks
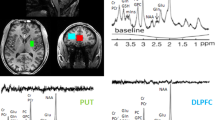
We calculated the grand average waveforms of oxyhaemoglobin (oxy-Hb) concentration changes while patients of both groups performed verbal fluency tasks (VFT) (Fig. 1). The grand waveforms of oxy-Hb concentration change increased while participants in the MAP group were performing the task. In contrast, the grand waveforms of oxy-Hb concentration showed little change in the participants in the schizophrenia group. We found group differences in the mean oxy-Hb measurements between the pre-task and post-task periods in the 24 channels (Fig. 2) (FDR-corrected, all P = 0.002 ~ 0.008). In the MAP group, the mean change in oxy-Hb between the pre-task and post-task periods was significantly larger than in the schizophrenia group for channels 8, 9 and 12. Those channels were located near the right dorsolateral prefrontal cortex (DLPFC). Overall, while performing the VFT, the MAP group exhibited smaller oxy-Hb changes in the prefrontal cortex compared to the schizophrenia group, particularly in the right DLPFC.
Location of the 24 channels in the near-infrared spectroscopy instrument:
(a) Corresponding anatomical site of each channel. (b) Arrangement of emitters and detectors, as well as definition of each channel. (c) Protocols and procedures for data analysis in each task. Pre- and post-task periods for all verbal fluency tasks (VFT) performed were 30 s and 90 s, respectively. For data analysis, we defined baseline as the mean levels of oxyhaemoglobin changes during the last 25 s of the pre-task period. The mean levels of oxyhaemoglobin changes during the 60-s task period of VFT were defined as activation levels.
Grand average waveforms showing changes in oxyhaemoglobin during the VFT.
Red lines represent the methamphetamine-induced psychosis group, while blue lines represent the schizophrenia group. The task was performed in the interval represented by green lines; the first green line indicates the beginning of the task and the second indicates the end. Abbreviations: Ch, channel.
Correlation between NIRS measurements and demographic characteristics
Because MAP and schizophrenia patients varied considerably in terms of characteristics, we performed Spearman’s ρ correlation calculations for NIRS measurements (Channels 8, 9 and 12), age, JART IQ, duration of illness, neuroleptics and PANSS subscales. There were no significant correlations between mean oxy-Hb changes and any other potential confounding factors, for any of the two groups.
We then compared mean oxy-Hb changes and working memory BACS scores. In the MAP group, we found a significant positive correlation at channel 8 (ρ = 0.565, p = 0.035). On the other hand, in the schizophrenia group, we found a significant negative correlation at channel 8 (ρ = −0.550, p = 0.018) (Fig. 3).
Comparison of the association between working memory and haemodynamic changes for the methamphetamine-induced psychosis and schizophrenia groups.
(a) Three-dimensional topographical mapping of the channels showing the location of significant differences in correlation between the two groups (channel #8, labelled in red; uncorrected P = 0.001. (b) Scatterplot of a typical channel presenting significantly different correlations for the two groups [Ch 8 (MAP) r = 0.565, (schizophrenia) r = −0.550, Z = 3.170, P = 0.002]. Abbreviations: BACS, brief assessment of cognition in schizophrenia; MAP, methamphetamine-induced psychosis.
Discussion
To the best of our knowledge, this is the first study investigating prefrontal haemodynamic response during VFT between patients with MAP and schizophrenia using NIRS. We found that oxy-Hb changes in the prefrontal cortex (channels 8, 9 and 12) during VFT were significantly larger in patients with MAP than they were in patients with schizophrenia. Our results suggest that patients with MAP have significantly different blood oxygenation in the right DLPFC compared with that in patients with schizophrenia and NIRS could be a useful measurement tool to distinguish the two disorders, which is consistent with the findings of a previous report26.
