Abstract
Hypoxia induces plant stress, particularly in cucumber plants under hydroponic culture. In plants, calcium is involved in stress signal transmission and growth. The ultimate goal of this study was to shed light on the mechanisms underlying the effects of exogenous calcium on the mitochondrial antioxidant system, the activity of respiratory metabolism enzymes and ion transport in cucumber (Cucumis sativus L. cv. Jinchun No. 2) roots under hypoxic conditions. Our experiments revealed that exogenous calcium reduces the level of reactive oxygen species (ROS) and increases the activity of antioxidant enzymes in mitochondria under hypoxia. Exogenous calcium also enhances the accumulation of enzymes involved in glycolysis and the tricarboxylic acid (TCA) cycle. We utilized fluorescence and ultrastructural cytochemistry methods to observe that exogenous calcium increases the concentrations of Ca2+ and K+ in root cells by increasing the activity of plasma membrane (PM) H+-ATPase and tonoplast H+-ATPase and H+-PPase. Overall, our results suggest that hypoxic stress has an immediate and substantial effect on roots. Exogenous calcium improves metabolism and ion transport in cucumber roots, thereby increasing hypoxia tolerance in cucumber.
Similar content being viewed by others
Introduction
Higher plants frequently experience limited oxygen availability, mainly due to flooding1, waterlogging2, irrigation or hydroponic culture conditions3. Approximately 16% of fertile land worldwide is affected by soil waterlogging4. Waterlogging or hydroponic culturing result in lowered levels of oxygen in the plant root zone due to the low diffusion rate of molecular oxygen in water5. The consequences of hypoxia, such as a decrease in the cellular energy charge, a drop in cytoplasmic pH and the accumulation of toxic end products from anaerobic respiration and reactive oxygen species (ROS) during recovery, are responsible for the reduced growth and yield observed in many agriculturally important crops exposed to flooding6. Plants subjected to hypoxia undergo dramatic metabolic changes. Defense mechanisms are induced to cope with the potential damage. Cucumber, which is sensitive to oxygen shortage, often faces hypoxic stress, resulting in enormous economic losses.
Oxygen is vital to the central energy-providing pathway of the cell and the presence or absence of oxygen determines metabolic activity and energy production7. Respiration is generally the first aspect of plant metabolism to be affected by oxygen shortage. Hypoxic stress interferes with electron transport chains and a lack of suitable electron acceptors leads to the saturation of redox chains, accumulation of NAD(P)H and decreased synthesis of ATP8. The mitochondrial electron transport chain, with its redox-active electron carriers, is the most likely candidate for the formation of intracellular ROS. ROS production results in damage to physiological metabolism and the cell membrane structure in plants9. The metabolic pathways in the mitochondria are also sensitive to environmental changes10. Accumulating evidence suggests that the mitochondria may act as ‘sensors’ in the overall plant stress response11.
Free Ca2+ is one of the key signal molecules in plants and animals and is involved in multiple signal transduction pathways, which are fundamental for many intercellular and intracellular interactions12,13. Intracellular Ca2+ signals are realized by spatially and temporally defined changes in the free Ca2+ concentration in the cytosol14. Environmental stimuli, such as salt stress15, hypoxia16 and chilling17, can alter the cellular Ca2+ concentration. Moreover, the involvement of calcium in hypoxia responses has been observed in many plants. For example, anoxia stress in the cells of maize, rice and wheat plants causes a rise in the cytoplasmic Ca2+ concentration18. Elevated calcium levels significantly influence the antioxidant system and nitrogen metabolism in hypoxia-stressed muskmelon roots19. The measurement of changes in free Ca2+ activity in the cytoplasm is crucial for the evaluation of the role of calcium in the transduction of external stimuli in cellular processes20.
As ATP generation by oxidative phosphorylation begins to diminish due to O2 limitation, an energetic deficit can be overcome by the activation of the anaerobic ATP supply. The reduction in ATP availability has major repercussions for root development, root nutrient uptake and root maintenance. Ion transport ATPases are a major ATP sink in plants cells and tissues21. When oxygen is in short supply, these energy-consuming processes affect cell metabolism and the overall plant nutritional status in response to stress. A previous study showed that both hypoxia and anoxia rapidly depolarize the plasma membrane (PM) of higher plants by approximately 50 mV, presumably by inhibiting electrogenic H+ pumps22. It remains to be determined whether all ion transporters are affected to the same extent by hypoxia. The spatial profile of ions in root tissues is also unknown23.
Results
The growth conditions of cucumber plants
The effect of hypoxia on cucumber plants was determined by visual observation. After 6 d of treatment, several morphological changes were observed in the stressed plants (Fig. 1). The roots of the hypoxia-treated plants were less dense and more stunted than control roots; hypoxia + calcium treatment reversed this condition. Plants receiving exogenous calcium also exhibited enhanced aboveground growth compared to hypoxia-treated plants, including less etiolated stems, larger leaf areas and an increased number of leaves. Our previous research also revealed that exogenous calcium enhanced the biomass and soluble protein content of cucumber seedlings under hypoxia24. Apparently, the growth of cucumber seedlings was significantly hindered. This effect could be partially rescued by the application of exogenous calcium.
The content of lipid peroxides, free radical production and antioxidant enzyme activity in mitochondria
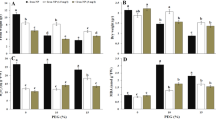
At 3 d and 6 d, the MDA content, H2O2 content and O2•- production rate in the mitochondria of cucumber roots significantly increased under hypoxia. The values of these components increased with increasing treatment times (Fig. 2A,D,G). Treatment with exogenous calcium resulted in a 34.6% reduction in the O2•- production rate, a 23.1% reduction in the H2O2 content and a 34.8% reduction in the MDA content compared to hypoxia-stressed plants at 6 d.
Determination of changes in O2•- production and MDA and H2O2 content (A); the activities of SOD, POD and CAT (B); plasma membrane (PM) H+-ATPase; and tonoplast H+-ATPase and H+-PPase in the mitochondria of cucumber roots at 3 d and 6 d after treatment. Samples were analyzed under normoxic conditions (Control), hypoxia treatment (Hypoxia) and hypoxia + 4 mM CaCl2 treatment (Hypoxia + Ca2+). Values are means ± SE of three independent experiments. Bars marked with dissimilar letters are significantly different according to Duncan’s multiple range tests (P < 0.05).
