Abstract
We report a first-principle study on electronic structure and simulation of the spin-polarized scanning tunneling microscopy graphic of a benzene/Fe4N interface. Fe4N is a compound ferromagnet suitable for many spintronic applications. We found that, depending on the particular termination schemes and interface configurations, the spin polarization on the benzene surface shows a rich variety of properties ranging from cosine-type oscillation to polarization inversion. Spin-polarization inversion above benzene is resulting from the hybridizations between C pz and the out-of-plane d orbitals of Fe atom.
Similar content being viewed by others
In recent years, combining inorganic and organic materials have opened many venues for the novel science and applications. The rising field of organic spintronics is one of them1,2,3,4,5,6. Organic materials, with long spin-flip diffusion length and weak spin-orbit coupling, are desirable in many spintronic applications2,7,8,9,10,11,12,13,14,15,16,17. Moreover, the functionalities of devices made of organic materials can be manipulated through relatively simple methods such as ligand modification18,19 and isomerization20,21.
A major topic in organic spintronics is the spin related properties at the interface between a ferromagnetic substrate and organic material, i.e. the spinterface22. Much effort has been made to clarify the underlying mechanisms that drive the peculiarities, such as spin polarization inversion, at various spinterfaces21,23,24,25,26. For the interfaces between Fe and benzene molecule (as well as C5H5 and C8H8), Atodiresei et al. argue that it is the pz-d Zener exchange-type mechanism that leads to the spin-polarization inversion24. In another study on the thiophene/cobalt(001) interface25, the strong spatial dependence of the spin polarization at the interface is attributed to the reduced molecular symmetry. The azobenzene isomer adsorbed on Fe surface has been reported recently, where the switch between two types of azobenzenes takes place by applying light and/or heat21. On the other hand, studies are extended to systems consisting of AFM substrates including benzene adsorbed on monolayer AFM Mn26 or metal phthalocyanine4,27,28,29, where the spin polarization modification has often been reported.
But, we are not aware of any investigations into the interfaces between an organic molecule and a compound ferromagnet, such as iron nitride (Fe4N). Fe4N carries a high spin polarization of nearly ~100%30 as well as a large saturation magnetization of 1200 emu/cm3 31. Its Curie temperature is about 760 K. Together with its high chemical stability32 and low coercivity33, Fe4N is a promising candidate for, among other, spin injection source34. In this work, we scrutinize the spinterface between a benzene molecule and Fe4N substrate. In particular, we will show that different termination schemes and adsorption configurations unique to the benzene/Fe4N interface enrich the properties of spin polarization.
Calculation details
Our first principles calculations are based on the density-functional theory (DFT) and the projector augmented wave method as implemented in the Vienna Ab initio Simulation Package code35,36. For the exchange and correlation functional, we use the Perdew-Burke-Ernzerhof spin-polarized generalized gradient approximation (PBE-GGA)37. The plane-wave basis set is converged using a 500 eV energy cutoff. A Γ-centered 3 × 3 × 1 k-mesh is used for the Brillouin-zone integrations. A Gaussian smearing of 0.02 eV is used for the initial occupations. It is worth pointing out that van der Waals force is excluded from our calculation. We do so not only because strong bonding exits between benzene molecule and Fe4N (as shown in the due discussion), but also recent studies suggest that it has a negligible effect on GGA optimized structure in, for example, azobenzene/Fe(110)21.
Bulk Fe4N has a cubic perovskite-type structure (Pm3m) with a lattice constant of 3.795 Ǻ34. Fe occupies the corner (FeI) or face-centered (FeII) position labeled structure graphic of bulk Fe4N, whereas N locates at the body-centered site34, as shown in Fig. 1(a). Our calculations give a lattice constant of 3.789 Ǻ, in agreement with the experimental value34. The Fe4N substrate is modeled by slabs of three atomic layers with a (3 × 3) flat surface. We concentrate, in this work, on the effect of different adsorption schemes on the spatial spin-polarization distribution. The subtleties due to the number of atomic layers will be reported in upcoming studies.