A number of researchers have used NIRS for the assessment of schizophrenia symptoms, although only one study has been published. Okada et al. reported that using NIRS, patients with MAP exhibited significantly smaller changes in oxy-Hb at the frontopolar prefrontal cortex during stop-signal tasks and higher impulsivity compared with patients with schizophrenia26. These findings are reasonable because the frontopolar cortex and its junction with the temporal lobe are considered the neuronal basis of impulsivity and moral judgment29,30,31. On the other hand, NIRS studies revealed that patients with schizophrenia show a reduction in prefrontal activation during the performance of VFT27,28. During VFT, which is a task of executive function, subjects are asked to generate as many words as possible for a given letter or category, during a limited time period. This requires a wide range of cognitive abilities, including evocation of appropriate words, suppression of incorrect words, remembering which words have already been used and attention to the task32,33. Patients with schizophrenia show poor performance in this complex task34, as well as low oxygenation of the prefrontal cortex. Interestingly, it had been never investigated how the prefrontal cortex responds to VFT in patients with MAP. Therefore, we examined haemodynamic changes during the performance of VFT in patients with MAP and schizophrenia. In the DLPFC, patients with schizophrenia exhibited significantly smaller changes in oxy-Hb compared to MAP patients. Since DLPFC is a region responsible for working memory and impaired working memory is a core symptom of schizophrenia35,36,37, hypo-oxygenation in the DLPFC in these patients can be reasonably expected. In spite of differential oxygenation of the DLPFC, scores of working memory in BACS domains were low in patients with both MAP and schizophrenia in this study, which is consistent with a previous study38.
A previous report showed that patients with MAP and those with schizophrenia presented an opposite correlation between impulsivity and haemodynamic changes in the bilateral premotor cortex26. Similar to these findings, our results showed that patients with MAP exhibited an opposite correlation between working memory and haemodynamic changes in channel 8 compared to patients with schizophrenia; there was a positive correlation in patients with MAP, while a negative correlation was observed in patients with schizophrenia. The interesting point in our study is that scores of working memory in BACS were identical for the two disorders, while scores of excitement (impulsivity) in PANSS were higher in MAP than in schizophrenia in a previous study24, which suggests that our NIRS analysis during VFT was able to distinguish between the two groups, even in cases in which clinical symptoms were completely identical.
There are several potential limitations in our study. First, the spatial resolution for the detection of haemodynamic responses from the scalp surface using NIRS is lower than that of more deeply penetrating functional imaging such as functional MRI. However, this limitation could be within an acceptable range because the differences in haemodynamic response in individuals with MAP and schizophrenia could be clearly observed using NIRS, although it would be interesting to investigate activity differences of the whole brain using functional MRI. Second, our sample size was relatively small and future studies should include a larger sample size to examine if such changes measured by NIRS are dose-dependent in patients with MAP. Third, all participants were receiving antipsychotic medications during the tests, although there was no significant difference in drug dose between the two groups.
Conclusion
To the best of our knowledge, this is the first NIRS study to examine prefrontal haemodynamic responses during the performance of VFT in participants with MAP and schizophrenia. We found that changes in oxy-Hb concentration in the prefrontal cortex were significantly higher in patients with MAP compared to those with schizophrenia. Our study indicates that patients with MAP might have less prefrontal dysfunction than patients with schizophrenia. Since the multi-channel NIRS system enables non-invasive functional mapping of the cerebral cortex and the measurement time is much shorter (about 5 min) than that in other functional brain imaging methodologies, this could be a powerful tool for the differential diagnosis of MAP and schizophrenia in cases that present similar clinical symptoms.
Methods
Participants
Fifteen MAP patients (9 males: mean ± SD age 42.6 ± 11.6 years; and 6 females: mean ± SD age 35.8 ± 9.3 years) and 19 schizophrenia patients (9 males: mean ± SD age 37.3 ± 8.0 years; and 10 females: mean ± SD age 40.7 ± 6.0 years) were recruited from the in- and outpatient clinics of the Department of Psychiatry at Nara Medical University, Japan (Table 1). For each patient, diagnosis was determined according to DSM-5 and ICD-10 research criteria for schizophrenia or methamphetamine psychosis. At least two experienced psychiatrists separately examined the patients and diagnostic consensus was reached. All participants were of Japanese descent and right-handed, as indicated by the Edinburgh Handedness Inventory39. Their premorbid IQs were assessed using the Japanese version of the National Adult Reading Test (JART)40. Exclusion criteria included neurological disorders, traumatic brain injury with known cognitive consequence or loss of consciousness for more than 5 min, history of electroconvulsive therapy, history of pervasive developmental disorder, a serious medical condition, or low intelligence. Seventeen out of the 19 patients with schizophrenia received atypical neuroleptics, while the other 2 patients received both typical and atypical neuroleptics. On the other hand, all patients with MAP received atypical neuroleptics. Mean chlorpromazine equivalent dosages for antipsychotics did not differ between subject groups.
The study protocols were approved by the appropriate Ethics Committees at Nara Medical University (No. 325-2) and was carried out in accordance with the Declaration of Helsinki. All study participants or their legal guardians provided informed consent for their participation prior to the start of the study.