Regarding antioxidant enzyme activities in mitochondria, the activities of SOD, POD and CAT slightly increased at 3 d under hypoxia treatment; however, these increases were not significant. The activities of these enzymes were decreased at 6 d under hypoxia treatment in comparison with the control (Fig. 2B,E,H). Conversely, hypoxia + CaCl2 treatment increased the content of these enzymes at both 3 d and 6 d compared to hypoxia-treated plants. At 6 d under treatment, the activity of SOD and POD under both hypoxia and hypoxia + CaCl2 treatments was lower than that in control plants. However, the extent of this decline in plants receiving the hypoxia + CaCl2 treatment was less than that observed in hypoxia-treated plants. The activity of CAT under hypoxia + CaCl2 was not significantly different compared to the control at 6 d.
The activity of tonoplast H+-ATPase and H+-PPase and plasma membrane H+-ATPase in cucumber roots
A decrease in the activities of PM H+-ATPase and tonoplast H+-ATPase (Fig. 2C,F) was observed in comparison with the control at 3 d and 6 d. In plants treated with exogenous calcium, the activity of the PM H+-ATPase and the tonoplast H+-ATPase increased by 28.9% and 25.3%, respectively, compared to the hypoxia-stressed plants at 3 d. At 6 d, the activity of PM H+-ATPase and tonoplast H+-ATPase in plants treated with exogenous calcium increased by 34.6% and 55.9%, respectively, compared to hypoxia-stressed plants. Nevertheless, the activity of these enzymes did not reach levels observed in control plants. The activity of tonoplast H+-PPase (Fig. 2I) significantly decreased under hypoxic conditions compared to the control. After applying exogenous calcium, the activity of this enzyme increased.
Enzymes involved in glycolysis and tricarboxylic acid metabolism
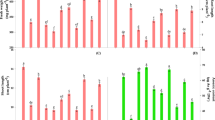
The enzymes that catalyze the key steps in glycolysis metabolism, such as hexokinase (HK), 6-phosphofructokinase (PFK) and pyruvate kinase (PK), were analyzed. To determine HK activity (Fig. 3A), we measured the levels of two isozymes in all treatments. Under hypoxic conditions, the expression of a1 and a2 were enhanced with respect to the control. Under hypoxia + CaCl2 treatment, the expression of HK was further enhanced. With respect to the accumulation of PFK (Fig. 3B) and PK (Fig. 3C), an increase in the expression of these enzymes was observed under hypoxic conditions; hypoxia + CaCl2 further enhanced their expression.
Expression of hexokinase (HK, A), 6-phosphofructokinase (PFK, B), pyruvate kinase (PK, C), isocitrate dehydrogenase (IDH, D), malate dehydrogenase (MDH, E) and succinate dehydrogenase (SDH, F) isozymes in cucumber roots exposed to one of three treatments for 3 d. These three treatments were normoxic conditions (Control), hypoxia treatment (Hypoxia) and hypoxia + 4 mM CaCl2 treatment (Hypoxia + Ca2+). Lines indicate the isozymes detected by staining.
Key enzymes involved in tricarboxylic acid metabolism were analyzed, including isocitrate dehydrogenase (IDH), malate dehydrogenase (MDH) and succinate dehydrogenase (SDH). Three IDH isozymes—d1, d2 and d3—were isolated from cucumber roots in all treatments (Fig. 3D). The three treatments had little effect on the expression of d1. However, the accumulation of d2 decreased remarkably under hypoxic stress. In plants treated with hypoxia + CaCl2, the expression of the d2 isozyme increased and a new band (d3) was observed. A new MDH band (e2) was observed under hypoxic conditions (Fig. 3E). Two new bands (e1 and e2) were observed under hypoxia + CaCl2 conditions; this result suggests that the isozymes e1 and e2 are sensitive to hypoxia and calcium, respectively. The expression of the SDH isozyme f1 was significantly enhanced under hypoxia + CaCl2 conditions and decreased in the control and in plants under hypoxic conditions (Fig. 3F). The f2 band was visually weak under the hypoxia treatment compared to the control, whereas exogenous calcium application increased its expression markedly.
We also quantified the activities of these enzymes in spectrophotometric assays to reinforce the findings described above (Fig. 4). The activities of HK, PFK, PK and PEPC were observed to increase under hypoxic conditions compared to the control. After applying exogenous calcium, the activities of IDH, MDH and SDH increased further to reach significant levels. Conversely, the activities of IDH, MDH and SDH decreased under hypoxia treatment. Hypoxia + CaCl2 treatment increased the activity of these enzymes. Except for SDH, the activities of these enzymes did not reach the levels observed in the control.
Changes in enzyme activities in cucumber roots at 3 d after treatment.
The activities of hexokinase (HK), 6-phosphofructokinase (6-PFK), pyruvate kinase (PK), phosphoenolpyruvate carboxylase (PEPC), isocitrate dehydrogenase (IDH), malate dehydrogenase (MDH) and succinate dehydrogenase (SDH) were determined in root material collected from cucumber plants grown under normoxic conditions (Control), hypoxia treatment (Hypoxia) and hypoxia + 4 mM CaCl2 treatment (Hypoxia + Ca2+). Values are means ± SE of three independent experiments. Bars marked with dissimilar letters are significantly different from each other according to Duncan’s multiple range tests (P < 0.05).
Fluorescence measurements and subcellular localization of Ca2+ in the root apex of cucumber
To trace the dynamics of free calcium ions in cucumber roots, the fluorescent indicator Ca2+ green 5N/AM was used for loading in the root tips. Detailed analysis under a confocal microscope revealed significant differences between the signal in the treated plants (hypoxia and hypoxia + CaCl2) and the control. After loading with Ca2+ green 5N/AM, green fluorescence was observed in the middle of the root tips in control plants; most fluorescence occurred in the elongation zone. The meristem region of control plants was practically devoid of green fluorescence (Fig. 5A). Under hypoxia treatment, the region of green fluorescence was more intense compared to the control (Fig. 5B). The greatest range and intensity of the green fluorescence signal were observed under the hypoxia + CaCl2 treatment. In plants that received this treatment, the whole root tip exhibited intense green labeling, including the elongation zone, the meristem region and the edge of the root tip (Fig. 5C). The Ca2+ content was quantified using the Calcium Colorimetric Assay Kit (BioVision, Mountain View, CA); the quantitative results revealed similar trends (Fig. 5D).
Detection of Ca2 + concertrations in cucumber roots tips at 3 d after treatment.
(A–C) Confocal images of cucumber roots tips by Fluo-3 AM. (A) Cucumber plants grown under normoxic conditions (Control). (B) Cucumber plants grown under hypoxic conditions (Hypoxia). (C) Cucumber plants grown under hypoxia + 4 mM CaCl2 treatment (Hypoxia + CaCl2). The signal corresponding to the incorporated Fluo-3 is green. (D) The Ca2+ content (μg•μl−1) of cucumber roots under normoxic conditions (control), hypoxic conditions (Hypoxia) and hypoxia + 4 mM CaCl2 treatment (Hypoxia + Ca2+). Values are means ± SE of three independent experiments. Bars marked with dissimilar letters are significantly different from each other according to Duncan’s multiple range tests (P < 0.05).
