Abstract
Disperse fine equiaxed α-Al2O3 nanoparticles with narrow size distribution are important materials in nanotechnology and nanomaterials, but syntheses of disperse fine equiaxed α-Al2O3 nanoparticles usually result in fine γ-Al2O3 nanoparticles or large α-Al2O3 nanoparticles larger than 15 nm. α-Al2O3 has a higher surface energy than γ-Al2O3 and becomes thermodynamically not stable with respect to γ-Al2O3 at specific surface areas larger than 100 m2/g (at sizes smaller than 15 nm for spherical particles) at room temperature. So disperse fine equiaxed α-Al2O3 nanoparticles smaller than 15 nm with narrow size distribution are extremely difficult to synthesise. Here we show the successful synthesis of disperse fine equiaxed α-Al2O3 nanoparticles with average sizes below 10 nm and narrow size distribution by selective corrosion and refined fractionated coagulation separation. An almost fully dense nanocrystalline α-Al2O3 ceramic with a relative density of 99.5% and an average grain size of 60 nm can be sintered from disperse fine equiaxed α-Al2O3 nanoparticles with narrow size distribution.
Similar content being viewed by others
Introduction
Disperse fine equiaxed α-Al2O3 nanoparticles (NPs) are important materials for applications such as cancer therapy1, abrasive polishing2 and nanocomposite materials3. Disperse fine equiaxed α-Al2O3 NPs with narrow size distribution are essential raw materials for sintering nanocrystalline α-Al2O3 ceramic which may exhibit low temperature ductility like nanocrystalline CaF2 and TiO2 ceramics with an average grain size of 8 nm showing large plastic deformations at 80 and 180 °C respectively4 and nanocrystalline TiO2 ceramic with an average grain size of 40 nm showing a large true strain of −0.6 at 800 °C5. Many efforts have been made to synthesise disperse fine equiaxed α-Al2O3 NPs with sizes below 15 nm and narrow size distribution6,7,8,9,10,11,12,13,14,15. Johnston et al. reported γ-Al2O3 NPs of 5–14 nm in size prepared by reactive laser ablation of aluminum in oxygen, but upon transformation into α-Al2O3 at 1200 °C, the particles grew to large particles of about 52 nm6. Noguchi et al. synthesised fine γ-Al2O3 NPs of about 6 nm in supercritical water by continuous hydrothermal flow reaction system7. Li et al. prepared vermicular α-Al2O3 particles with sizes of about 100 nm agglomerated from primary particles of about 10 nm by precipitation method using α-Al2O3 seeding and calcination at 900 °C8. Das et al. synthesised porous α-Al2O3 powders of the hard-agglomerated particles with primary particle sizes of about 20 nm by the thermal decomposition of an aqueous solution of aluminum nitrate and sucrose and calcination at 600 °C9. Laine et al. converted θ-Al2O3 NPs to 29 nm (86% α and 14% θ) Al2O3 NPs by liquid-feed flame spray pyrolysis and sintered a 99.5% dense α-Al2O3 ceramic with a grain size of about 350 nm from the Al2O3 NPs10. Zhang et al. prepared α-Al2O3 nanostructures of about 150 nm in size consisting of nanorods of about 15 nm in diametre and about 150 nm in length by calcining fine boehmite powders at 1000 °C11. Yoo et al. reported vermicular α-Al2O3 particles with primary particle sizes of about 35 nm synthesised by vapor-phase hydrolysis of AlCl3 and calcination at 1200 °C12. Karagedov et al. prepared disperse equiaxed α-Al2O3 NPs with an average size of 25 nm by ball-milling α-Al2O3 powders and boiling the ball-milled α-Al2O3 powders in HCl13 and α-Al2O3 NPs of about 50 nm by precipitation method using 25 nm α-Al2O3 NP seeding and calcination at 930 °C14. Borsella et al. synthesised vermicular α-Al2O3 NPs with primary particle sizes of about 15 nm by laser synthesis from gaseous precursors15. So syntheses of disperse fine equiaxed α-Al2O3 NPs usually result in fine γ-Al2O3 NPs6,7 or large α-Al2O3 NPs larger than 15 nm8,9,10,11,12,13,14,15.