Neurocognitive evaluation
The Brief Assessment of Cognition in Schizophrenia (BACS) scale was used to evaluate the neurocognitive functions of all patients41. BACS is a newly developed instrument that evaluates the elements of cognition that are most commonly impaired and strongly connected with real world functioning aspects in patients with schizophrenia42. The BACS evaluation takes about 30 min, yields a high completion rate in patients and has high test-retest reliability. BACS evaluation included such tests as the List Learning Test, Digit Sequencing Task, Token Motor Task, Category Instances Test, Controlled Oral World Association Test, Symbol Coding and Tower of London Test, which measure verbal memory, working memory, motor speed, verbal fluency, attention, processing speed and executive function, respectively. The primary measure of each BACS domain was standardized creating z-scores and the composite score is the Z-score of that sum43.
Psychopathological evaluation
The Japanese version of the Positive and Negative Symptom Scale (PANSS)44 was used to evaluate symptoms in the participants with schizophrenia and MAP. A seven point Likert-scale, in which higher scores indicate greater severity, was used to rate all of the items. Subscale scores were calculated using small sets of variables based on the three domains of PANSS: positive, negative and general psychopathological symptoms45.
Cognitive activation task
Patients were administered the 160→190 block VFT (letter version). In the present study, we chose VFT because previous studies have demonstrated deficits in brain cortical activity over bilateral frontotemporal regions during VFT in patients with schizophrenia, using NIRS measurement. The 190-s block VFT contains three different time periods: a 40-s pre-task, a 60-s task, a 90-s post-task periods. In the 25→40 pre-task and 90-s post-task periods, patients were instructed to say Japanese vowels (/a/, /i/, /u/, /e/ and /o/) repeatedly to control for and remove task-related motion artefacts during the task periods. During the 60-s task period, patients were instructed to say as many words as possible that started with a phonological syllable presented as an audible instruction by a computer. The task period comprised three continuous 20-s subperiods (/a/. /ki/ and /ha/). Transitions between the 20-s subperiods were continuous, which encouraged patients to produce continuous vocalizations (Fig. 1(c)). We recorded the total number of correct words generated during the task as an index of VFT performance.
NIRS measurements
Oxy-Hb increases and deoxy-Hb decreases, as measured using NIRS, have been shown to reflect cortical activation. In animal studies, oxy-Hb has been shown to be the most sensitive indicator of regional cerebral blood flow, because the direction of change in deoxy-Hb is determined by the degree of change in venous blood oxygenation and volume46. Therefore, we decided to focus on changes in oxy-Hb. In this study, oxy-Hb was measured with a 24-channel NIRS machine (Hitachi ETG-100, Hitachi Medical Corporation, Tokyo, Japan), determining the absorption of two wavelengths of near infrared light (760 and 840 nm). Oxy-Hb was calculated as previously described47. The inter-probe intervals of the machine were 3.0 cm and it was determined that the machine measured points 2-3 cm beneath the scalp, which is the depth of the surface of the cerebral cortices48. Participants maintained a natural sitting position during NIRS measurements. The NIRS probes were placed on the participant’s prefrontal regions and were arranged to measure relative oxy-Hb concentration changes at 24 measurement points in an 8 × 8 cm area (Fig. 1(a)). The lowest probes were positioned along the Fp1-Fp2 line, according to the international 10–20 system used in electroencephalography. The probe positions and measurement points on the cerebral cortex were confirmed by overlaying the probe positions on a three-dimensionally reconstructed magnetic resonance imaging (MRI) scan of the cerebral cortex of a representative participant from the control group (Fig. 1(b)). The absorption of near-infrared light was measured with a time resolution of 0.1 s and data were analysed using the “integral mode”. The pre-task baseline was determined as the mean during the 10 s immediately preceding the task performance and the post-task baseline was determined as the mean during the 25 s immediately following completion of the task. Linear fitting was performed on the data between the two baselines. Moving average methods (moving average window, 5 s) were used to exclude short-term motion artefacts in the analysed data. We tried to minimize motion artefacts by closely monitoring subjects and by instructing them to avoid artefact-evoking body movements, such as neck movements, strong biting and blinking. Particular attention was paid to blinking because it was identified as the most influential source of motion artefacts in a preliminary artefact-evoking study. Examiners who were blind to the diagnosis of the participants evaluated the NIRS results.