To study the subcellular distribution of the Ca2+ ions, we used the cytochemical method. This method is used to localize free and loosely bound calcium. Precipitates were mainly localized to the plasma membrane and in intercellular spaces. In the control, a few Ca2+ deposits were found in the plasma membrane (Fig. 6A,B). The Ca2+ level increased in the plasma membrane and in the intercellular spaces after 3 d of hypoxia (Fig. 6D,E). The karyotheca appeared to have contracted and presented with a wavy shape. The number of the mitochondria decreased compared to the control (Fig. 6F). In hypoxia + CaCl2-treated samples, a rich pool of fine and dense Ca2+ precipitates were localized in the plasma membrane and intercellular spaces; a few Ca2+ precipitates were also observed in the cytosol and mitochondria (Fig. 6G,H). The application of exogenous calcium alleviated these changes in the nucleus; more mitochondria were observed compared to in hypoxia-treated samples (Fig. 6I).
Subcellular localization of Ca2+ (A, B, D, E, G, H, J and K) and subcellular distribution (C, F, I and L) in cucumber roots at 3 d after treatment.
Samples comprised 3 mm of the root tips. (A–C) Cucumber plants grown under normoxic conditions (Control). (D–F) Cucumber plants grown under hypoxic conditions. (G–I) Cucumber plants grown under hypoxia + 4 mM CaCl2 treatment (Hypoxia + Ca2+). (J–L) Cucumber plants grown under hypoxia + 50 μM LaCl3 treatment (Hypoxia + La3+). Arrows indicate Ca2+ ion precipitates in the root cells.
X-ray microanalysis of transverse sections of cucumber roots for [K+] and [Ca2+]
Transverse sections of the roots of cucumber seedlings were scanned using X-ray microanalysis. Sectioning began with the outermost tissues and proceeded inward in the following order: epidermal cells, exodermal cells, cortical cells, endodermal cells and the stelar parenchyma. All of these spectra were transformed into data using professional software supplied with the S-3000N scanning electron microscope. Relative amounts of [K+] and [Ca2+] in the samples comprising the three treatments in five tissues are shown in Fig 7. In the roots of hypoxia-treated plants, Ca2+ levels increased in the epidermal cells, exodermal cells and the stelar parenchyma compared with the control. After applying exogenous calcium, [Ca2+] significantly increased at the outer root tissues and toward the middle tissues; such an increase was especially evident in epidermal and exodermal cells. Conversely, [K+] greatly decreased under hypoxic stress and increased under the hypoxia + Ca2+ treatment (Fig. 7). Exodermal cells, cortical cells and the stelar parenchyma exhibited higher levels of K+; both [K+] and [Ca2+] were asymmetrically distributed. Images of the distrobution of Ca2+ and K+ in cucumber roots were obtained by map-scanning an X-ray and showed a similar trend (Fig. 8).
Ca2+ and K+ level in five different tissues (epidermal cells, exodermis cells, cortical cells, endodermal cell and the stelar parenchyma) of cucumber roots detected using point-scanning of an X-ray.
The plants were grown under normoxic conditions (Control), hypoxic conditions (Hypoxia) and hypoxia + 4 mM CaCl2 treatment (Hypoxia + Ca2+) for 3 d. Samples comprised 1 cm of the root tips. Values are means ± SE of three independent experiments. Bars marked with dissimilar letters are significantly different from each other according to Duncan’s multiple range tests (P < 0.05).
Images of Ca2+ and K+ distribution in cucumber roots detected using map-scanning of an X-ray.
The plants were grown under normoxic conditions (Control), hypoxic conditions (Hypoxia) and hypoxia + 4 mM CaCl2 treatment (Hypoxia + Ca2+) for 3 d. Samples comprised 1 cm of the root tips. Denser points indicate higher ion concentrations.
Discussion
To date, there have been few reports concerning the interplay among exogenous calcium, mitochondrial function and respiratory metabolism. However, a previous study revealed that calcium is involved in short-term hypoxic tolerance in muskmelons by promoting NO3− uptake and accelerating its transformation into amino acids, heat-stable proteins or polyamines as well as by preventing polyamine degradation19. In the present study, cucumber seedlings were shown to be tolerant to hypoxic stress after exogenous calcium was applied (Fig. 1). Growth inhibition is a common phenomenon in plants under hypoxia. This phenomenon has also been observed in waterlogged Lotus japonicas25. These morphological changes can be explained as part of the adaptive response of plants to hypoxia; plants limit their energy demand and respiratory oxygen consumption by down-regulating the synthesis of assimilate products such as starch and protein24. If the demand for respiratory oxygen consumption decreases, anoxia in plant tissues can be postponed or even prevented26.
The resistance to stress and the response to a variety of stress signals are influenced by the mitochondria status in plants27. The role of calcium in the mitochondria under hypoxic conditions was discussed in a review by Stael et al.28. The root apoplast is another important site for ROS generation and scavenging. Central to ROS signaling is the induction of Ca2+ influx across the plasma membrane. ROS such as •OH can also stimulate both the outward K+ current and hyperpolarization-activated Ca2+-permeable channels29. The production and detoxification of ROS likely play widespread roles in signaling and damage at the onset of and release from low-oxygen stress30. The major ROS-scavenging enzymes in plants include SOD, POD, CAT, APX, glutathione peroxidase and peroxiredoxin31. A recent study demonstrated that the Arabidopsis PPR40 protein is associated with the mitochondrial inner membrane complex III and its malfunction results in ROS accumulation, SOD activation, lipid peroxidation and the altered expression of stress-responsive genes32. A study based on the expression of a peroxisome-targeted chameleon probe (a Ca2+ reporter protein construct) revealed that plant peroxisomes undergo Ca2+ fluxes33. In the present study, low oxygen also enhanced ROS levels in the mitochondria of cucumber roots (Fig. 2). When CaCl2 was applied, the ROS content in mitochondria decreased compared to that under the hypoxia treatment at both 3 d and 6 d. This result suggests that exogenous calcium decreases lipid peroxidation by alleviating the increase in ROS levels under hypoxic stress. A decline in the activity of antioxidant enzymes was observed at 6 d in hypoxia-treated samples compared to the control (Fig. 2). This finding indicates that a longer period of hypoxic stress destroyed the antioxidant system in mitochondria. Exogenous calcium enhanced hypoxic tolerance in cucumber plants by promoting the activity of antioxidant enzymes and decreasing ROS production in mitochondria. Similar results were also obtained in the roots of cucumber seedlings under hypoxic stress34 and in eggplant seedlings under chilling17.
An alteration in the isozymic pattern usually occurs under abiotic stress35. Antioxidant isozymes, glutamate dehydrogenase isozymes and many other isozymes have been analyzed by gel electrophoresis in previous studies36. However, the key isoenzymatic patterns of the glycolytic and tricarboxylic acid (TCA) cycle have rarely been reported. Moore et al.37 observed that Arabidopsis plants use a specific hexokinase (HXK1) as a glucose sensor to interrelate nutrient, light and hormone signaling networks to control growth and development in response to a changing environment. PFK is a major enzyme that controls glycolytic flux and is likely a rate-limiting enzyme of glycolysis under anoxia38. The activity of PFK increased in peach fruits in response to anoxia39. In our experiments, all of the tested enzymes involving glycolytic metabolism were up-regulated under hypoxia (Figs 3,4). This result suggests that the glycolytic pathway, a major source of energy, was up-regulated under anoxic circumstances24. Increased glycolytic flux is also needed to support pyruvate demand in the fermentative pathways induced by anoxia39. After applying CaCl2, the expression and activity of most enzymes, including HK, PFK and PK, increased compared to those in hypoxia-treated samples. These results indicated that exogenous calcium further increases the activity of glycolytic enzymes to increase the rate of glycolytic flux40, thereby increasing the tolerance of cucumber seedlings to hypoxia. The TCA cycle is a fundamental component of mitochondrial respiration and links glycolysis and/or extramitochondrial malate synthesis to the mitochondrial electron transport chain. As part of an ongoing project, a wide range of transgenics and mutants deficient in the expression of TCA cycle enzymes were analyzed. Growing plants deficient in the expression of mitochondrial MDH under short-day conditions resulted in a dwarf phenotype41. In a previous study, the activities of IDH, MDH and SDH decreased in Lotus japonicas under waterlogging24. Moreover, calcium acts to maintain higher MDH and SDH activities and a certain level of aerobic respiration in pepper42. The accumulation and activity of IDH, MDH and SDH were all induced by exogenous calcium (Figs 3,4). In our experiments, Ca2+ positively affected ATP production by up-regulating the expression of major limiting enzymes of the TCA cycle. Such activity enhanced hypoxic tolerance in cucumber.
K+ fluxes have been extensively evaluated in plants under environmental stresses such as salinity43 and hypoxia23. As K+ uptake is disturbed by oxygen deprivation21, an improved K+ uptake in roots may be critical to overall plant performance under hypoxia. Latz et al.44 have shown that Ca2+-dependent protein kinases activate the vacuolar K+ channel TPK1 under salt stress to maintain higher cytosolic K+ levels. Hypoxia also led to depolarization-induced K+ efflux via outward-rectifying depolarization-activated PM K+ channels (GORK/SKOR)45. However, Ca2+ is a potent blocker of these channels. Extracellular Ca2+ can ameliorate NaCl-induced K + loss in Arabidopsis root and leaf cells by controlling PM K + -permeable channels46. Our observation that K+ concentrations decrease in roots under hypoxia and increase after the application of exogenous Ca2+ may indicate that similar mechanisms are also active under hypoxia (Figs 7,8). Our findings are also consistent with previous reports showing that hypoxia significantly inhibits K+ uptake in barley roots23. Pre-treatment with vanadate, a known inhibitor of the PM H+-ATPase, significantly increased net H+ in barley roots. This finding supports the hypothesis that a substantial component of H+ transport under hypoxia is due to changes in the activity of PM H+-ATPase. A decrease in the activity of proton-pumping ATPases not only reduced K+ uptake but also led to the acidification of the cytosol under anoxic conditions. Such acidification results from protons released through ATP hydrolysis and from the low ATP concentration, which reduces the activity of the proton pump. It has also been suggested that pyrophosphate (PPi) can substitute for ATP as an energy source, which would represent an important acclimation mechanism in anoxia-tolerant species47. For example, the activity of tonoplast H+-PPiase increased 75-fold in rice seedlings after 6 d under anoxia48. The expression of one gene encoding tonoplast H+-PPase (Os02g55890) was up-regulated by 35-fold in anoxic rice coleoptiles49. Calcium-binding mitochondrial carrier proteins (APC1, APC2 and APC3) can function as mitochondrial ATP-importers50. Mitochondrial ATP import might be a first line of defense against hypoxia, keeping the mitochondrial membrane proton gradient and the electron transport chain intact to prevent ROS generation. In our experiment, we also observed that hypoxia inhibits the activity of PM H+-ATPase and tonoplast H+-ATPase and slightly induces the activity of tonoplast H+-PPase; exogenous calcium increases the activity of these enzymes (Fig. 2). Exogenous calcium resulted in the maintenance of a high K+ level via an increase in the activity of enzymes involved in respiratory metabolism; mitochondrial homeostasis was maintained and sufficient ATP was supplied to these ion transport ATPases (Fig. 9). A high K+ level resulted in better ion homeostasis and regulated cell turgor, as K+ significantly affects the activity of vacuolar ion channels51.
A simplified metabolic scheme and signal transduction pathway for Ca2 + in cucumber under hypoxia.
The model is based on the results presented here. The red arrow with a straight line and + symbol indicates up-regulation. The blue arrow with a curve and − symbol indicates down-regulation. GORK: guard cell outward rectifying K+ channel; SKOR: stelar K+ outward rectifier.
In summary, our results indicate that hypoxia signal transduction pathways are distinct in terms of Ca2+ elevation. External Ca2+ is involved in hypoxia signaling in cucumber roots. Exogenous calcium, through decreased ROS levels, enhanced the antioxidant system in mitochondria and enhanced mitochondrial homeostasis. Furthermore, exogenous calcium increased the accumulation of respiratory metabolism enzymes and mediated the transport of Ca2+ and K+ to strengthen hypoxia tolerance in cucumber roots.