α-Al2O3 (corundum) is the thermodynamically stable phase of bulk Al2O3 at common pressure and temperature conditions. Simulation and experimental studies showed that α-Al2O3 has a higher surface energy (2.64 J/m2) than γ-Al2O3 (1.67 J/m2) and becomes thermodynamically not stable with respect to γ-Al2O3 at specific surface areas larger than 125 m2/g (at specific surface areas larger than 100 m2/g at room temperature)16,17 (specific surface areas of 125 and 100 m2/g correspond to diametres of 12 and 15 nm for spherical particles respectively). Perrotta et al. synthesised α-Al2O3 particles with a specific surface area of 150 m2/g by the dehydration of diaspore to α-Al2O318,19 and attributed the formation of the high surface area α-Al2O3 NPs to the chemisorbed H2O which could stabilise the high surface area of α-Al2O3 NPs18, but these high surface area α-Al2O3 particles have a platy morphology20. So the synthesis of disperse fine equiaxed α-Al2O3 NPs smaller than 15 nm with narrow size distribution is extremely difficult and remains a challenge so far.
In this article, we present the successful synthesis of disperse fine equiaxed α-Al2O3 NPs with different average particle sizes below 10 nm and narrow size distribution widths by the refined fractionated coagulation separations of disperse equiaxed α-Al2O3 NPs with a wide size distribution width. Disperse equiaxed α-Al2O3 NPs with a wide size distribution width were prepared by removing the matrixes in the α-Al2O3-NPs-embedded composites through selective corrosion. The α-Al2O3-NPs-embedded composite powders were synthesised by mechanochemical method. Our primary sintering experiments demonstrate that green compacts of the α-Al2O3 NPs with an average particle size of 7.9 nm and a size distribution width of 4–14 nm were sintered to an almost fully dense nanocrystalline α-Al2O3 ceramic with a relative density of 99.5% and an average grain size of 60 nm by a non-optimised two-step pressureless sintering.
Results
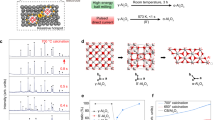
To prepare disperse fine equiaxed α-Al2O3 NPs, α-Al2O3-NPs-embedded composite powders were synthesised at first by mechanochemical method. Stoichiometric mixtures of Al and Fe2O3 powders (according to Fe2O3 + 2Al = 2Fe + Al2O3) were milled under the optimised ball milling conditions [a main disk rotation speed of 300 revolutions per minute (rpm), a ball-to-powder ratio (BPR) of 20:1 and a milling duration of 20 h] [see Methods and Supplementary Information] and characterized by x-ray diffraction (XRD) and transmission electron microscopy (TEM). The XRD pattern of the ball-milled powders shows the overlapped XRD patterns of α-Al2O3 and α-Fe without any other diffraction peaks (Fig. 1a), indicating that the ball-milled powders consist of α-Al2O3 and α-Fe phases. The dark-field TEM observations reveal equiaxed α-Al2O3 NPs of 2–250 nm in size in the ball-milled powders. The dark-field TEM image of the ball-milled powders in Fig. 1b shows that α-Al2O3 NPs (bright) in the ball-milled powders are 3 to 20 nm in size and equiaxed in shape. The XRD and dark-field TEM analyses demonstrate that the ball-milled powders are the composites of α-Al2O3 NPs embedded in the Fe matrix. The volume fraction of α-Al2O3 in the composites, estimated from the stoichiometry of 2Al + Fe2O3 = 2Fe + Al2O3, is about 64%. α-Al2O3-NPs-embedded composite powders can also be prepared using Al and CoO powders as starting powders by mechanochemical method.
Composites of α-Al2O3 NPs embedded in Fe matrix.
(a) XRD pattern of the composite powders obtained by ball-milling stoichiometric mixtures of Al and Fe2O3 powders (according to 2Al + Fe2O3 = 2Fe + Al2O3) at a BPR of 20:1 and a main disk rotation speed of 300 rpm for 20 h, showing coexistence of α-Al2O3 and α-Fe. (b) Dark-field TEM image of the ball-milled composite powders showing fine equiaxed α-Al2O3 NPs (bright) in the composite. Scale bar, 100 nm.
To obtain disperse fine equiaxed α-Al2O3 NPs, the matrixes in the α-Al2O3-NPs-embedded composites were removed through selective corrosion. The composite powders of α-Al2O3 NPs embedded in the Fe matrix prepared by high-energy ball milling were corroded with hydrochloric acid at room temperature and at 120 °C (Methods and Supplementary Information). The powders obtained by selective corrosion were analysed by XRD, TEM, energy dispersive x-ray spectroscopy (EDS) and inductively coupled plasma-atomic emission spectrometry (ICP-AES). The powders yield a typical XRD pattern of α-Al2O3 without any additional diffraction peaks (Fig. 2a), indicating that the powders are pure α-Al2O3. This phase identification was supported by the selected area electron diffraction (SAED) pattern of the powders obtained by selective corrosion (Supplementary Fig. S1a). The TEM image in Fig. 2b and the low magnification TEM image in Supplementary Fig. S1b reveal that the powders obtained by selective corrosion are disperse equiaxed α-Al2O3 NPs of sizes ranging from 3 to 200 nm without agglomeration. The average particle size and size distribution of the α-Al2O3 NPs were statistically determined from the TEM observations; and the size distribution histogram of the α-Al2O3 NPs is shown in Fig. 2c. According to the size distribution histogram of the α-Al2O3 NPs (Fig. 2c), most of α-Al2O3 NPs (~69%) are smaller than 15 nm whereas few of α-Al2O3 NPs (~31%) are larger than 15 nm and the average particle size and size distribution width of the α-Al2O3 NPs are 14.3 and 2–250 nm respectively. The purity of the disperse equiaxed α-Al2O3 NPs, determined by EDS and ICP-AES analyses (Supplementary Fig. S2 and Supplementary Tables S1 and S2), is 99.6% (mass percent) though the starting Al and Fe2O3 powders only have a purity of 99.0% (mass percent).
α-Al2O3 NPs obtained by removing the Fe matrix in the composites.
(a) XRD pattern of the α-Al2O3 NPs obtained by removing the Fe matrix in the composites of α-Al2O3 NPs embedded in the Fe matrix through selective corrosion, showing a typical XRD pattern of α-Al2O3. (b) TEM image of the α-Al2O3 NPs obtained by removing the Fe matrix in the composites, revealing disperse equiaxed NPs. Scale bar, 50 nm. (c) Size distribution histogram (inset: enlarged part for large particles) of the α-Al2O3 NPs obtained by removing the Fe matrix in the composites, showing that most of α-Al2O3 NPs are smaller than 15 nm.
Disperse equiaxed α-Al2O3 NPs, obtained by removing the Fe matrix in the α-Al2O3-NPs-embedded composites through selective corrosion, have a small average particle size of 14.3 nm but a quite wide size distribution width from 2 to 250 nm (Fig. 2c). For many applications, for example, sintering dense nanocrystalline ceramics, a narrow particle size distribution width is desired because large particles will grow at the expense of small particles (Ostwald ripening) during sintering21,22,23, resulting in coarse-grained ceramics rather than expected nanocrystalline ceramics. To obtain disperse fine equiaxed α-Al2O3 NPs with a narrow size distribution width, the disperse equiaxed α-Al2O3 NPs with a wide size distribution width of 2–250 nm should be size-selectively separated. Fractionated coagulation was applied to separate Au NPs of 7.5 and 80 nm using NaNO3 as coagulating agent24. The critical coagulation concentration decreases when the particle size increases24. The disperse equiaxed α-Al2O3 NPs with a wide size distribution width of 2–250 nm were size-selectively separated by refined fractionated coagulation (Methods). Here refined fractionated coagulation refers to fractionated coagulation by decreasing the concentration of coagulating agent in a small interval to decrease size distribution widths of coagulation-separated NPs. Hydrochloric acid was used as coagulating agent for the size-selective separations of α-Al2O3 NPs. The particle size of coagulation increases when the HCl concentration decreases for disperse equiaxed α-Al2O3 NPs. By stepwise decreasing HCl concentration from 1.4 to 0.8 mol/L at a step of 0.2 mol/L, namely at HCl concentrations of 1.4, 1.2, 1.0 and 0.8 mol/L, disperse fine equiaxed α-Al2O3 NPs with an average size of 5.2 nm and a size distribution width of 2–9 nm, an average size of 6.5 nm and a size distribution width of 3–11 nm, an average size of 7.9 nm and a size distribution width of 4–14 nm as well as an average size of 9.6 nm and a size distribution width of 5–15 nm were separated by refined