Statistical Analyses
We used Student’s t-tests to compare oxy-Hb changes between the two groups by calculating the grand average waveforms every 0.1 s in each channel. This analysis enabled a more detailed comparison of oxy-Hb changes along the time course of the task. Since we performed 24 paired t tests, we performed a correction for multiple comparisons using the false discovery rate (FDR) (two-tailed; we set the value of q specifying the maximum FDR to 0.05, so that on average, there would be no more than 5% false positives49). We used PASW Statistics 18.0 J for Windows (SPSS, Tokyo, Japan) for statistical analysis.
Additional Information
How to cite this article: Yamamuro, K. et al. Differential patterns of blood oxygenation in the prefrontal cortex between patients with methamphetamine-induced psychosis and schizophrenia. Sci. Rep. 5, 12107; doi: 10.1038/srep12107 (2015).
References
Kishimoto, M. et al. The dysbindin gene (DTNBP1) is associated with methamphetamine psychosis. Biological psychiatry 63, 191–196, 10.1016/j.biopsych.2007.03.019 (2008).
Matsuzawa, D. et al. Identification of functional polymorphisms in the promoter region of the human PICK1 gene and their association with methamphetamine psychosis. The American journal of psychiatry 164, 1105–1114, 10.1176/appi.ajp.164.7.1105 (2007).
Farrell, M., Marsden, J., Ali, R. & Ling, W. Methamphetamine: drug use and psychoses becomes a major public health issue in the Asia Pacific region. Addiction (Abingdon, England) 97, 771–772 (2002).
McKetin, R., McLaren, J., Lubman, D. I. & Hides, L. The prevalence of psychotic symptoms among methamphetamine users. Addiction (Abingdon, England) 101, 1473–1478, 10.1111/j.1360-0443.2006.01496.x (2006).
Mahoney, J. J., 3rd, Kalechstein, A. D., De La Garza, R., 2nd & Newton, T. F. Presence and persistence of psychotic symptoms in cocaine- versus methamphetamine-dependent participants. The American journal on addictions/American Academy of Psychiatrists in Alcoholism and Addictions 17, 83–98, 10.1080/10550490701861201 (2008).
Batki, S. L. & Harris, D. S. Quantitative drug levels in stimulant psychosis: relationship to symptom severity, catecholamines and hyperkinesia. The American journal on addictions/American Academy of Psychiatrists in Alcoholism and Addictions 13, 461–470, 10.1080/10550490490512834 (2004).
Curran, C., Byrappa, N. & McBride, A. Stimulant psychosis: systematic review. The British journal of psychiatry : the journal of mental science 185, 196–204, 10.1192/bjp.185.3.196 (2004).
Sato, M., Numachi, Y. & Hamamura, T. Relapse of paranoid psychotic state in methamphetamine model of schizophrenia. Schizophrenia bulletin 18, 115–122 (1992).
Snyder, S. H. Amphetamine psychosis: a “model” schizophrenia mediated by catecholamines. The American journal of psychiatry 130, 61–67 (1973).
Weich, L. & Pienaar, W. Occurrence of comorbid substance use disorders among acute psychiatric inpatients at Stikland Hospital in the Western Cape, South Africa. African journal of psychiatry 12, 213–217 (2009).
Srisurapanont, M. et al. Comparisons of methamphetamine psychotic and schizophrenic symptoms: a differential item functioning analysis. Progress in neuro-psychopharmacology & biological psychiatry 35, 959–964, 10.1016/j.pnpbp.2011.01.014 (2011).
Srisurapanont, M. et al. Psychotic symptoms in methamphetamine psychotic in-patients. The international journal of neuropsychopharmacology/official scientific journal of the Collegium Internationale Neuropsychopharmacologicum 6, 347–352, 10.1017/s1461145703003675 (2003).
Tomiyama, G. Chronic schizophrenia-like states in methamphetamine psychosis. The Japanese journal of psychiatry and neurology 44, 531–539 (1990).
Green, M. F. What are the functional consequences of neurocognitive deficits in schizophrenia? The American journal of psychiatry 153, 321–330, 10.1176/ajp.153.3.321 (1996).
Horan, W. P. et al. Impaired implicit learning in schizophrenia. Neuropsychology 22, 606–617, 10.1037/a0012602 (2008).
Mueser, K. T. & McGurk, S. R. Schizophrenia. Lancet 363, 2063–2072, 10.1016/s0140-6736(04)16458-1 (2004).
Kahn, R. S. & Keefe, R. S. Schizophrenia is a cognitive illness: time for a change in focus. JAMA psychiatry 70, 1107–1112, 10.1001/jamapsychiatry.2013.155 (2013).