Materials and Methods
Plant materials and growth conditions
Cucumber (Cucumis sativus L. cv. Jinchun No. 2, hypoxia sensitive52) seeds were sterilized with 0.5% (W/V) sodium hypochlorite solution for 10 min and then washed thoroughly with deionized water. The washed seeds were sown in two layers of wet filter paper and incubated in the dark at 28 °C for 24 h. The germinated seedlings were transplanted to plastic trays (41 × 41 × 5 cm) containing quartz sand and grown at 25–30 °C (day) and 15–18 °C (night), with 60–75% relative humidity (RH), in a greenhouse at Nanjing Agriculture University in 2012. The seedlings were supplied with half-strength Hoagland nutrient solution (pH 6.5 ±0.1, EC 2.0–2.2 dS m−1). When the second leaves were fully expanded, relatively uniform seedlings were transferred to tanks containing half-strength Hoagland nutrient solution. The solution was renewed every 3 d. The solution in the tanks was kept at 20–25 °C and aerated with an air pump at an interval of 20 min to maintain the dissolved oxygen (DO) level at 8.0 ±0.2 mg l−1 (the optimum DO level for cucumber). When the 3rd leaf developed, seedlings were subjected to one of the following three treatments: (1) Control: half-strength Hoagland solution (containing 2 mM Ca2+) with a DO level of 8.0 ±0.2 mg l−1; (2) Hypoxia treatment: half-strength Hoagland solution (containing 2 mM Ca2+) with a DO level of 1.0 ±0.1 mg l−1, which was prepared by pumping N2 gas into the nutrient solutions; the oxygen concentration in the nutrient solutions was monitored with an automatic DO control system (Quantum-25, Quantum Analytical Instruments Inc., USA); (3) Hypoxia + CaCl2 treatment: half-strength Hoagland solution +4 mM CaCl2 with DO of 1.0 ±0.1 mg l−1; the oxygen concentration in the nutrient solutions was controlled as in the hypoxia treatment.
The roots of the control and treated seedlings were sampled after 3 and 6 d of treatment. Tissues were immediately frozen in liquid nitrogen and stored at −80 °C for further experiments.
Determination of lipid peroxidation, free radical production and antioxidant enzyme activity in mitochondria
Intact mitochondria were isolated using a method described previously with a slight modification53,54. The levels of lipid peroxides in the mitochondria were determined by measuring the malondialdehyde (MDA) content from the thiobarbituric acid (TBA) reaction as described previously. A 1 ml suspension of mitochondria was added to 2 ml of a solution containing 20% trichloroacetic acid (TCA) and 0.6% TBA. Tubes were placed in a 95 °C water bath for 20 min and then immediately cooled on ice for 10 min. The samples were centrifuged at 8000 × g at 4 °C for 15 min. The MDA concentration was calculated using the A532 value and the nonspecific turbidity of the measurements was corrected by subtracting the value at A600. MDA values were expressed in nmol MDA/mg protein. The superoxide production rate was measured according to a modified version of the method described previously. The measurement was performed at A530. The O2 ·- formation rate was calculated from a standard curve of NaNO2 and expressed as nmol/min/mg protein. The H2O2 content in the mitochondria was based on the absorbance change of the titanium peroxide complex measured at A415. The H2O2 content was expressed as μmol/mg protein.
One unit of superoxide dismutase (SOD, EC 1.15.1.1) activity was defined as the amount of enzyme required to cause 50% inhibition of the photochemical reduction of nitroblue tetrazolium (NBT). The total volume of the assay mixture was 3 ml and contained 50 mM PBS-Na (pH 7.8), 15 mM methionine, 2.25 mM NBT, 60 μM riboflavin, 30 mM EDTA and 0.01 ml of a suspension of mitochondria. The SOD activity was monitored at 560 nm using a spectrophotometer (WFZ ultraviolet/VIS-2600; UNIC, Shanghai, China) and expressed as U/mg protein. The activity of peroxidase (POD, EC 1.11.1.7) was measured as previously described. One unit of activity was defined as the amount of enzyme required to increase the optical density at 470 nm·min−1 by 1 absorbance unit. Measurements were expressed as U/mg protein. The catalase (CAT, EC 1.11.1.11) activity was calculated by measuring the disappearance of H2O2 over 1 min at 240 nm. One unit of activity was defined as the amount of enzyme required to decrease the optical density by 0.1 absorbance units. Measurements were expressed as U/mg protein.
Native gel electrophoresis for the visualization of isozymes and the assay of enzyme activity
Frozen root samples (0.2 g) were finely ground into a powder in liquid nitrogen and homogenized on ice in 0.6 ml Tris-HCl buffer (0.1 M pH 7.0) containing 15% sucrose, 0.2% β-mercaptoethanol and 1% PVP. The homogenate was centrifuged at 12,000 × g for 20 min at 4 °C. The supernatant was centrifuged a second time under the same conditions for 10 min and used as directed for native polyacrylamide gel electrophoresis (PAGE). The PAGE gel consisted of a 3.1% stacking gel and a 7.5% separating gel. The gel was electroporated at 80 V for approximately 60 min at 4 °C (in a refrigerator) and then at 180 V for approximately 4 h. The staining solutions for each isozyme were as follows55:
-
(I) Hexokinase (HK, EC 2.7.1.1): 50 ml of a 0.1 M Tris-HCl (pH 7.8) solution containing 100 mg D-glucose, 60 mg ATP-Na2, 30 mg NAD, 10 mg MTT, 2 mg PMS, 20 mg MgCl2•6H2O and 40 U G-6-PD was used. The gel was soaked in the Tris solution in the dark at 37 °C until dark blue bands were visible. Coloration was then stopped by the addition of 25% ethanol.
-
(II) 6-Phosphofructokinase (PFK, EC 2.7.1.11): 50 ml of a 0.1 M Tris-HCl (pH 8.3), solution containing 100 mg ATP-Na2, 80 mg D-fructose-6-phosphate, 70 mg phosphoenolpyruvate, 30 mg NADH, 80 mg MgCl2•6H2O, 120 mg KCl, 180 U pyruvate kinase and 250 U lactate dehydrogenase (LDH) was used. The gel was incubated at 37 °C and observed under long-wave ultraviolet light.
-
(III) Pyruvate kinase (PK, EC 2.7.1.40): 50 ml of a 0.2 M Tris-HCl (pH 8.0) solution containing 125 mg ADP-Na2, 75 mg phosphoenolpyruvate, 35 mg NADH, 150 mg MgCl2•6H2O, 200 mg KCl and 250 U LDH was used. The gel was incubated at 37 °C for 30–60 min and observed under long-wave ultraviolet light.
-
(IV) Isocitrate dehydrogenase (IDH, EC 1.1.1.42): 100 ml of a 0.1 M Tris-HCl (pH 8.0) solution containing 3 ml 0.1 M isocitrate, 20 mg NADP, 10 mg NBT, 3 mg PMS and 0.4 ml 0.25 M MnCl2 was used. The gel was soaked in the mixture in the dark at 37 °C until dark blue bands were visible. Coloration was then stopped by the addition of 25% ethanol.
-
(V) Malate dehydrogenase (MDH, EC 1.1.1.37): 100 ml of a 0.1 M Tris-HCl (pH 8.0) solution containing 250 mg L-malic acid, 30 mg NAD, 25 mg NBT and 2 mg PMS was used. The gel was soaked in the mixture in the dark at 37 °C until dark blue bands were visible. Coloration was then stopped by the addition of 25% ethanol.
-
(VI) Succinate dehydrogenase (SDH, EC 1.3.99.1): The gel was soaked in mixture A (containing 50 ml 200 mM PBS at pH 7.5, 20 ml 200 mM succinate, 20 ml 10 mM KCN, 5 ml 32.5 mg/ml PMS and 5 ml 5 mg/ml MTT) and solution B (2% agar solution at 60 °C).
The stained gels were scanned using Image Scanner III software (GE Healthcare, Piscataway, NJ).
The activities of enzymes were measured using enzyme kits. The kits for measuring HK, PFK, PK, PEPC (phosphoenolpyruvate carboxylase), IDH, MDH, SDH and soluble proteins were purchased from Comin Biotechnology Co. Ltd (Suzhou, China) and the manufacturer’s instructions were followed.
Transmission electron microscope analysis to determine Ca2+ localization and subcellular distribution in the cells of root tips
Three-millimeter-long root tip segments were fixed according to the method previously described56. The cut root tips were immersed in a solution containing 2% glutaraldehyde, 2.5% paraformaldehyde, 2% potassium pyroantimonate (K[Sb(OH)]6) and 0.1% tannic acid in a 0.1 M phosphate buffer (pH 7.6) for 6 h. The materials were rinsed three times with 0.1 M phosphate buffer containing 2% K[Sb(OH)]6. Post-fixation was carried out overnight (approximately 16 h) at 4 °C in 1% OsO4 and 2% K[Sb(OH)]6. After dehydration in an ethanol-acetone series, the materials were embedded in spur resin. Ultrathin sections for electron microscopy were prepared and stained before being examined using a transmission electron microscope (Transmission Electron Microscope H-7650; Hitachi, Japan).
X-ray microanalysis
Roots of seedlings at the three-leaf stage from different treatments were washed with distilled water three times. Root segments, including the tip and 1 cm or more of the root, were dipped in 5% agar, inserted to a depth of 1 cm in a copper holder and immediately sliced free-hand with a razor blade to obtain transverse sections; the sections were then frozen in liquid nitrogen57. The samples were freeze dried, gold coated in a high-vacuum sputter coater and stored in a desiccator. The samples were analyzed under a scanning electron microscope (S-3000N Hitachi, Japan) equipped with an energy-dispersive X-ray detector58. Five tissues—epidermal cells, exodermal cells, cortical cells, endoderm cells and the stelar parenchyma—from each root section were analyzed. Three transverse sections from each treatment were observed and three locations within the same tissue in each section were analyzed. Both map scanning and line scanning were performed.
Laser scanning confocal microscopy analyses of Ca2+ concentrations in root tips
To analyze Ca2+ levels, cucumber root tips were loaded with the Ca2+ indicator Ca2+ green 5N/AM (Molecular Probes, Inc.) and incubated at 28 °C for 60 min in the dark. The roots were washed carefully to remove excess fluorophore. Changes in intracellular Ca2+ levels were measured using a digital imaging system equipped with a laser confocal scanning microscope (Leica TCS SP2, Germany).
Quantification of Ca2+ content
Ca2+ content was measured using the Calcium Colorimetric Assay Kit (BioVision, Mountain View, CA) following the manufacturer’s instructions. The Ca2+ content was measured using a TECAN Infinite M200 Pro Reader (TRCAN, Switzerland) and was expressed as the optical density (OD) at 575 nm in μg per well. The final Ca2+ measurements were calculated according to the manufacturer’s protocol and were given in μg per μl of sample. A standard curve was prepared using known amounts of the Ca2+ standard included in the kit.
Determination the activities of tonoplast H+-ATPase and H+-PPase and plasma membrane H+-ATPase
With reference to the previous method59, the activity of plasma membrane (PM) H+-ATPase was measured in a 0.5 ml reaction volume containing 30 mM Hepes-Tris (pH 6.5), 3 mM MgSO4, 0.1 mM (NH4)MoO4, 50 mM KNO3, 0.5 mM NaN3 and 0.2% Triton X-100. The reaction was initiated by the addition of 50 μl 30 mM Na2-ATP and terminated by the addition 50 μl 55% TCA after incubation at 37 °C for 30 min. The solution was incubated at room temperature for 15 min; 2.5 ml of inorganic phosphorus protection agent containing 16 mM EDTA-Na2, 4% (NH4)MoO4, 1 mM PVP, 172 mM (H2NOH)2H2SO4, 87.5 mM H2SO4 and 0.25 ml 6.47 mM NaOH was then added. After letting the reaction continue for 40 min, we measured the OD at 660 nm using a spectrophotometer. The activity of the plasma membrane H+-ATPase was expressed as Pi•mg−1 protein•h−1. The method used to measure the activity of tonoplast H+-ATPase was similar to that used to measure the activity of plasma membrane H+-ATPase. A 0.5 ml aliquot of reaction buffer contained 30 mM Hepes-Tris (pH 7.5), 50 mM KCl, 3 mM MgSO4, 0.1 mM (NH4)2MoO4, 0.1 mM Na3VO4 and 0.5 mM NaN3. The reaction was started by adding 50 μl of 30 mM ATP-Na2. The remaining steps were identical to the method used to measure the activity of the plasma membrane H+-ATPase. The method used to measure the activity of the tonoplast H+-PPase was also identical to the method used to measure the plasma membrane H+-ATPase, except that the reaction buffer contained 30 mM Hepes-Tris (pH 8.0) and 50 μl 3 M Na4PPi was used to start the reaction60.
Additional Information
How to cite this article: He, L. et al. The effect of exogenous calcium on mitochondria, respiratory metabolism enzymes and ion transport in cucumber roots under hypoxia. Sci. Rep. 5, 11391; doi: 10.1038/srep11391 (2015).
References
Bailey-Serres, J. & Voesenek, L. A. C. J. Flooding stress: acclimations and genetic diversity. Annu. Rev. Plant Biol. 59, 313–339 (2008).
Sairam, R., Kumutha, D., Ezhilmathi, K., Deshmukh, P. & Srivastava, G. Physiology and biochemistry of waterlogging tolerance in plants. Biologia Plantarum 52, 401–412 (2008).
Kang Y.Y., Guo S.R. & Duan, J.J. Effects of root zone hypoxia on respiratory metabolism of cucumber seedlings roots. Chinese Journal of Ecology 19, 583–587 (2008).
Ahsan, N. et al. A proteomic screen and identification of waterlogging-regulated proteins in tomato roots. Plant and Soil 295, 37–51 (2007).
Christianson, J. A., Llewellyn, D. J., Dennis, E. S. & Wilson, I. W. Global gene expression responses to waterlogging in roots and leaves of cotton (Gossypium hirsutum L.). Plant and Cell Physiol. 51, 21–37, (2010).
Yang, C.Y., Hsu, F.C., Li, J.P., Wang, N.N. & Shih, M.C. The AP2/ERF transcription factor AtERF73/HRE1 modulates ethylene responses during hypoxia in Arabidopsis. Plant Physiol. 156, 202–212 (2011).
Dennis, E. S. et al. Molecular strategies for improving waterlogging tolerance in plants. J. Exp. Bot. 51, 89–97 (2000).
Kennedy, R. A., Rumpho, M. E. & Fox, T. C. Anaerobic metabolism in plants. Plant Physiol. 100, 1 (1992).
Li, J., Sun, J., Yang, Y., Guo, S. & Glick, B. R. Identification of hypoxic-responsive proteins in cucumber roots using a proteomic approach. Plant Physiol. Biochem. 51, 74–80, (2012).
Li, C.R. et al. Unravelling mitochondrial retrograde regulation in the abiotic stress induction of rice ALTERNATIVE OXIDASE 1 genes. Plant Cell Environ. 36, 775–788, (2013).
Vanlerberghe, G. C., Cvetkovska, M. & Wang, J. Is the maintenance of homeostatic mitochondrial signaling during stress a physiological role for alternative oxidase? Physiologia Plantarum 137, (2009).
Lord, E. M. & Russell, S. D. The mechanisms of pollination and fertilization in plants. Annu. Rev. Cell and Develop. Biol. 18, 81–105 (2002).
Boavida, L. C., Vieira, A. M., Becker, J. D. & Feijó, J. A. Gametophyte interaction and sexual reproduction: how plants make a zygote. Inter. J. Develop. Biol. 49, 615–632 (2005).
Hashimoto, K. & Kudla, J. Calcium decoding mechanisms in plants. Biochimie 93, 2054–2059 (2011).
Henriksson, E. & Nordin Henriksson, K. Salt-stress signalling and the role of calcium in the regulation of the Arabidopsis ATHB7 gene. Plant Cell Environ 28, 202- 210 (2005).
Aurisano, N., Bertani, A. & Reggiani, R. Involvement of calcium and calmodulin in protein and amino acid metabolism in rice roots under anoxia. Plant and Cell Physiol. 36, 1525–1529 (1995).
Gao, H., Chen, G., Han, L. & Lin, H. Calcium influence on chilling resistance of grafting eggplant seedlings. J. Plant Nutr. 27, 1327- 1339 (2005).
Yemelyanov, V., Shishova, M., Chirkova, T. & Lindberg, S. Anoxia-induced elevation of cytosolic Ca2+concentration depends on different Ca2+sources in rice and wheat protoplasts. Planta 234, 271–280 (2011).
Gao, H. et al. Exogenous calcium affects nitrogen metabolism in root-zone hypoxia-stressed muskmelon roots and enhances short-term hypoxia tolerance. J. Plant Physiol. 168, 1217–1225 (2011).
Qu, H., Jiang, X., Shi, Z., Liu, L. & Zhang, S. Fast loading ester fluorescent Ca2+ and pH indicators into pollen of Pyrus pyrifolia. J. Plant Res. 125, 185–195, (2012).
Pang, J. & Shabala, S. in Waterlogging Signalling and Tolerance in Plants (eds Stefano Mancuso & Sergey Shabala ) Ch. 10, [197–219] (Springer Berlin Heidelberg, 2010).
Greenway, H. & Gibbs, J. Review: Mechanisms of anoxia tolerance in plants. II. Energy requirements for maintenance and energy distribution to essential processes. Functional Plant Biol. 30, 999–1036 (2003).
Pang, J. Y., Newman, I. A. N., Mendham, N., Zhou, M. & Shabala, S. Microelectrode ion and O2 fluxes measurements reveal differential sensitivity of barley root tissues to hypoxia. Plant Cell Environ 29, 1107–1121 (2006).
Rocha, M. et al. Glycolysis and the tricarboxylic acid cycle are linked by alanine aminotransferase during hypoxia induced by waterlogging of lotus japonicus. Plant Physiol. 152 (2010).
He, L. Z., Guo, S. R ., LU, X. M., Wang, L. P. & Yang, Y. J. Effects of calcium on soluble protein expression of cucumber seedlings under roo-zoon hypoxia stress. Journal of Nanjing Agricultural University 35, 21–25 (2012).
Gupta, K. J., Zabalza, A. & Van Dongen, J. T. Regulation of respiration when the oxygen availability changes. Physiologia Plantarum 137, 383–391 (2009).
Millar, A. H., Whelan, J., Soole, K. L. & Day, D. A. Organization and regulation of mitochondrial respiration in plants. Annu. Rev. Plant Biol. 62, 79–104 (2011).
Stael, S. et al. Plant organellar calcium signalling: an emerging field. J. Exp. Bot. 63, 1525–1542 (2012).
Pottosin, I. et al.Cross-talk between reactive oxygen species and polyamines in regulation of ion transport across the plasma membrane:implication for plant adaptive responses. J.Exp. Bot. 65, 1271–1283 (2014).
Semenza, G. L. Oxygen-dependent regulation of mitochondrial respiration by hypoxia-inducible factor 1. Biochem J 405, 1–9 (2007).
Tian, J., Wang, L.P., Yang, Y.J., Sun, J. & Guo, S.-R. Exogenous spermidine alleviates the oxidative damage in cucumber seedlings subjected to high temperatures. J. American Society for Horticultural Sci. 137, 11–19 (2012).
Zsigmond, L. et al. Arabidopsis PPR40 connects abiotic stress responses to mitochondrial electron transport. Plant Physiol. 146, 1721–1737 (2008).
Costa, A. et al. H2O2 in plant peroxisomes: an in vivo analysis uncovers a Ca2+‐dependent scavenging system. Plant J. 62, 760–772 (2010).
He, L. et al. Proteomic analysis of the effects of exogenous calcium on hypoxic-responsive proteins in cucumber roots. Proteome Sci. 10, 42 (2012).
Loulakakis, K. A. & Roubelakis-Angelakis, K. A. The seven NAD(H)-glutamate dehydrogenase isoenzymes exhibit similar anabolic and catabolic activities. Physiologia Plantarum 96, 29–35 (1996).