fractionated coagulation respectively (Table 1). The TEM images of the α-Al2O3 NPs with an average size of 5.2 nm (Fig. 3a and Supplementary Fig. S3a) show that the α-Al2O3 NPs are fully disperse without any agglomeration, 3 to 8 nm in size and equiaxed in shape. The SAED pattern in the inset of Fig. 3a supports the phase identification of α-Al2O3 by XRD analysis. The size distribution width of the α-Al2O3 NPs with an average size of 5.2 nm (Fig. 3b) is narrow (2–9 nm), compared with that before the size-selective separation (2–250 nm) in Fig. 2c. The high resolution TEM (HRTEM) image in Fig. 3c shows the two-dimensional hexagonal lattice image of a single α-Al2O3 NP among the α-Al2O3 NPs with an average size of 5.2 nm along the [0001] direction. The TEM images of the α-Al2O3 NPs with average particle sizes (and size distribution widths) of 6.5 (3–11), 7.9 (4–14) and 9.6 (5–15) nm in Fig. 3d–f and Supplementary Fig. S3b-d reveal disperse fine equiaxed α-Al2O3 NPs with narrow size distribution widths (Supplementary Fig. S4). The XRD analysis of the α-Al2O3 NPs with an average size of 7.9 nm shows the broad diffraction peaks of pure α-Al2O3 (Supplementary Fig. S5). The average grain size estimated from the XRD peak widths using Scherrer equation is 7.5 nm, close to the average particle size of the α-Al2O3 NPs (7.9 nm) determined statistically by TEM analysis. The specific surface area of the α-Al2O3 NPs with an average size of 7.9 nm, measured by N2 adsorption at 77 K using the Brunauer-Emmett-Teller (BET) method (Supplementary Fig. S6), is 170 m2/g.
α-Al2O3 NPs with different average particle sizes and narrow size distribution widths.
(a,b) TEM image (scale bar, 20 nm; inset: SAED pattern, scale bar, 5 nm−1) (a) and size distribution histogram (b) of the α-Al2O3 NPs obtained by refined fractionated coagulation separation with 1.4 mol/L HCl, showing disperse fine equiaxed α-Al2O3 NPs with an average particle size of 5.2 nm and a size distribution width of 2–9 nm. (c) HRTEM image (the beam direction in [0001]; scale bar, 2 nm) of a single α-Al2O3 NP among the α-Al2O3 NPs with an average size of 5.2 nm, showing the hexagonal lattice of α-Al2O3. (d–f) TEM images (scale bar, 20 nm) of the α-Al2O3 NPs with average particle sizes of 6.5 (d), 7.9 (e) and 9.6 nm (f) obtained by refined fractionated coagulation separation with 1.2, 1.0 and 0.8 mol/L HCl respectively. Low magnification TEM images of the α-Al2O3 NPs with average sizes of 5.2, 6.5, 7.9 and 9.6 nm are shown in Supplementary Fig. S3a-d.
To check the sintering activity of disperse fine equiaxed α-Al2O3 NPs with narrow size distribution widths (separated by refined fractionated coagulation), our disperse fine equiaxed α-Al2O3 NPs were pressed into green compacts and sintered (Methods). Chen at al. developed the two-step pressureless sintering method to suppress the final-stage grain growth and sintered dense nanocrystalline Y2O3 ceramic with a grain size of 60 nm25. Our α-Al2O3 green compacts pressed from the α-Al2O3 NPs with an average particle size of 7.9 nm and a size distribution width of 4–14 nm at 600 MPa were sintered in air by a two-step sintering (heating to 1,230 °C without hold, then decreasing to 1,080 °C with a 40 h hold). The SEM observations and Archimedes measurements reveal that the sintered bodies have a relative density of 99.5%, an average grain size of 60 nm and a grain size distribution width of 20–130 nm (Fig. 4 and Supplementary Fig. S7). This two-step pressureless sintering process (1,230 °C–1,080 °C for 40 h) was not optimised, yet the sintered nanocrystalline α-Al2O3 ceramic exhibits a relative density as high as 99.5% and an average grain size as small as 60 nm.
Microstructure of almost fully dense nanocrystalline α-Al2O3 ceramic with an average grain size of 60 nm.
SEM cross-sectional image of the sintered body of a green compact pressed from the disperse fine equiaxed α-Al2O3 NPs with an average particle size of 7.9 nm and a size distribution width of 4–14 nm at 600 MPa and sintered by a non-optimised two-step pressureless sintering (heating to 1,230 °C without hold and decreasing to 1,080 °C with a 40 h hold in air) (after additional thermal etching). Scale bar, 100 nm. The sintered nanocrystalline α-Al2O3 ceramic has a relative density of 99.5%, an average grain size of 60 nm and a grain size distribution width of 20–130 nm. A low magnification SEM image of the sintered nanocrystalline α-Al2O3 ceramic is shown in Supplementary Fig. S7.
Discussion
The disperse fine equiaxed α-Al2O3 NPs separated by refined fractionated coagulation have narrow size distribution widths and average particle sizes below 10 nm, much smaller than the particle sizes of disperse equiaxed α-Al2O3 NPs achieved so far (25 nm α-Al2O3 NPs prepared by ball-milling and HCl boiling14 or 29 nm (86% α and 14% θ) Al2O3 NPs synthesised by liquid-feed flame spray pyrolysis10). The specific surface area of the α-Al2O3 NPs with an average size of 7.9 nm (170 m2/g) is close to a specific surface area of 178 m2/g, estimated from the size distribution histogram of the α-Al2O3 NPs with an average size of 7.9 nm (Supplementary Fig. S4) by assuming a spherical shape for the α-Al2O3 NPs and much higher than those of high surface area platy α-Al2O3 particles synthesised by the dehydration of diaspore (150 m2/g)18,19, (86% α) Al2O3 NPs synthesised by liquid-feed flame spray pyrolysis (40–60 m2/g)10 and α-Al2O3 NPs prepared by ball-milling and HCl boiling (57 m2/g)14. It is also much higher than 100 m2/g above which α-Al2O3 becomes thermodynamically not stable with respect to γ-Al2O3 at room temperature17. Moreover, the refined fractionated coagulation separation method is more efficient and much simpler than other size-selective separation methods such as gel electrophoresis26 and density gradients27 and can be scaled up for large-scale separations of α-Al2O3 NPs with narrow size distribution widths and average particle sizes below 10 nm (and for large-scale separations of α-Al2O3 NPs with narrow size distribution widths and average particle sizes above 10 nm as well, see Supplementary Fig. S3e-h) from α-Al2O3 NPs with a wide size distribution width.
α-Al2O3 in bulk form is thermodynamically stable at common pressure and temperature conditions. However, α-Al2O3 has a higher surface energy than γ-Al2O3, equiaxed α-Al2O3 NPs smaller than 15 nm are thermodynamically not stable (equiaxed γ-Al2O3 NPs smaller than 15 nm are thermodynamically stable) at room temperature17. The formation of fine equiaxed α-Al2O3 NPs smaller than 15 nm in the α-Al2O3-NPs-embedded composites during mechanochemical synthesis is thermodynamically unclear. On one hand, the impact of the balls in the high-energy ball milling can bring about a high energy in the collision regions28 which may favor the formation of thermodynamically not stable fine equiaxed α-Al2O3 NPs. Metastable metallic, intermetallic and oxide phases can form in high-energy ball milling, as reported in literature28. On the other hand, as the Fe2O3 + 2Al = 2Fe + Al2O3 reaction appears to take place on atomic scale in the mechanochemical synthesis, the fine equiaxed α-Al2O3 NPs formed in the α-Al2O3-NPs-embedded composites should be surrounded by α-Fe, as shown in Fig. 1b. The α-Al2O3/α-Fe interfaces in the α-Al2O3-NPs-embedded composites may have an interface energy29 lower than the surface energy of the free surfaces of α-Al2O3 NPs, which may stabilise the high interface area of fine equiaxed α-Al2O3 NPs in the α-Al2O3-NPs-embedded composites and conduce to the formation of the fine equiaxed α-Al2O3 NPs smaller than 15 nm. The successful synthesis of disperse fine equiaxed α-Al2O3 NPs smaller 15 nm, which are thermodynamically not stable, exemplifies that thermodynamically not stable nanomaterials may be producible.
The almost fully dense nanocrystalline α-Al2O3 ceramic with a relative density of 99.5% and an average grain size of 60 nm, sintered from the disperse fine equiaxed α-Al2O3 NPs with a narrow size distribution width by two-step pressureless sintering (1,230 °C–1,080 °C for 40 h), exhibits the finest average grain size for a 99.5% dense nanocrystalline α-Al2O3 ceramic (compared with a relative density of 99.5% and an average grain size of 350 nm10 or a relative density of 95% and an average grain size of 70 nm30) achieved so far by pressureless sintering. Therefore, disperse fine equiaxed α-Al2O3 NPs with narrow size distribution widths, prepared by our mechanochemistry-selective corrosion-refined fractionated coagulation separation approach, show a high sintering activity. Moreover, the successful pressureless sintering of an almost fully dense nanocrystalline α-Al2O3 ceramic with an average grain size as fine as 60 nm, from the disperse fine equiaxed α-Al2O3 NPs with a fine average particle size and a narrow size distribution width, reveals that disperse fine equiaxed α-Al2O3 NPs with a fine average particle size and a narrow size distribution width are an essential prerequisite for the pressureless sintering of dense nanocrystalline α-Al2O3 ceramic with a fine grain size.
In summary, this work presents a simple approach to synthesis of disperse fine equiaxed α-Al2O3 NPs with average particle sizes below 10 nm and narrow size distribution widths. Disperse fine equiaxed α-Al2O3 NPs with average particle sizes below 10 nm and narrow size distribution widths were separated from disperse equiaxed α-Al2O3 NPs with a wide size distribution width by refined fractionated coagulation. Disperse equiaxed α-Al2O3 NPs with a wide size distribution width were prepared by removing the matrixes in the α-Al2O3-NPs-embedded composites through selective corrosion. The α-Al2O3-NPs-embedded composite powders were synthesised by mechanochemical method. The green compacts of α-Al2O3 NPs with an average size of 7.9 nm and a size distribution of 4–14 nm were sintered by a non-optimised two-step pressureless sintering to a almost fully dense nanocrystalline α-Al2O3 ceramic with a relative density of 99.5% and an average grain size of 60 nm, the finest grain size achieved so far by pressureless sintering. Our mechanochemistry-selective corrosion-refined fractionated coagulation separation approach may be scaled up for large-scale production of disperse fine equiaxed α-Al2O3 NPs with average particle sizes below 10 nm and narrow size distribution widths.
Methods
Fe2O3 and Al powders mixed stoichiometrically according to Fe2O3 + 2Al = 2Fe + Al2O3 were ball-milled in a high-purity argon atmosphere using a high-energy planetary ball mill to synthesise α-Al2O3-NPs-embedded composites. The ball milling conditions were optimised to achieve α-Al2O3 NPs with finest particle sizes and lowest impurity contents as well as for a reasonable production efficiency (See Supplementary Information for experimental details). The optimised ball milling conditions are a main disk rotation speed of 300 rpm, a BPR of 20:1 and a milling duration of 20 h.
To remove the Fe matrixes (and other metal impurities from the vials and balls of the ball mill) in the composite powders and to obtain pure α-Al2O3 NPs, the ball-milled composite powders synthesised by high-energy ball milling were corroded with 12 mol/L hydrochloric acid at room temperature for 10 h and centrifuged. This room temperature acid corrosion was repeated totally three times. Then the powders were corroded with 4 mol/L hydrochloric acid in sealed hydrothermal synthesis reactors at 120 °C for 10 h (See Supplementary Information for experimental details).
In order to obtain disperse fine equiaxed α-Al2O3 NPs with narrow size distribution widths, the disperse equiaxed α-Al2O3 NPs obtained by selective corrosion were size-selectively separated by refined fractionated coagulation using hydrochloric acid as a coagulating agent. The disperse fine equiaxed α-Al2O3 NPs with an average particle size of 14.3 nm and a size distribution width of 2–250 nm were suspended in deionised water in ultrasonic bath and centrifuged at 10000 rpm to remove α-Al2O3 NPs larger than 100 nm. The obtained α-Al2O3 NPs smaller than 100 nm were suspended in a 1.4 mol/L HCl solution, then larger α-Al2O3 NPs coagulated and deposited whereas smaller α-Al2O3 NPs remained stable in the upper clear suspension. The deposited larger α-Al2O3 NPs were centrifuged and used for the next coagulation separation (referred to as the mother powder). A 12 mol/L HCl solution was added into the upper clear suspension; all the α-Al2O3 NPs in the upper clear suspension coagulated and deposited. After centrifuging, washing and drying of the deposited powder, the α-Al2O3 NPs with average particle sizes of 5.2 nm were obtained. In similar ways, the mother powders of the previous refined fractionated coagulation separations were suspended in order in the HCl solutions of 1.2, 1.0 and 0.8 mol/L concentrations and the α-Al2O3 NPs with average particle sizes of 6.5, 7.9 and 9.6 nm were separated respectively.
The α-Al2O3 NPs with an average particle size of 7.9 nm and a size distribution width of 4–14 nm were pressed into green compacts at 600 MPa. The green compacts were heated in air at a heating rate of 10 °C/min to 1,230 °C without hold, then cooled at a rate of 5 °C/min down to 1,080 °C with a 40 h hold and finally cooled at a rate of 10 °C/min down to room temperature.
Phases in α-Al2O3-NPs-embedded composite powders, α-Al2O3 NPs obtained by removing the Fe matrix in the composites and α-Al2O3 NPs size-selectively separated by refined fractionated coagulation were examined by XRD analysis. The morphology, microstructure and lattice structure of α-Al2O3-NPs-embedded composite powders, α-Al2O3 NPs obtained by removing the Fe matrix in the composites and α-Al2O3 NPs size-selectively separated by refined fractionated coagulation were analysed by TEM (or HRTEM) observations. SAED analysis was performed during TEM analysis. The average particle size and size distribution of the α-Al2O3 NPs obtained by removing the Fe matrix in the composites were statistically determined from more than 5000 particles observed in the TEM images of the different areas of the samples. The average particle sizes and size distributions of the α-Al2O3 NPs size-selectively separated by refined fractionated coagulation were statistically determined from more than 1500 particles observed in the TEM images of the different areas of the samples. The compositions of α-Al2O3 NPs were determined by EDS and ICP-AES elemental analyses. The specific surface areas of the α-Al2O3 NPs were measured by N2 adsorption at 77 K using the BET method. Before the BET measurements, the powder samples were degassed at 200 °C for 5 h. Relative densities of the green compacts and sintered bodies were measured by Archimedes' method. Microstructure of sintered bodies was analysed by SEM observations. The average grain size and grain size distribution of sintered nanocrystalline α-Al2O3 ceramic samples were statistically determined from about 1000 grains observed in the SEM images of the different areas of the samples.
Additional Information
How to cite this article: Pu, S. et al. Disperse fine equiaxed alpha alumina nanoparticles with narrow size distribution synthesised by selective corrosion and coagulation separation. Sci. Rep. 5, 11575; doi: 10.1038/srep11575 (2015).
References
Li, H., Li, Y., Jiao, J. & Hu, H.-M. Alpha-alumina nanoparticles induce efficient autophagy-dependent cross-presentation and potent antitumour response. Nat. Nanotechnol. 6, 645–650 (2011).
Lei, H. & Zhang, P. Preparation of alumina/silica core-shell abrasives and their CMP behavior. Appl. Surf. Sci. 253, 8754–8761 (2007).
Mazahery, A. & Ostadshabani, M. Investigation on mechanical properties of nano-Al2O3-reinforced aluminum matrix composites. J. Compos. Mater. 45, 2579–2586 (2011).
Karch, J., Birringer, R. & Gleiter, H. Ceramics ductile at low temperature. Nature 330, 556–558 (1987).
Hahn, H. & Averback, R. S. Low-temperature creep of nanocrystalline titanium (IV) oxide. J. Am. Ceram. Soc. 74, 2918–2921 (1991).
Johnston, G. P., Muenchausen, R., Smith, D. M., Fahrenholtz, W. & Foltyn, S. Reactive laser ablation synthesis of nanosize alumina powder, J. Am. Ceram. Soc. 75, 3293–3298 (1992).
Noguchi, T., Matsui, K., Islam, N. M., Hakuta, Y. & Hayashi, H. Rapid synthesis of γ-Al2O3 nanoparticles in supercritical water by continuous hydrothermal flow reaction system. J. Supercrit. Fluids 46, 129–136 (2008).
Li, J. G. & Sun, X. Synthesis and sintering behavior of a nanocrystalline α-alumina powder. Acta Mater. 48, 3103–3112 (2000).
Das, R. N., Bandyopadhyay, A. & Bose, S. Nanocrystalline α-Al2O3 using sucrose. J. Am. Ceram. Soc. 84, 2421–2423 (2001).
Laine, R. M., Marchal, J. C., Sun, H. P. & Pan, X. Q. Nano-α-Al2O3 by liquid-feed flame spray pyrolysis. Nat. Mater. 5, 710–712 (2006).
Zhang, X., Ge, Y., Hannula, S.-P., Levänen, E. & Mäntylä, T. Nanocrystalline α-alumina with novel morphology at 1000°C. J. Mater. Chem. 18, 2423–2425 (2008).
Yoo, Y. S., Park, K. Y., Jung, K. Y. & Cho, S. B. Preparation of α-alumina nanoparticles via vapor-phase hydrolysis of AlCl3 . Mater. Lett. 63, 1844–1846 (2009).
Karagedov, G. R. & Lyakhov, N. Z. Preparation and sintering of nanosized α-Al2O3 powder. Nanostruct. Mater 11, 559–572 (1999).
Karagedov, G. R. & Myz, A. L. Preparation and sintering pure nanocrystalline α-alumina powder, J. Euro. Ceram. Soc. 32, 219–225 (2012).
Borsella, E. et al. Laserdriven synthesis of nanocrystalline alumina powders from gasphase precursors. Appl. Phys. Lett. 63, 1345–1347 (1993).
Blonski, S. & Garofalini, S. H. Molecular dynamics simulations of α-alumina and γ-alumina surfaces. Surf. Sci. 295, 263–274 (1993).
McHale, J. M., Auroux, A., Perrotta, A. J. & Navrotsky, A. Surface energies and thermodynamic phase stability in nanocrystalline aluminas, Science 277, 788–791 (1997).
McHale, J. M., Navrotsky, A. & Perrotta, A. J. Effects of increased surface area and chemisorbed H2O on the relative stability of nanocrystalline γ-Al2O3 and α-Al2O3 . J. Phys. Chem. B 101, 603–613 (1997).
Perrotta, A. J. Nanosized corundum synthesis. Mater. Res. Innovat. 2, 33–38 (1998).
Smith, R. L., Yanina, S, V., Rohrer, G. S. & Perrotta, A. J. Inhibition of sintering and surface area loss in phosphorus-doped corundum derived from diaspore. J. Am. Ceram. Soc. 85, 2325–2330 (2002).
Shi, J. L. Relations between coarsening and densification and mass transport path in solid-state sintering of ceramics: model analysis. J. Mater. Res. 14, 1378–1388 (1999).
Shi, J.-L., Deguchi, Y. & Sakabe, Y. Relation between grain growth, densification and surface diffusion in solid state sintering—a direct observation. J. Mater. Sci. 40, 5711–5719 (2005).
Fang, Z. Z. & Wang, H. Densification and grain growth during sintering of nanosized particles. Int. Mater. Rev. 53, 326–352 (2008).
Frens, G. Particle size and sol stability in metal colloids. Kolloid-Z. u. Z. Polymere 250, 736–741 (1972).
Chen, I.-W. & Wang, X.-H. Sintering dense nanocrystalline ceramics without final-stage grain growth. Nature 404, 168–171 (2000).
Hanauer, M., Pierrat, S., Zins, I., Lotz, A. & Sönnichsen, C. Separation of nanoparticles by gel electrophoresis according to size and shape. Nano Lett. 7, 2881–2885 (2007).
Bai, L. et al. Rapid separation and purification of nanoparticles in organic density gradients. J. Am. Chem. Soc. 132, 2333–2337 (2010).
Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 46, 1–184 (2001).
Fei, G. T. et al. Structure and thermal stability of Fe:Al2O3 nanocomposite films. J. Phys. D: Appl. Phys. 35, 916–922 (2002).
Li, J. & Ye, Y. Densification and grain growth of Al2O3 nanoceramics during pressureless sintering. J. Am. Ceram. Soc. 89, 139–143 (2006).
Acknowledgements
This research was supported by the National Natural Science Foundation of China (50872046, 51071079 and 51272098). We thank Tianfei Zhu, Lianwen Wang and Yong Peng for helpful discussions.
Author information
Authors and Affiliations
Contributions
J.L. proposed and supervised the project. J.L. and S.P. designed the experiments. S.P., L.L., J.M. and F.L. performed the experiments. All authors discussed the results. J.L., L.L. and S.P. wrote the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Pu, S., Li, L., Ma, J. et al. Disperse fine equiaxed alpha alumina nanoparticles with narrow size distribution synthesised by selective corrosion and coagulation separation. Sci Rep 5, 11575 (2015). https://doi.org/10.1038/srep11575
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep11575
This article is cited by
-
Spherical aluminum oxide nanoparticle synthesis and monolayer film assembly
Journal of Materials Science (2023)
-
Low-temperature synthesis of α-Al2O3 single crystal platelets by one-step thermal decomposition of Al-urea complex
Journal of Thermal Analysis and Calorimetry (2023)
-
Homogeneous nucleation of corundum nanocrystallites by rapid heating of aluminum formate hydroxide-based precursor powder
Scientific Reports (2019)
-
Adsorption isotherms and thermal behavior of hybrids based on quercetin and inorganic fillers
Journal of Thermal Analysis and Calorimetry (2019)
-
Photoluminescence of carbon dots prepared by ball milling and their application in Hela cell imaging
Applied Physics A (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.