The structure of bulk Fe4N and the side and top views of four benzene/Fe4N(001) structures. The adsorption energies (Eabs) are labeled in Fig. 1(b).
Termination schemes and interface models
The benzene/Fe4N interfaces are modeled by placing benzene on top of the Fe4N(001) surface. The lattice structure of Fe4N allows us to have the interfaces with two types of terminations, namely, FeIIN and FeIFeII. For FeIIN termination, Fe4N surface is the plane across the body-centered site parallel to Fe4N(001). For FeIFeII termination, Fe4N surface refers to the plane across the face-centered site. For each termination scheme, we further consider two stacking models based on whether N or Fe atom, in the first layer, is right beneath the center of benzene molecule: models FeIIN-C and FeIFeII-C are named after the terminations with N or Fe atom in the Fe4N surface, locating right beneath the center of benzene molecule, whereas models FeIIN-NC and FeIFeII-NC are referring to the ones without, see Fig. 1. During the lattice structure relaxation, the atoms in the bottom layer of slab are fixed at their bulk positions, whereas other atoms are fully relaxed until the force is weaker than 0.03 eV/Å. In order to decouple adjacent slabs, a thick vacuum layer of 15 Ǻ is included in the direction perpendicular to the surface. To illustrate the nature of the spin-polarization inversion in the real space, we calculate the spin-polarization distribution by the constant-height spin-polarized scanning tunneling microscopy (SP-STM) simulation38.
Results and discussion
To illustrate the system, we also define the surface (inter layer, fixed layer) of Fe4N slab as I (II, III) layer and the benzene as M layer. We call the zone above benzene as ‘benzene surface’, between benzene and Fe4N as ‘interfaces’ and atoms of I layer, in Fe4N slab, as ‘Fe4N surface’. The sites where the Fe ion located right below the C atom are defined as the top (_t) sites; the ones where the Fe ion located under the C-C bond are defined as the bridge (_b) sites. At the top or bridge sites, we call the Fe (C) atom by Fe_t (C_t) or Fe_b (C_b). The atom located right under the center of benzene is Fe_c or N_c. Figure 1(c–f) show the side and top views of the four optimized stacking models. After the structure relaxation, benzene plane is no longer flat in all models except FeIIN-NC. Especially, the hydrogen atoms in FeIIN-C and FeIFeII-NC models lie fairly further away from the slab surface than carbon atoms, agreeing with earlier reports18,24. The C-C bonds become longer than those in the isolated benzene ring.
The Fe4N slab also experiences the structural changes. In both FeIIN-C and FeIFeII-NC models, the Fe_t and Fe_b atoms move out of the Fe plane of the surface, see Fig. 1(c,f). In the FeIFeII-C model, Fe_c moves up, as shown in Fig. 1(e). In the exception arises from the FeIIN-NC model, where the benzene plane is still flat, yet the C-C bonds are equal to that in the isolated benzene. The benzene plane is moving away from the Fe4N surface, as shown in Fig. 1(d). The data from our calculation is in the Table 1.
The adsorption energy (Eabs) of different models is labeled in Fig. 1(b). According to the adsorption energy, 4 adsorption models fall into two categories: the endothermic adsorption (two FeIIN terminals models) and exothermic adsorption (two FeIFeII terminals models). The FeIIN-C model has the maximum adsorption energy (0.74 eV). This implies that, at high temperatures, it is the most easily formed model among the four. On the other hand, FeIFeII-NC shows an exothermic adsorption with the minimum adsorption energy (−2.14 eV), implying that its stability favours low temperatures.