Enríquez-Arredondo, C. et al. The plasma membrane H+-ATPase of maize embryos localizes in regions that are critical during the onset of germination. Plant Sci. 169, 11–19 (2005).
Moore, B. et al. Role of the Arabidopsis glucose sensor HXK1 in nutrient, light and hormonal signaling. Science 300, 332–336 (2003).
Huang, S., Colmer, T. D. & Millar, A. H. Does anoxia tolerance involve altering the energy currency towards PPi? Trends in Plant Sci. 13, 221–227 (2008).
Lara, M. V. et al. Peach (Prunus Persica) fruit response to anoxia: reversible ripening delay and biochemical changes. Plant and Cell Physiol. 52, 392–403 (2011).
Mancuso, S. & Marras, A. M. Adaptative response of vitis root to anoxia. Plant and Cell Physiol. 47, 401–409 (2006).
Nunes-Nesi, A., Sulpice, R., Gibon, Y. & Fernie, A. R. The enigmatic contribution of mitochondrial function in photosynthesis. J. Exp. Bot. 59, 1675–1684 (2008).
Zhang, E.R., Ren, Y.Y., Hu, H.Q., Liu, Y.H. & Chen, S.S. Effects of calcium on growth and respiratory metabolism of hot pepper seedling roots under flood stress. Acta Horticulturae Sinica 36, 1749–1754 (2009).
Pandolfi, C., Pottosin, I., Cuin, T., Mancuso, S. & Shabala, S. Specificity of polyamine effects on NaCl-induced ion flux kinetics and salt stress amelioration in plants. Plant and Cell Physiol. 51, 422–434 (2010).
Latz, A. et al. Salt stress triggers phosphorylation of the Arabidopsis vacuolar K+ channel TPK1 by calcium-dependent protein kinases (CDPKs). Mol. Plant 6, 1274–1289 (2013).
Shabala, A. & Pottosin, l. Regulation of potassium transport in plants under hostile conditions: implications for abiotic and biotic stress tolerance. Physiologia Plantarum 151, 257–279 (2014).
Shabala, A. et al. Extracellular Ca2+ ameliorates NaCl-induced K+ loss from Arabidopsis root and leaf cells by controlling plasma membrane K+-permeable channels. Plant Phsiol. 141, 1653–1665 (2006).
Drew, M. C. Oxygen deficiency and root metabolism: injury and acclimation under hypoxia and anoxia. Annu. Rev. Plant Physiol. and Plant Mol. Biol. 48, 223–250 (1997).
Carystinos, G. D., MacDonald, H. R., Monroy, A. F., Dhindsa, R. S. & Poole, R. J. Vacuolar H+-Translocating Pyrophosphatase is induced by anoxia or chilling in seedlings of rice. Plant Physiol. 108, 641–649 (1995).
Lasanthi-Kudahettige, R. et al. Transcript profiling of the anoxic rice coleoptile. Plant Physiol. 144, 218–231 (2007).
Stael, S. et al. Arabidopsis calcium-binding mitochondrial carrier proteins as potential facilitators of mitochondrial ATP-import and plastid SAM-import. FEBS Letters 585, 3935–3940 (2011).
Shabala, S. & Cuin, T. A. Potassium transport and plant salt tolerance. Physiologia Plantarum 133, 651–669 (2008).
Ma, Y.H. & Guo, S.R. On different hypoxia tolerance of thirteen cucumber varieties. Jiangsu Agricultural Sciences 5, 68–70 (2004).
Wagner, A. M. & Wagner, M. J. Changes in mitochondrial respiratory chain components of petunia cells during culture in the presence of antimycin A. Plant Physiol. 115, 617–622 (1997).
Qian, Q.Q., Zai, W.S., He, Y., Wang, Y.C. & Zhu, Z.J. Protection of exogenous silicon and CoQ10 on mitochondria in cucumber (Cucumis sativus L.) roots under salt stress Sci. Agr. Sinica 39, 1208–1214 (2006).
Manchenko, G. P. Handbook of detection of enzymes on electrophoretic gels. 2 edn, [223–235] (CRC Press, 1994).
Zhang, H., Zhang, D., Han, S., Zhang, X. & Yu, D. Identification and gene mapping of a soybean chlorophyll-deficient mutant. Plant Breeding 130, 133–138 (2011).
Peng, Y. H. et al. Alkali grass resists salt stress through high [K+] and an endodermis barrier to Na+. J. Exp. Bot. 55, 939–949 (2004).
Tomos, A., Hinde, P., Richardson, P., Pritchard, J. & Fricke, W. Microsampling and Measurements of Solutes in Single Cells. (Oxford University Press, 1994).
Blumwald, E. & Poole, R. J. Salt Tolerance in suspension cultures of sugar beet: induction of Na+/H+ antiport activity at the tonoplast by growth in salt. Plant Physiol. 83, 884–887 (1987).
Zabalza, A. et al. Regulation of respiration and fermentation to control the plant internal oxygen concentration. Plant Physiol. 149, 1087–1098 (2009).
Acknowledgements
This work was supported by the National Basic Research Program of China (973 Program) (No. 2009CB119000), the China earmarked fund for Modern Agro-industry Technology Research System (CARS-25-C-03) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). The authors are very grateful to Prof. Juan Li for helping us to revise our manuscript.
Author information
Authors and Affiliations
Contributions
S.G. designed and managed the research and improved the manuscript. L.H., B.L., X.L., L.Y., Ya.Y., Yi.Y. and J.D. performed the experiments. L.H. and B.L. wrote the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
He, L., Li, B., Lu, X. et al. The effect of exogenous calcium on mitochondria, respiratory metabolism enzymes and ion transport in cucumber roots under hypoxia. Sci Rep 5, 11391 (2015). https://doi.org/10.1038/srep11391
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep11391
This article is cited by
-
Physiological and Biochemical Mechanisms of Prunus sachalinensis Root for Coping with Short-term Waterlogging and Subsequent Recovery
Journal of Soil Science and Plant Nutrition (2024)
-
CaCl2 priming promotes sorghum seed germination under salt stress by activating sugar metabolism
Plant Growth Regulation (2023)
-
Paenibacillus polymyxa NSY50 Improves Defense Against Fusarium oxysporum by Increasing Photosynthetic Efficiency, Sucrose Metabolism, and Antioxidant Capacity in Cucumber
Journal of Plant Growth Regulation (2023)
-
Improving yield and quality of rice under acid rain stress by regulating nitrogen assimilation with exogenous Ca2+
Environmental Science and Pollution Research (2022)
-
Temperature effect on water dynamics in tetramer phosphofructokinase matrix and the super-arrhenius respiration rate
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.