Jacobs, E., Fujii, D., Schiffman, J. & Bello, I. An exploratory analysis of neurocognition in methamphetamine-induced psychotic disorder and paranoid schizophrenia. Cognitive and behavioral neurology:official journal of the Society for Behavioral and Cognitive Neurology 21, 98–103, 10.1097/WNN.0b013e31816bdf90 (2008).
Scott, J. C. et al. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychology review 17, 275–297, 10.1007/s11065-007-9031-0 (2007).
Bramness, J. G. et al. Amphetamine-induced psychosis--a separate diagnostic entity or primary psychosis triggered in the vulnerable? BMC psychiatry 12, 221, 10.1186/1471-244x-12-221 (2012).
Medhus, S., Mordal, J., Holm, B., Morland, J. & Bramness, J. G. A comparison of symptoms and drug use between patients with methamphetamine associated psychoses and patients diagnosed with schizophrenia in two acute psychiatric wards. Psychiatry research 206, 17–21, 10.1016/j.psychres.2012.09.023 (2013).
Smith, J. L., Mattick, R. P., Jamadar, S. D. & Iredale, J. M. Deficits in behavioural inhibition in substance abuse and addiction: a meta-analysis. Drug and alcohol dependence 145, 1–33, 10.1016/j.drugalcdep.2014.08.009 (2014).
Hughes, M. E., Fulham, W. R., Johnston, P. J. & Michie, P. T. Stop-signal response inhibition in schizophrenia: behavioural, event-related potential and functional neuroimaging data. Biological psychology 89, 220–231, 10.1016/j.biopsycho.2011.10.013 (2012).
Callaghan, R. C. et al. Methamphetamine use and schizophrenia: a population-based cohort study in California. The American journal of psychiatry 169, 389–396, 10.1176/appi.ajp.2011.10070937 (2012).
Rounsaville, B. J. DSM-V research agenda: substance abuse/psychosis comorbidity. Schizophrenia bulletin 33, 947–952, 10.1093/schbul/sbm054 (2007).
Okada, N. et al. Characterizing prefrontal cortical activity during inhibition task in methamphetamine-associated psychosis versus schizophrenia: a multi-channel near-infrared spectroscopy study. Addiction biology, 10.1111/adb.12224 (2015).
Koike, S. et al. Different hemodynamic response patterns in the prefrontal cortical sub-regions according to the clinical stages of psychosis. Schizophrenia research 132, 54–61, 10.1016/j.schres.2011.07.014 (2011).
Takizawa, R. et al. Reduced frontopolar activation during verbal fluency task in schizophrenia: a multi-channel near-infrared spectroscopy study. Schizophrenia research 99, 250–262, 10.1016/j.schres.2007.10.025 (2008).
Tsujimoto, S., Genovesio, A. & Wise, S. P. Frontal pole cortex: encoding ends at the end of the endbrain. Trends in cognitive sciences 15, 169–176, 10.1016/j.tics.2011.02.001 (2011).
Yang, Y., Raine, A., Colletti, P., Toga, A. W. & Narr, K. L. Abnormal temporal and prefrontal cortical gray matter thinning in psychopaths. Molecular psychiatry 14, 561-562, 555, 10.1038/mp.2009.12 (2009).
Crockett, M. J. et al. Restricting temptations: neural mechanisms of precommitment. Neuron 79, 391–401, 10.1016/j.neuron.2013.05.028 (2013).
Broome, M. R. et al. Neural correlates of executive function and working memory in the ‘at-risk mental state’. The British journal of psychiatry : the journal of mental science 194, 25–33, 10.1192/bjp.bp.107.046789 (2009).
Henry, J. D. & Crawford, J. R. A meta-analytic review of verbal fluency performance following focal cortical lesions. Neuropsychology 18, 284–295, 10.1037/0894-4105.18.2.284 (2004).
Curtis, V. A. et al. Attenuated frontal activation during a verbal fluency task in patients with schizophrenia. The American journal of psychiatry 155, 1056–1063 (1998).
Lewis, R. Should cognitive deficit be a diagnostic criterion for schizophrenia? Journal of psychiatry & neuroscience : JPN 29, 102–113 (2004).
Kang, S. S., Sponheim, S. R., Chafee, M. V. & MacDonald, A. W., 3rd . Disrupted functional connectivity for controlled visual processing as a basis for impaired spatial working memory in schizophrenia. Neuropsychologia 49, 2836–2847, 10.1016/j.neuropsychologia.2011.06.009 (2011).
Van Veelen, N. M., Vink, M., Ramsey, N. F. & Kahn, R. S. Left dorsolateral prefrontal cortex dysfunction in medication-naive schizophrenia. Schizophrenia research 123, 22–29, 10.1016/j.schres.2010.07.004 (2010).
Chen, C. K. et al. Persistence of psychotic symptoms as an indicator of cognitive impairment in methamphetamine users. Drug and alcohol dependence 148, 158–164, 10.1016/j.drugalcdep.2014.12.035 (2015).
Oldfield, R. C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9, 97–113 (1971).
Matsuoka, K., Uno, M., Kasai, K., Koyama, K. & Kim, Y. Estimation of premorbid IQ in individuals with Alzheimer’s disease using Japanese ideographic script (Kanji) compound words: Japanese version of National Adult Reading Test. Psychiatry and clinical neurosciences 60, 332–339, 10.1111/j.1440-1819.2006.01510.x (2006).
Keefe, R. S. et al. The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity and comparison with a standard neurocognitive battery. Schizophrenia research 68, 283–297, 10.1016/j.schres.2003.09.011 (2004).
Keefe, R. S., Poe, M., Walker, T. M., Kang, J. W. & Harvey, P. D. The Schizophrenia Cognition Rating Scale: an interview-based assessment and its relationship to cognition, real-world functioning and functional capacity. The American journal of psychiatry 163, 426–432, 10.1176/appi.ajp.163.3.426 (2006).
Keefe, R. S. et al. Norms and standardization of the Brief Assessment of Cognition in Schizophrenia (BACS). Schizophrenia research 102, 108–115, 10.1016/j.schres.2008.03.024 (2008).
Kay, S. R., Fiszbein, A. & Opler, L. A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia bulletin 13, 261–276 (1987).
Sawamura, J., Morishita, S. & Ishigooka, J. Is there a linear relationship between the Brief Psychiatric Rating Scale and the Clinical Global Impression-Schizophrenia scale? A retrospective analysis. BMC psychiatry 10, 105, 10.1186/1471-244x-10-105 (2010).
Hoshi, Y., Kobayashi, N. & Tamura, M. Interpretation of near-infrared spectroscopy signals: a study with a newly developed perfused rat brain model. Journal of applied physiology (Bethesda, Md. : 1985) 90, 1657–1662 (2001).
Schweitzer, J. B. et al. Alterations in the functional anatomy of working memory in adult attention deficit hyperactivity disorder. The American journal of psychiatry 157, 278–280 (2000).
Toronov, V. et al. Investigation of human brain hemodynamics by simultaneous near-infrared spectroscopy and functional magnetic resonance imaging. Medical physics 28, 521–527 (2001).
Singh, A. K. & Dan, I. Exploring the false discovery rate in multichannel NIRS. NeuroImage 33, 542–549, 10.1016/j.neuroimage.2006.06.047 (2006).
Acknowledgements
We wish to thank the participants for their valuable involvement in the study. The authors would also like to thank Hitachi Medical Corporation for the ETG-4000 equipment and the skilled technical and methodical support.
Author information
Authors and Affiliations
Contributions
K.Y., M.M., S.K., N.K., T.M., T.O., M.T., S.U., K.M., Y.T., O.K., T.T., M.T., Y.T., Y.N., Y.M. and H.Y. were involved in the collection of the data. K.Y. and M.M. wrote the manuscript. S.K., M.M., J.I. and T.K. supervised the entire project. All authors have read and approved the final manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Yamamuro, K., Makinodan, M., Kimoto, S. et al. Differential patterns of blood oxygenation in the prefrontal cortex between patients with methamphetamine-induced psychosis and schizophrenia. Sci Rep 5, 12107 (2015). https://doi.org/10.1038/srep12107
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep12107
This article is cited by
-
Shared and differential cortical functional abnormalities associated with inhibitory control in patients with schizophrenia and bipolar disorder
Scientific Reports (2018)
-
Cognitive profile of ketamine-dependent patients compared with methamphetamine-dependent patients and healthy controls
Psychopharmacology (2018)
-
Differential therapeutic effects of atomoxetine and methylphenidate in childhood attention deficit/hyperactivity disorder as measured by near-infrared spectroscopy
Child and Adolescent Psychiatry and Mental Health (2017)
-
Association of fronto-temporal function with cognitive ability in schizophrenia
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.