The moment and charge are listed in Table 2. The charge value is calculated using Bader analysis39,40,41. We note that the FeII moment in layer II, see the 3rd row in Table 2, is smaller than 2.29 μB in bulk Fe4N, where μB is Bohr magneton. This is due to a stronger yet more localized hybridization between N and FeII in the second layer42,43,44. Apart from the exception in the FeII ions in layer II, in FeIFeII terminal, we observe that, while it gains more charge, the FeII moment tends to be larger than that in bulk Fe4N. But in the FeIIN terminal, this relation no longer holds; no prominent relationship between charge and moment is present.
To understand the bonding mechanisms, we analyze the charge density difference defined by  , where
, where  ,
,  and
and  are the charge densities of the full system, isolated benzene and Fe4N surface, respectively. Charge accumulation (depletion) is in yellow (blue). In the FeIIN-C model, the charge accumulates on the C-Fe bonds, as Fig. 2(a) shows. In Fig. 2(b), the interface has little charge accumulation between C and Fe ions, indicating that C atoms do not form bonds with Fe4N slab. This is consistent with the large distance between the benzene and Fe4N surface. Figure 2(c) displays a large charge accumulation in the region right below benzene in the FeIFeII-C model. In the FeIFeII-NC model, the charge depletion distributes around the centerline perpendicular to Fe4N surface and significant charge accumulation appears around the C_t-Fe_t and C_b-Fe_b bonds, as shown in Fig. 2(d). The C atom captures only approximately 0.1 ~ 0.2|e|, suggesting the covalent characteristics of the C-Fe bonds.
are the charge densities of the full system, isolated benzene and Fe4N surface, respectively. Charge accumulation (depletion) is in yellow (blue). In the FeIIN-C model, the charge accumulates on the C-Fe bonds, as Fig. 2(a) shows. In Fig. 2(b), the interface has little charge accumulation between C and Fe ions, indicating that C atoms do not form bonds with Fe4N slab. This is consistent with the large distance between the benzene and Fe4N surface. Figure 2(c) displays a large charge accumulation in the region right below benzene in the FeIFeII-C model. In the FeIFeII-NC model, the charge depletion distributes around the centerline perpendicular to Fe4N surface and significant charge accumulation appears around the C_t-Fe_t and C_b-Fe_b bonds, as shown in Fig. 2(d). The C atom captures only approximately 0.1 ~ 0.2|e|, suggesting the covalent characteristics of the C-Fe bonds.
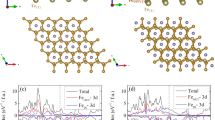
A general picture of bonding mechanism between the benzene and different Fe4N terminations can be further extracted from the spin-resolved density-of-states (DOS), as Figs. 3 and 4 show. The 3d orbitals can be divided into two classes according to the symmetry: the out-of-plane orbitals ( , dxz + dyz) and the in-plane ones (
, dxz + dyz) and the in-plane ones ( ). In the FeIIN-C model, for the top sites, Fe_t
). In the FeIIN-C model, for the top sites, Fe_t  and dxz + dyz hybridize with C_t pz in the energy interval of −4.25~−3.5 eV for both the spin-up and spin-down states. At about 2.79 eV, the hybridization is just for the spin down states. Meanwhile, the Fe_t spin-up
and dxz + dyz hybridize with C_t pz in the energy interval of −4.25~−3.5 eV for both the spin-up and spin-down states. At about 2.79 eV, the hybridization is just for the spin down states. Meanwhile, the Fe_t spin-up  has hybridization with C_t pz at 1.80 eV.
has hybridization with C_t pz at 1.80 eV.
For the bridge sites, we note that the spin-up and spin-down π orbitals of benzene are mixed with Fe_b  and dxz + dyz in −4.14~−3.5 eV energy interval and with Fe_b spin-down dxz + dyz at 2.54 eV. Besides the strong hybridization that mentioned above, a series of hybridizations between the C pz and Fe d states are drawn in Fig. 3(a). For Fe at both the top and bridge sites, its s and d orbitals, for both spin species, hybridize with the N_c p orbitals in the energy interval −7.6~−3.8 eV. The degenerated px + py orbitals of N_c hybridize with Fe
and dxz + dyz in −4.14~−3.5 eV energy interval and with Fe_b spin-down dxz + dyz at 2.54 eV. Besides the strong hybridization that mentioned above, a series of hybridizations between the C pz and Fe d states are drawn in Fig. 3(a). For Fe at both the top and bridge sites, its s and d orbitals, for both spin species, hybridize with the N_c p orbitals in the energy interval −7.6~−3.8 eV. The degenerated px + py orbitals of N_c hybridize with Fe  in the energy interval 2.0~5.0 eV. And this hybridization between the spin-down pz of C and
in the energy interval 2.0~5.0 eV. And this hybridization between the spin-down pz of C and  ,
,  of Fe_t and Fe_b is fairly strong at −0.43 eV.
of Fe_t and Fe_b is fairly strong at −0.43 eV.
In Fig. 3(b) for the FeIIN-NC model, the benzene π orbitals originating from the C pz orbitals do not hybridize with Fe. The slab keeps mostly the properties of a clean surface. The N px and py orbitals are degenerate. The DOS of Fe_t is almost the same as that of Fe_b. Meanwhile, the FeII dxz + dyz orbitals hybridize with the N pz orbitals in the energy interval −4.1~−6.6 eV and at Fermi energy (EF). The FeII  and s orbitals are mixed with the degenerate N px, py orbitals at −6.6~−7.2 eV.
and s orbitals are mixed with the degenerate N px, py orbitals at −6.6~−7.2 eV.
In the FeIFeII terminations, the intensity of local benzene π orbitals peak become weak gradually and the peak becomes wider. Figure 4 shows the DOS of two FeIFeII terminations. In the FeIFeII-C model, Fe_c dxz + dyz hybridizes with C pz at −5.1 eV and with C px, py at −7.9 eV, as shown in Fig. 4(a). The Fe_c  orbitals hybridize with C pz in the energy interval −6.7~−6.3 eV. The hybridization between the Fe_c spin-up
orbitals hybridize with C pz in the energy interval −6.7~−6.3 eV. The hybridization between the Fe_c spin-up  and C pz is strengthened in the energy interval of 1.1 ~ 2.1 eV. At the energy level above 2.5 eV, Fe_c spin-down
and C pz is strengthened in the energy interval of 1.1 ~ 2.1 eV. At the energy level above 2.5 eV, Fe_c spin-down  weakly hybridizes with C_t pz.
weakly hybridizes with C_t pz.
In the FeIFeII-NC model, we see a rather weak mixture between the C_t pz and Fe_t  , dxz + dyz states in the interval −7.4~−6.0 eV. We note that C_t pz orbitals tend to degenerate with the px orbitals, yet C_b shows no such tendency. In the interval −5.5~−4.0 eV, the prime conjugate peaks consist of Fe_b s and C_b pz orbitals. At −1.75 eV, the Fe_b spin-down dxz + dyz and
, dxz + dyz states in the interval −7.4~−6.0 eV. We note that C_t pz orbitals tend to degenerate with the px orbitals, yet C_b shows no such tendency. In the interval −5.5~−4.0 eV, the prime conjugate peaks consist of Fe_b s and C_b pz orbitals. At −1.75 eV, the Fe_b spin-down dxz + dyz and  have hybridization with the C_b pz orbitals.
have hybridization with the C_b pz orbitals.
We note a trend from the above hybridization schemes. As the benzene molecule moves towards the Fe4N surface, the hybridization of different orbitals depends on the termination schemes. In the FeIIN-C and FeIFeII-C models, the Fe dxz + dyz and  orbitals hybridize strongly with the C pz state, leading to spin-polarization inversion. In the FeIFeII-NC model, the hybridization between both spin species of the C pz and Fe s,
orbitals hybridize strongly with the C pz state, leading to spin-polarization inversion. In the FeIFeII-NC model, the hybridization between both spin species of the C pz and Fe s,  orbitals is stronger than that between C pz and Fe (dxz + dyz,
orbitals is stronger than that between C pz and Fe (dxz + dyz,  ), unable to reverse the spin polarization. This is consistent with the report in Ref. 17. We are thus led to conclude that the spin-polarization inversion at benzene surface is a result of hybridizations between the pz orbital of C and the out-of-plane Fe d orbitals.
), unable to reverse the spin polarization. This is consistent with the report in Ref. 17. We are thus led to conclude that the spin-polarization inversion at benzene surface is a result of hybridizations between the pz orbital of C and the out-of-plane Fe d orbitals.
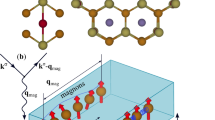
We show, in Fig. 5, the spatial distribution of spin-polarization Pspace, defined as

for an energy interval of [ε, EF].  is the spin-up (down) charge density in real space, at position
is the spin-up (down) charge density in real space, at position  and a distance z from the surface. Here, the value of ε is either EF−0.4 eV or EF + 0.4 eV 21,28. In this figure, we focus on two energy intervals, [EF−0.4 eV, EF] and [EF, EF + 0.4 eV]. In each energy interval, the spin-polarization is projected onto the plane that is parallel to the Fe4N surface, see Fig. 5(b,c); the distance between the plane and benzene surface is labeled in Fig. 5(b). For the 4 models discussed in this work, we plot, in Fig. 5(a,d), the spin polarization along a few selected lines defined in Fig. 5(b,c).
and a distance z from the surface. Here, the value of ε is either EF−0.4 eV or EF + 0.4 eV 21,28. In this figure, we focus on two energy intervals, [EF−0.4 eV, EF] and [EF, EF + 0.4 eV]. In each energy interval, the spin-polarization is projected onto the plane that is parallel to the Fe4N surface, see Fig. 5(b,c); the distance between the plane and benzene surface is labeled in Fig. 5(b). For the 4 models discussed in this work, we plot, in Fig. 5(a,d), the spin polarization along a few selected lines defined in Fig. 5(b,c).
Spin polarization nearby the benzene plane in the vacuum for these four models. (a) and (b) represent spin polarization distribution in [EF−0.4 eV, EF] energy interval; (c) and (d) represent spin-polarization distribution in [EF, EF + 0.4 eV] energy interval. (a)/(d) is line profiles of the spin-polarization selected in (b)/(c) for different structures, respectively. The height of the spin-polarization distribution plane relative to the benzene plane are labeled under (b) for each model.
For the FeIIN-C model, the highest spin polarization is ~80% and the lowest value is ~−60% via line 2 and line 3, see Fig. 5(a). The intensity of inversion is much stronger than benzene adsorbed antiferromagnetic Mn26, due to the hybridization between the pz states and  ,
,  orbitals of Fe_t and Fe_b at −0.43 eV. This hybridization enhances the population of the spin-down species and is thus reversing the spin polarization. It is interesting to note that the spin-polarization distributions in the energy interval [EF −0.4 eV, EF] and [EF, EF + 0.4 eV] are rather different, even with opposite signs. This suggests that the sign of spin polarization can be reversed by simply shifting the EF by, for example, applying a gate voltage.
orbitals of Fe_t and Fe_b at −0.43 eV. This hybridization enhances the population of the spin-down species and is thus reversing the spin polarization. It is interesting to note that the spin-polarization distributions in the energy interval [EF −0.4 eV, EF] and [EF, EF + 0.4 eV] are rather different, even with opposite signs. This suggests that the sign of spin polarization can be reversed by simply shifting the EF by, for example, applying a gate voltage.
In the FeIIN-NC model, line 2 exhibits the spin-polarization inversion, as shown in Fig. 5(b). The DOS, in Fig. 3(b), however, points to a weak adsorption. The spin-polarization distribution in this model is thus similar to that in vacuum (above a clean Fe4N surface). For the FeIIN-NC model, the spin polarization of line 1 with a cosine-type distribution is shown in Fig. 5(a).
In both FeIFeII terminal models, spin-polarization inversion happens, but the strong spin-polarization inversion in the neighbourhood of benzene happens only in FeIFeII-C. In FeIFeII terminations, Fe4N surface distort significantly, FeII ions are not located right above N atoms, as shown in Figs. 1(e,f). Then positive spin polarization of N atoms extends into benzene surface. In FeIFeII-C model, the most interesting feature is that the positive spin-polarization distributes along the C-C bonds, see Fig. 5(b). In Fig. 6, a positive spin polarization of benzene appears at EF in the FeIFeII-C model. On the other hand, in the FeIFeII-NC model spin polarization is approximate 0%. Meanwhile spatial spin-polarization, for FeIFeII-NC, in the neighbourhood of benzene is almost 0%, see Fig. 5(b). So, the atomic scale spin-polarization, at benzene surface, is modulated by N and C atoms.
Figure 7 is the spin-polarization plane of FeIIN-C structure. It’s across the top sites and parallel to Fe4N(100). From this figure, benzene hampers the extension of N position spin polarization and realizes the spatial spin polarization inversion. The reason is the overlap of pz and out-of-plane components of d.
Conclusion
In summary, we have shown that at the spinterface formed by benzene adsorbed on Fe4N, depending on the specific termination schemes, a variety of spin polarization, including spin polarization inversion, can take place. The spin-polarization inversion finds its origin in the hybridization between the out-of-plane components of Fe d orbitals and the benzene π orbitals (the pz orbital, in particular). The presence of N atoms partition the adsorption into two categories: the endothermic (adsorption) FeIIN terminal models and the exothermic FeIFeII terminal ones. With these results, we can see that adsorptions rely on the temperature. The FeIIN-C with the maximum adsorption energy will be easier to be formed than others under high temperature and has significant spin-polarization inversion, which is desired for the spintronic devices.
Additional Information
How to cite this article: Zhang, Q. et al. Spin Polarization Inversion at Benzene-Absorbed Fe4N Surface. Sci. Rep. 5, 10602; doi: 10.1038/srep10602 (2015).
References
Xiong, Z. H., Wu, D. Z., Vardeny, V. & Shi, J. Giant magnetoresistance in organic spin-valves. Nature 427, 821–824 (2004).
Dediu,V., Murgia, M., Matacotta, F. C., Taliani, C. & Barbanera, S. Room temperature spin polarized injection in organic semiconductor. Solid State Commun. 122, 181–184 (2002).
Drew, A. J. et al. Direct measurement of the electronic spin diffusion length in a fully functional organic spin valve by low-energy muon spin rotation. Nat. Mater. 23, 109–114 (2008).
Mugarza, A. et al. Electronic and magnetic properties of molecule-metal interface: Transition-metal phthalocyanines adsorbed on Ag(100). Phys. Rev. B 85, 155437 (2012).
Rybicki, J. et al. Tuning the performance of organic spintronic devices using X-Ray generated traps. Phys. Rev. Lett. 109, 076603 (2012).
Sun, D. et al. Giant magnetoresistance in organic spin valves. Phys. Rev. Lett. 104, 236602 (2010).
Dediu, V., Hueso, L. E., Bergenti, I. & Taliani, C. Spin routes in organic semiconductors. Nature Mater. 8, 707–716 (2009).
Xu, W. et al. Tunneling magnetoresistance observed in La0.67Sr0.33MnO3/organic molecule/Co junctions. Appl. Phys. Lett. 90, 072506 (2007).
Vinzelberg, H. et al. Low temperature tunneling magnetoresistance on (La, Sr)MnO3/Co junctions with organic spacer layers. Appl. Phys. