Abstract
A silver nanoparticle-decorated N,S-co-doped TiO2 nanocomposite was successfully prepared and used as an efficient photoanode in high-performance dye-sensitized solar cells (DSSCs) with N719 dye. The DSSCs assembled with the N,S-TiO2@Ag-modified photoanode demonstrated an enhanced solar-to-electrical energy conversion efficiency of 8.22%, which was better than that of a DSSC photoanode composed of unmodified TiO2 (2.57%) under full sunlight illumination (100 mWcm−2, AM 1.5 G). This enhanced efficiency was mainly attributed to the reduced band gap energy, improved interfacial charge transfer and retarded charge recombination process. The influence of the Ag content on the overall efficiency was also investigated and the optimum Ag content with N,S-TiO2 was found to be 20 wt%. Because of the enhanced solar energy conversion efficiency of the N,S-TiO2@Ag nanocomposite, it should be considered as a potential photoanode for high-performance DSSCs.
Similar content being viewed by others
Introduction
Renewable energy sources have become important approaches for gaining independence from fossil fuels. Utilizing solar energy is certainly one of the most viable ways to solve the world’s energy crisis. Dye-sensitized solar cells (DSSCs) have emerged as promising candidates for harnessing solar power because of their low cost, flexibility, ease of production, relatively high energy conversion efficiency and low toxicity to the environment1. Several efforts have been made to fabricate highly efficient DSSCs by the introduction of novel components such as electrolytes, dyes, counter electrodes and semiconductor photoanodes. Among these DSSC components, the photoanode plays a vital role in determining the DSSC performance. So far, a titanium dioxide (TiO2) is one of the most commonly used photoanode materials and it is promising material for a DSSC because of its low cost, ease of fabrication, relatively high energy conversion efficiency, high specific surface area and non-toxicity. However, the major limitation associated with using TiO2 as a photoanode is its random electron transport, which will cause the electron–hole recombination process and hence hinder the cell performance2,3. In order to solve this serious issue, designing a photoanode with an efficient charge transport pathway from the photoinjected carriers to the current collector seems to be a possible alternative to enhance the performance of DSSCs. With this aim, surface modifications of TiO2 with metal doping, semiconductor coupling and hybridization with a carbon material has have been attempted and shown better results4,5,6,7.
The modification of TiO2 with a noble metal such as gold (Au)8, silver (Ag)9, or platinum (Pt)10 has been actively reported for the photoanodes in DSSC applications to prevent the recombination of the photogenerated electron–hole pairs and improve the charge transfer efficiency. In the present decade, Ag nanoparticle-modified TiO2 has been applied as a photoanode with the aim of improving the efficiency of a DSSC. The Ag nanoparticles play dual roles in the DSSC performance, including the enhancement of the absorption coefficient of the dye and optical absorption due to surface plasmonic resonance11,12,13. Moreover, they act as an electron sink for photoinduced charge carriers, improve the interfacial charge transfer process and minimize the charge recombination, thereby enhancing the electron transfer process in a DSSC14,15,16. Hence, the performance of a DSSC with Ag@TiO2 plasmonic nanocomposite material-modified photoanodes has been actively investigated14,15,16. On the other hand, to improve the light absorption of a TiO2 material in the visible region, non-metals such as nitrogen (N), carbon (C), sulfur (S) and phosphorus (P) have been widely studied as dopants for TiO217. Among these, nitrogen doped TiO2 possesses good photovoltaic properties and hence has received much attention due to the narrowing of the band gap and shift to the visible region18. Hence, the co-deposition of Ag on doped TiO2 showed enhanced photovoltaic properties due to the synergistic effect between the Ag and doped TiO219,20.
In the present investigation, we successfully developed a facile route to synthesize N,S-TiO2@Ag nanocomposite materials as a photoanode for high-performance dye-sensitized solar cells. The influence of the Ag content in the N,S-TiO2@Ag on the DSSC performance was also investigated. The introduction of plasmonic Ag nanoparticles on the N,S-TiO2 surface showed multifunctional properties, including a surface plasmon resonance effect, a reduction in the band-gap, an improved interfacial charge transfer and the minimization of the charge recombination process as a result of the synergistic photovoltaic performance.
Results and Discussion
Morphological studies of N,S-TiO2@Ag nanocomposite
Figure 1 shows the FESEM microscopic morphology of the as-prepared samples and Fig. 1a shows an FESEM image of N,S-TiO2, which appeared to be spherical, with a uniform size. Upon the addition of Ag nanoparticles, no significant change in the morphology was observed for the N,S-TiO2@Ag film (Fig. 1b). An energy dispersive X-ray spectrometry (EDS) mapping analysis of the N,S-TiO2@Ag thin film also confirmed that the N,S-TiO2@Ag nanocomposite, composed of Ag, N and S elements, was dispersed on the surface of the TiO2 nanoparticles with Ti and O signals (Fig. 1c). Further, TEM images of the N,S-TiO2 were also recorded and are shown in Fig. 2a. The TEM images show that the N,S-TiO2 nanoparticles are spherical in shape, with a TiO2 particle size range of 20–25 nm. Figure 2b clearly shows that small spherical Ag nanoparticles with a particle size range of 2–5 nm were well distributed and decorated on the surface of the N,S-TiO2 nanoparticles. Figure 2c depicts the selected area electron diffraction (SAED) pattern of the N,S-TiO2@Ag nanocomposite. The pattern clearly reveals bright concentric rings, which were due to the diffraction from the (211), (200), (004) and (101) planes of the anatase TiO2. In the HRTEM image of the N,S-TiO2@Ag (Fig. 2d), lattice fringes with d-spacing values of 3.51 Ǻ, 1.34 Ǻ and 1.89 Ǻ were observed, which corresponds to the (101), (220) and (200) planes of the anatase TiO2, respectively; while the inter-planar d-spacing value of 2.05 Ǻ was assigned to the (210) plane of rutile TiO2.
Crystalline studies of N,S-TiO2@Ag nanocomposite
The XRD pattern obtained for the N,S-TiO2@g is shown in Fig. S1, along with those of TiO2 and N,S-TiO2 for comparison. The TiO2, N,S-TiO2 and N,S-TiO2@Ag films were composed of mixed anatase and rutile phases, which agreed with reference patterns JCPDS 83-2243 and 21-1276, respectively. The diffraction peaks observed at 25.4°, 38.4°, 48.2° and 74.5° corresponded to the anatase phase of TiO2 and were assigned to the (101), (004), (200) and (215) crystallographic planes. In contrast, the peaks at 54.09°, 63.2° and 64.9° agreed well with the rutile phase of TiO2 and were assigned to the (220), (002) and (310) crystallographic planes. It was difficult to differentiate the Ag from the TiO2 signals because of the overlapping of the Ag signal with the rutile plane in the N,S-TiO2@Ag nanocomposite. The XRD patterns clearly confirmed the anatase and rutile phases were retained without a phase change after the nitrogen and sulfur doping and the deposition of Ag nanoparticles. The additional peaks were due to the ITO substrate.
To further evaluate the phases in the prepared films, Raman studies were performed in the range of 100–1000 cm−1 and the results are shown in Fig. 3a. The values of 147.48, 199, 395.28, 515.74, 639.25 and 710.18 cm−1 were due to the anatase phase TiO221,22. The rutile TiO2 phase was observed at 444 cm−1 21,22. This clearly indicated that the TiO2 and N,S-TiO2 nanoparticles contained a mixture of the anatase and rutile phases. The Raman signals of TiO2 did not change after adding nitrogen, which indicated that no phase transition occurred. However, no signals related to Ag particles were identified for the samples because of the relatively low concentration of Ag loaded onto the TiO2 and its weak Raman scattering power. An interesting observation was that the peak intensities increased with the deposition of Ag, while the position of the Raman signal remained the same. This indicates that there was an interaction between the Ag and TiO2 that affected the Raman resonance of TiO223,24. This observation shows that the deposition of Ag on TiO2 and N,S-TiO2 does not cause any phase transition, but may bring about an electronic environment change in the surroundings21,22.
XPS studies of N,S-TiO2@Ag nanocomposite
The XPS spectra of the TiO2, N,S-TiO2 and N,S-TiO2@Ag were recorded in order to analyze and determine the chemical composition and identify the chemical states of the N, S, Ti, O and Ag elements and are shown in Fig. 4. Figure 4a shows the Ti 2p spectra for the samples, in which two peaks are observed at 454.1 and 460 eV, corresponding to the binding energies of Ti 2p3/2 and Ti2 p1/2 core levels due to the presence of the Ti(IV) state. Figure 4b shows the O1s spectra of TiO2 and Ag@TiO2 and the binding energy of the O 1s state of the samples was located at 531.1 eV, which was assigned to the bulk oxides (O2−) in the P25 lattice. The N 1s peak at 399.2 eV was assigned to the anionic N-doping, where O was replaced by N atoms involving substitutional N-doping and the peak at 398.4 eV was also due to anionic N-doping but incorporated in the TiO2 as an N–Ti–O structural feature25−27 (Fig. 4c). Figure 4d shows the corresponding high-resolution XPS spectrum of the S 2p region and the binding energy peak that appeared at around 164.1 eV corresponds to the Ti–S bond due to the fact that sulfur atoms replaced some of the oxygen atoms on the TiO2 surface28,29. The binding energies found for the Ag 3d5/2 and Ag 3d3/2 levels were 368.4 and 374.5 eV, respectively (Fig. 4e), with a peak separation of 6 eV due to the metallic silver. The XPS analysis provided support for the existence of elements such as Ti, O, N, S and Ag in the nanocomposite materials.
Optical properties of N,S-TiO2@Ag nanocomposite
The optical absorption properties of the TiO2, N,S-TiO2 and N,S-TiO2@Ag were studied by recording the UV–visible absorption spectra, which are shown in Fig. S2(A). It can be seen that the absorption edge of TiO2 was red shifted after the doping with N and S. The further deposition of Ag nanoparticles on the surface of the N,S-TiO2 significantly influenced the visible light absorption and an additional peak was also observed at 517 nm. The enhanced absorption in the visible region was due to the surface plasmon resonance (SPR), which was aroused by the collective oscillation of electrons in the Ag nanoparticles stimulated by optical excitation30. The band-gap energy (Ebg) values of the TiO2, N,S-TiO2 and N,S-TiO2-Ag were calculated using the well-known Tauc’s plot method31. The calculated Ebg values of TiO2, N,S-TiO2 and N,S-TiO2@Ag were 3.36, 3.20 and 2.90 eV, respectively (Fig. S2(B–D)). A remarkable reduction in the Ebg value of N,S-TiO2 was observed due to the substitution of oxygen by nitrogen and sulfur in the TiO2 lattice. The mixing of the N 2p and S 2p states with O 2p states resulted in a band gap narrowing of TiO217. When Ag nanoparticles were added to the N,S-TiO2, the Ebg value was further decreased. This was due to the free electron properties being exhibited with a downward shift in the conduction band and upward shift in the valence band, which then led to a decrease in the Ebg value31,32.
Understanding the charge recombination process of a semiconductor is crucial because it significantly influences the photoelectrochemical properties and DSSC performance. Photoluminescence (PL) is a suitable tool to study the efficiency of charge carrier trapping, migration and transfer and to understand the fate of electron–hole pairs in semiconductor particles because PL emissions result from the recombination of free carriers. The TiO2 will absorb the incident photons with sufficient energy equal to or higher than the band-gap energy, which will produce photoinduced charge carriers (h+…e−). In addition, the recombination of photoinduced electrons and holes releases energy in the form of PL emission spectra. Hence, a lower PL intensity indicates less charge recombination. The observed PL spectra of TiO2, N,S-TiO2 and N,S-TiO2-Ag are shown in Fig. 5. A broad peak with a maximum emission at around 580 nm can be observed for TiO2, N,S-TiO2 and N,S-TiO2-Ag. The TiO2 showed a higher PL intensity due to the rapid recombination of photoinduced charge carriers. The PL intensity decreased when N, S and Ag were introduced to TiO2. This can mainly be attributed to the formation of a Schottky barrier at the Ag and TiO2 interface, which could act as an electron sink to efficiently prevent the electronhole recombination process33.
Photovoltaic performance of N,S-TiO2@Ag photoanode-modified DSSCs
The photovoltaic performances of TiO2, N,S-TiO2 and N,S-TiO2@Ag photoanode-modified DSSCs under simulated solar irradiation of AM 1.5 G were studied by recording the J-V profiles, which are shown in Fig. 6. The corresponding photovoltaic parameters are summarized in Table 1. A significant boost in the short-circuit current up to 29.05 mA/cm2 can be observed from the J-V curve of N,S-TiO2@Ag compared to those of the DSSCs based on TiO2 (6.27 mA/cm2) and N,S-TiO2 (9.78 mA/cm2). The N,S-TiO2@Ag-based DSSC showed a conversion efficiency (η = 8.22%) that was 145% and 231% higher than those of the DSSCs based on N,S-TiO2 (η = 3.35%) and pure TiO2 (η = 2.57%). This significant improvement in the photovoltaic performance was due to the synergetic effect of the N, S and Ag. The presence of N in the TiO2 successfully reduced the band gap and extended the absorption of TiO2 into the visible region. The existence of S facilitated the electron transfer process and consequently increased the forward reduction reaction. The electrons from the conduction band could transfer to the more electropositive S atoms, which were reduced from S6+ to S4+. The electrons were then transferred to the Ag to retain the system at S6+ 19,20. Moreover, the deposition of Ag onto the N,S-TiO2 resulted in a change in the Fermi energy level. A large number of the electrons accumulated on the surfaces of the Ag nanoparticles due to the surface plasmon resonance effect. The accumulation of these electrons on the Ag nanoparticles shifted the position of the Fermi level closer to the conduction band of TiO234. The electrons in the Ag nanoparticles that were excited due to the SPR effect were transferred to the conduction band of the TiO2 and collected by the current collector (ITO), which improved the photocurrent under irradiation in the visible region35. In this way, the photovoltaic performance significantly improved with the enhancement of absorption in the visible region. The synergetic effect of Ag and S as a redox couple played a potential role in the enhanced DSSC performance. The controlled experiments also carried out for N,S-TiO2@Ag sample without dye loading and the result is shown (Fig. S3). The photocurrent response of ~0.15 mA/cm2 was observed due to the plasmonic excitation of Ag nanoparticles and it suggests that coupling of Ag nanoparticles with N,S-TiO2 in the presence of N719 sensitizer can produce synergistic effect in DSSC.
Influence of Ag content on photovoltaic performance of N,S-TiO2@Ag photoanode-modified DSSCs
The loading of the Ag content in the N,S-TiO2@Ag photoanode was varied to obtain a high-performance DSSC. The photocurrent density–photovoltage (J-V) curves were recorded for the N,S-TiO2@Ag nanocomposite-modified photoanodes with different Ag contents and are shown in Fig. 7. Their corresponding photovoltaic parameters were also evaluated and are listed in Table 2. The obtained conversion efficiency for the N,S-TiO2 photoanode without Ag showed a value of 3.39%, whereas N,S-TiO2 with 20% Ag showed an efficiency of 8.22%. Those with 5%, 10% and 40% Ag showed conversion efficiencies of 5.42%, 5.90% and 4.40%, respectively. The observed results clearly revealed that the conversion efficiency of the DSSC was increased with an increase in the Ag content in the photoanode until it reached a maximum of 20% and a further increase in the Ag content eventually led to a decrease in the conversion efficiency (Fig. 8a and Table 2). The decrease in efficiency at a high Ag loading was due to the free standing/excess Ag in the composite, which could oxidize to Ag(I)16,36 and erode to the redox electrolyte16. The oxidation of the Ag would have acted as a new recombination center, thus reducing the number of charge carriers, which led to a decrease in the Jsc and Voc. Consequently, the overall conversion efficiency of the DSSC deteriorated. The Jsc was increased by an increase in the Ag content and attained a maximum of 29.05 mA/cm2 with 20% (Fig. 8b). Then, Jsc decreased to 16.65 mA/cm2 with 40% Ag loading in the photoanode of the DSSC. It could also be seen that the Voc trend was almost the same, within the range of ~0.69–0.75 V. The Jmax and Vmax values of the device also followed trends similar to those of Jsc and Voc (Fig. 8c).
Electrochemical behavior of N,S-TiO2@Ag photoanode-modified DSSCs
For the interfacial charge transfer process within the fabricated DSSC with the N,S-TiO2@Ag photoanode, the electrochemical impedance spectra (EIS) were recorded in the frequency range between 0.01 Hz and 100 KHz and are shown in Fig. 9. Well-defined semicircles in the middle frequency region for the DSSCs with N,S-TiO2@Ag nanocomposite-modified photoanodes with different Ag contents were observed in the Nyquist plots (Fig. 9(A)). The intersection of the high-frequency semicircle at the real axis represents the equivalent series resistance of the device (Rs); the arc in the middle frequency range between 1 and 1000 Hz represents the charge transfer resistance (Rct) between the dye-adsorbed photoanode and electrolyte interface2,37. The Rs values varied because different concentrations of Ag used in the photoanode. An increase in the Rct value of the N,S-TiO2@Ag-based photoanode was observed with the increasing Ag content. Thus, a photoanode that contained a high amount of Ag with a higher Rct value corresponded to an ineffective electron transfer process between the photoanode and electrolyte interface. The changes in Rs and Rct could mainly be attributed to the changes in the Ag content of the photoanode, which contributed the most to the internal impedance. The Nyquist plot for different types of photoanodes is shown in Fig. 9(B). As demonstrated in the Nyquist plot, both the real (Z’) and imaginary (Z”) parts of the total impedance, as well as the Rct value, increased in the order N,S-TiO2 < TiO2 < N,S-TiO2@Ag, which showed the crucial dependence of the charge transport on the dopants (N and S) and Ag incorporation .The Rs value also increased with the addition of N, S and Ag. Therefore, the origin of the higher Jsc in N,S-TiO2@Ag is expected to have arisen from the device resistance (Rs), Rct and charge transport dynamics determined by the electron lifetime (τn). Based on the Bode phase plots (Fig. 9(C,D)), the frequency was apparently shifted to the lower frequency region with the addition of N, S and Ag. The maximum frequencies (ωmax) in the middle frequency region of the Bode plots for TiO2 and N,S-TiO2@Ag were 792.44 Hz and 316.23 Hz, respectively. Since ωmax is inversely associated with the electron lifetime τn = 1/(2πf)38,39, the decrease in ωmax indicated a reduced rate for the charge-recombination process in the DSSC. Hence, electrons with longer τn values were prevented from recombining, characterized by a larger Rct.
Furthermore, Table 3 and Fig. 10 summarize the results obtained from the Nyquist plot. The N,S-TiO2@Ag photoanode exhibited a faster electron transport time (τs = Rs*Cμ)38,40,41 than TiO2. Hence, its electron lifetime (τn = Rct*Cμ))38,40,41 was significantly increased and they were prevented from recombining. The photovoltaic performance of the DSSC was clearly reflected by the charge collection efficiency (ηc) derived from ηc = (1+Rs/Rct)−1 38,40,41. Eventually, the charge collection efficiency was significantly increased upon the addition of N, S and Ag. Therefore, we can conclude that as a result of the longer τn and larger Rct, the devices fabricated using N,S-TiO2@Ag showed improved Jsc values compared to the TiO2 and N,S-TiO2 photoanode-based DSSCs.
The electrocatalytic activity of the Pt counter electrode was investigated by cyclic voltammetry (CV) technique in 10 mM LiI, 1 mM I2 and 0.1 M LiClO4 acetonitrile solution. The observed more negative cathodic peak due to the reduction of tri-iodide and the anodic peak corresponds to the reverse reaction42. Further, the role of Pt counter electrode on the photovoltaic performance of DSSC was studied by electrochemical impedance spectroscopy (EIS) analysis (Fig. S4(b and c)). The charge transfer resistance (RCT) of the electrode was calculated as 103.41 Ω from the half the real impedance of the high-frequency side semicircle and thus indicate the higher electrocatalytic activity of Pt counter electrode.
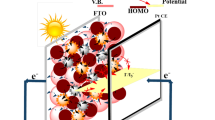
Operation principle of N,S-TiO2@Ag photoanode-modified DSSCs
The operation principle of the N,S-TiO2@Ag nanocomposite photoanode-modified DSSC under illumination is shown in Fig. 11a. Upon illumination, the adsorbed dye molecules (N719) underwent photo-excitation. The excited electrons were injected into the conduction band of the N,S-TiO2 nanocomposite. The dye+ became oxidized by receiving electrons from the electrolyte through the redox system and was regenerated. The electrolyte itself was regenerated via the platinum counter electrode by electrons passing through the external circuit. In our study, the deposition of Ag onto the surface of N,S-TiO2 not only acted as an electron sink for efficient charge transfer, but could also be used as a scattering element for plasmonic scattering to trap the light in the near field coupled with the dye molecules43. This will eventually improve the optical absorption of the dye, resulting in a significant photocurrent enhancement (Fig. 11b). In addition, the inclusion of Ag nanoparticles resulted in a change in the Fermi energy level. The electrons in the conduction band of the TiO2 could be effectively captured by the Ag until a Fermi level equilibrium was obtained and the charge recombination process was minimized, which improved the DSSC performance. The ideal property of Ag nanoparticles and the presence of N and S also successfully reduced the band gap and helped to shift the optical absorbance toward the visible region. The incorporation of N in the TiO2 lattice in the form of N-Ti-O partially converted the system from Ti4+ to Ti3+ and effectively contributed to the visible light absorption44,45. Furthermore, the existence of S also played a vital role in the photovoltaic performance, facilitated the electron transfer process and consequently increased the forward reduction reaction. The Ag and S acted as co-catalysts and effectively minimized the electron recombination process. The attractiveness of the present photoanode is that it combines all of the photovoltaic fundamental criteria within its electronic environment.
Conclusion
A simple approach to prepare Ag nanoparticle-deposited N,S-TiO2 via a chemical reduction method to fabricate a novel photoanode for a DSSC was demonstrated. The N,S-TiO2@Ag nanocomposite was characterized using UV–visible absorption, PL, Raman, XRD, XPS, SEM, EDAX and TEM analyses. The DSSC fabricated with an N,S-TiO2@Ag-modified photoanode showed an enhanced solar-to-electrical energy conversion efficiency of 8.22% compared to the photoanode of a DSSC composed of unmodified TiO2 (2.57%) under simulated solar irradiation of 100 mWcm−2 with AM 1.5 G. The enhancement in the photovoltaic performance was mainly attributed to the plasmonic Ag nanoparticles, which enhanced the visible light adsorption as a result of their light harvesting property in the visible range due the surface plasmon effect. These Ag nanoparticles also significantly promoted interfacial charge transfer, which minimized the charge recombination process. The optimal Ag content in the N,S-TiO2 to obtain an efficient photoanode was found to be 20% Ag. In addition, the presence of the N and S dopants also helped to reduce the band gap, shift the optical absorbance toward the visible region and also suppress the charge recombination. The synergetic effect of the Ag nanoparticles, surface plasmon effect, reduction of the band gap and effective charge transfer ameliorated the photocurrent generation and conversion efficiency of the DSSC.
Experimental Methods
Materials
Titanium dioxide (P25) was purchased from Acros Organics. Silver nitrate (AgNO3) and Thiourea were purchased from Merck and Fluka, respectively. Sodium borohydride (NaBH4) was purchased from R&M chemicals. Indium tin oxide (ITO) conducting glass slides (7 Ω/sq) were purchased from Xin Yan Technology Limited, China. N719 (Ruthenizer 535-bisTBA) and iodolyte Z-100 were received from Solaronix.
Materials characterization techniques
The crystalline phase of each sample was determined via X-ray diffraction (XRD; D5000, Siemens), using copper Kα radiation (λ = 1.5418 Å) at a scan rate of 0.02° s−1. The morphology of each film was examined using field emission scanning electron microscopy (Hitachi, SU 8000), transmission electron microscopy TEM (Hitachi, HT-7700) and high-resolution TEM (JEOL JEM-2100 F). The optical absorption properties in the spectral region of 190–900 nm were assessed using a Thermo Scientific Evolution 300 UV–vis spectrophotometer. The photoluminescence and Raman spectra were collected using a Renishaw inVia 2000 system with an argon ion laser emitting at 325 and 532 nm, respectively. X-ray photoelectron spectroscopy (XPS) measurements were performed using synchrotron radiation from beamline no. 3.2 at the Synchrotron Light Research Institute, Thailand.
Synthesis of N,S-TiO2-Ag nanocomposite
The N,S-TiO2@Ag nanocomposite was prepared as follows. Initially, TiO2 and thiourea at a 1:1 weight ratio were mixed and ground for at least 30 min in a mortar. The mixture was then annealed in a furnace at 400 °C with a heating rate of 10 °C/min in air for 1 h. Subsequently, the pale yellow N,S-TiO2 was obtained. The N,S-TiO2@Ag nanocomposite was prepared using a chemical reduction method. A 500-mg quantity of N,S-TiO2 was added to an aqueous solution containing the AgNO3. The weight percentage of the AgNO3 to N,S-TiO2 varied from 0% to 5%, 10%, 20% and 40%. Each mixture was vigorously stirred for 30 min at room temperature. The reduction of Ag+ was carried out by the drop-wise addition of a freshly prepared ice-cold NaBH4 solution until the color of the mixture changed. A yellowish green solution indicated the formation of the N,S-TiO2@Ag nanocomposite, after which and the solution continued to be stirred for another 30 min. The nanocomposite was collected and washed with distilled water and ethanol several times by centrifugation. Finally, the product was dried in an oven at 60 °C.
Fabrication of N,S-TiO2@Ag-modified photoanode
The N,S-TiO2@Ag-modified photoanodes were fabricated using the following procedure. Initially, 300 mg of the N,S-TiO2@Ag nanocomposite was mixed in an ethanolic solution and stirred for 30 min. A 0.1 M quantity of TTIP was slowly introduced into the above reaction mixture and stirred until a homogenous solution was obtained. Finally, the N,S-TiO2@Ag nanocomposite was coated on a conducting side of the ITO using the doctor-blade technique. In order to obtain a stable photoanode, the film was dried at room temperature, sintered at 150 °C for 30 min in a muffle furnace and then allowed to cool naturally to room temperature.
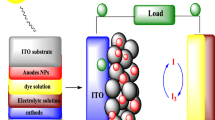
Fabrication of DSSCs and evaluation of their performances
The prepared N,S-TiO2@Ag nanocomposite photoanodes were immersed into an ethanolic solution of 0.3 mM N719 (Ruthenizer 535-bisTBA) dye for 24 h at room temperature. The dye-adsorbed photoanode was withdrawn from the solution and immediately cleaned with ethanol. A platinum-sputtered ITO was placed on the dye-absorbed photoanode and they were clamped firmly together. An electrolyte (Iodolyte Z-100, Solaronix) solution was introduced into the cell assembly by capillary action. A 150-W Xenon arc lamp (Newport, Model 69907) containing a simulated AM 1.5-G filter was used as a light source throughout the experiments. Prior to testing the photovoltaic parameter, an Avaspec-2048 fiber optic spectrophotometer was used to measure the light illumination intensity. The photocurrent signal measurements (J-V and J-T curves) were carried out with an active electrode area of 0.5 cm2 using a computer-controlled VersaSTAT 3 electrochemical workstation (Princeton Applied Research, USA).
Additional Information
How to cite this article: Lim, S. P. et al. Boosting Photovoltaic Performance of Dye-Sensitized Solar Cells Using Silver Nanoparticle-Decorated N,S-co-doped-TiO2 Photoanode. Sci. Rep. 5, 11922; doi: 10.1038/srep11922 (2015).
References
O’Regan, B. & Gratzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 353, 737–740 (1991).
van de Lagemaat, J., Park, N. G. & Frank, A. J. Influence of electrical potential distribution, charge transport and recombination on the photopotential and photocurrent conversion efficiency of dye-sensitized nanocrystalline TiO2 solar cells: A study by electrical impedance and optical modulation techniques. J. Phys. Chem.B 104, 2044–2052 (2000).
Kopidakis, N. et al. Spatial location of transport-limiting traps in TiO2 nanoparticle films in dye-sensitized solar cells. Appl. Phys. Lett. 87, 202106-202106-3 (2005).
Lai, Y. et al. Fabrication of uniform Ag/TiO2 nanotube array structures with enhanced photoelectrochemical performance. New J. Chem. 34, 1335–1340 (2010).
Macak, J. M., Schmidt-Stein, F. & Schmuki, P. Efficient oxygen reduction on layers of ordered TiO2 nanotubes loaded with Au nanoparticles. Electrochem. Commun. 9, 1783–1787 (2007).
Yang, L., He, D., Cai, Q. & Grimes, C. A. Fabrication and Catalytic Properties of Co−Ag−Pt Nanoparticle-Decorated Titania Nanotube Arrays. J. Phys. Chem. C 111, 8214–8217 (2007).
Zhao, H., Chen, Y., Quan, X. & Ruan, X. Preparation of Zn-doped TiO2 nanotubes electrode and its application in pentachlorophenol photoelectrocatalytic degradation. Chin. Sci. Bull. 52, 1456–1461 (2007).
Muduli, S. et al. TiO2–Au plasmonic nanocomposite for enhanced dye-sensitized solar cell (DSSC) performance. Sol. Energy 86, 1428–1434 (2012).
Gao, Y. et al. Enhancement of stability of N-doped TiO2 photocatalysts with Ag loading. Appl Surf Sci 265, 796–801 (2013).
Huang, L. H., Sun, C. & Liu, Y. L. Pt/N-codoped TiO2 nanotubes and its photocatalytic activity under visible light. Appl. Surf. Sci 253, 7029–7035 (2007).
Eagen, C. F. Nature of the enhanced optical absorption of dye-coated Ag island films. Applied Optics 20, 3035–3042 (1981).
Mock, J. J. et al. Shape effects in plasmon resonance of individual colloidal silver nanoparticles. J. Chem. Phys 116, 6755–6759 (2002).
Schaadt, D. M., Feng, B. & Yu, E. T. Enhanced semiconductor optical absorption via surface plasmon excitation in metal nanoparticles. Appl. Phys. Lett. 86, 063106 (2005).
Lee, K.-C. et al. Size effect of Ag nanoparticles on surface plasmon resonance. Surf. Coat. Technol. 202, 5339–5342 (2008).
Wen, C., Ishikawa, K., Kishima, M. & Yamada, K. Effects of silver particles on the photovoltaic properties of dye-sensitized TiO2 thin films. Sol. Energy Mater. Sol. Cells 61, 339–351 (2000).
Zhao, G., Kozuka, H. & Yoko, T. Effects of the incorporation of silver and gold nanoparticles on the photoanodic properties of rose bengal sensitized TiO2 film electrodes prepared by sol-gel method. Sol. Energy Mater. Sol. Cells 46, 219–231 (1997).
Zhang, J. et al. Development of modified N doped TiO2 photocatalyst with metals, nonmetals and metal oxides. Energy Environ. Sci. 3, 715–726 (2010).
Kang, S. H., Kim, H. S., Kim, J.-Y. & Sung, Y.-E. Enhanced photocurrent of nitrogen-doped TiO2 film for dye-sensitized solar cells. Mater. Chem. Phys. 124, 422–426 (2010).
Pany, S., Naik, B., Martha, S. & Parida, K. Plasmon induced nano Au particle decorated over S,N-modified TiO2 for exceptional photocatalytic hydrogen evolution under visible light. ACS Appl. Mater. Interfaces 6, 839–846 (2013).
Zhang, J. C. et al. N, S-doped TiO2 anode effect on performance of dye-sensitized solar cells. J. Phys. Chem. Solids 72, 1239–1244 (2011).
Chiarello, G. L., Aguirre, M. H. & Selli, E. Hydrogen production by photocatalytic steam reforming of methanol on noble metal-modified TiO2 . J. Catal. 273, 182–190 (2010).
Sivaranjani, K. & Gopinath, C. S. Porosity driven photocatalytic activity of wormhole mesoporous TiO2−xNx in direct sunlight. J. Mater. Chem. 21, 2639–2647 (2011).
Lim, S. P., Huang, N. M., Lim, H. N. & Mazhar, M. Aerosol assisted chemical vapour deposited (AACVD) of TiO2 thin film as compact layer for dye-sensitised solar cell. Ceram. Int. 40, 8045–8052 (2014).
Su, C. et al. Fabrication of Ag/TiO2 nanoheterostructures with visible light photocatalytic function via a solvothermal approach. CrystEngComm 14, 3989–3999 (2012).
Chen, X. et al. Formation of oxynitride as the photocatalytic enhancing site in nitrogen-doped titania nanocatalysts: comparison to a commercial nanopowder. Adv. Funct. Mater. 15, 41–49 (2005).
Saha, N. C. & Tompkins, H. G. Titanium nitride oxidation chemistry: An X-ray photoelectron spectroscopy study. J. Appl. Phys. 72, 3072–3079 (1992).
Sathish, M., Viswanathan, B. & Viswanath, R. P. Alternate synthetic strategy for the preparation of CdS nanoparticles and its exploitation for water splitting. Int. J. Hydrogen Energy 31, 891–898 (2006).
Zhou, M. & Yu, J. Preparation and enhanced daylight-induced photocatalytic activity of C,N,S-tridoped titanium dioxide powders. J. Hazard. Mater. 152, 1229–1236 (2008).
Yu, J. C. et al. Efficient Visible-Light-Induced photocatalytic dDisinfection on sulfur-doped nanocrystalline titania. Environ. Sci. Technol. 39, 1175–1179 (2005).
Subramanian, V., Wolf, E. E. & Kamat, P. V. Catalysis with TiO2/gold nanocomposites. Effect of metal particle size on the fermi level equilibration. J. Am. Chem. Soc. 126, 4943–4950 (2004).
Lim, S. P., Huang, N. M., Lim, H. N. & Mazhar, M. Surface modification of aerosol-assisted CVD produced TiO2 thin film for dye sensitised solar cell. Int. J. Photoenergy 2014, 1–12 (2014).
Lim, S. P., Pandikumar, A., Huang, N. M. & Lim, H. N. Silver/titania nanocomposite-modified photoelectrodes for photoelectrocatalytic methanol oxidation. Int. J. Hydrogen Energy 39, 14720–14729 (2014).
Du, J. et al. Controlled synthesis of Ag/TiO2 core−shell nanowires with smooth and bristled surfaces via a one-step solution route. Langmuir 22, 1307–1312 (2006).
Ni, M., Leung, M. K. H., Leung, D. Y. C. & Sumathy, K. A review and recent developments in photocatalytic water-splitting using for hydrogen production. Renew. Sust. Energ. Rev. 11, 401–425 (2007).
Christopher, P., Ingram, D. B. & Linic, S. Enhancing Photochemical Activity of Semiconductor Nanoparticles with Optically Active Ag Nanostructures: Photochemistry Mediated by Ag Surface Plasmons. J. Phys. Chem. C 114, 9173–9177 (2010).
Guo, L. et al. Highly monodisperse polymer-capped ZnO nanoparticles: Preparation and optical properties. Appl. Phys. Lett. 76, 2901–2903 (2000).
Wang, H., Leonard, S. L. & Hu, Y. H. Promoting effect of graphene on dye-sensitized solar cells. Ind. Eng. Chem. Res. 51, 10613–10620 (2012).
Kim, S. G. et al. Nb-doped TiO2 nanoparticles for organic dye-sensitized solar cells. RSC Adv. 3, 16380–16386 (2013).
Archana, P. S., Gupta, A., Yusoff, M. M. & Jose, R. Tungsten doped titanium dioxide nanowires for high efficiency dye-sensitized solar cells. Phys. Chem. Chem. Phys. 16, 7448–7454 (2014).
Nissfolk, J., Fredin, K., Hagfeldt, A. & Boschloo, G. Recombination and transport processes in dye-sensitized solar sells investigated under working conditions. J. Phys. Chem. B 110, 17715–17718 (2006).
Wang, Q., Zhang, Z., Zakeeruddin, S. M. & Grätzel, M. Enhancement of the performance of dye-sensitized solar cell by formation of shallow transport levels under visible vight illumination. J. Phys. Chem. C 112, 7084–7092 (2008).
Lan, J.-L. et al. The simple and easy way to manufacture counter electrode for dye-sensitized solar cells. Curr. Appl Phys. 10, S168–S171 (2010).
Lin, S., Lee, K., Wu, J. & Wu, J. Plasmon-enhanced photocurrent in dye-sensitized solar cells. Solar Energy 86, 2600–2605 (2012).
Pany, S., Parida, K. M. & Naik, B. Facile fabrication of mesoporosity driven N-TiO2@CS nanocomposites with enhanced visible light photocatalytic activity. RSC Adv. 3, 4976–4984 (2013).
Naik, B., Parida, K. M. & Gopinath, C. S. Facile synthesis of N- and S-incorporated nanocrystalline TiO2 and direct solar-light-driven photocatalytic activity. J. Phys. Chem. C 114, 19473–19482 (2010).
Acknowledgements
This work was financially supported by a University of Malaya Research Grant Programme (RP007C/13AFR), Science Fund from the Ministry of Science, Technology and Innovation (06-01-04-SF1513), a High Impact Research Grant from the Ministry of Higher Education of Malaysia (UM.C/625/1/HIR/MOHE/05) and a University Malaya Flagship project (FL017-2011). The authors are grateful to the Synchrotron Light Research Institute, Nakhon Ratchasima, Thailand, for the XPS measurements.
Author information
Authors and Affiliations
Contributions
L.S.P. designed and carried out the experiment. L.S.P. analyzed and wrote the main manuscript. A.P., R.R., H.N.M. and L.H.N. reviewed and commented on the paper. All the authors participated in the discussion and commented on the paper.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Lim, S., Pandikumar, A., Lim, H. et al. Boosting Photovoltaic Performance of Dye-Sensitized Solar Cells Using Silver Nanoparticle-Decorated N,S-Co-Doped-TiO2 Photoanode. Sci Rep 5, 11922 (2015). https://doi.org/10.1038/srep11922
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep11922
This article is cited by
-
Enhanced visible light photocatalytic VOC oxidation via Ag-loaded TiO2/SiO2 materials
Journal of Materials Science (2024)
-
A doped heterojunction photoanode: a strategy-driven approach to improve the charge carrier dynamics and photoconversion performance of the TiO2-based dye-sensitized solar cell
Journal of Materials Science: Materials in Electronics (2024)
-
Synergistic effect of Ga(NO3)3 & TiCl4 post-treatment on photovoltaic performance of dye-sensitized solar cells
Applied Physics A (2023)
-
Role of rare-earth oxides, conjugated with \({\mathrm{TiO}}_{2}\), in the enhancement of power conversion efficiency of dye sensitized solar cells (DSSCs)
Environmental Science and Pollution Research (2023)
-
Plasmonic Engineering of TiO2 Photoanodes for Dye-Sensitized Solar Cells: A Review
Journal of Electronic Materials (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.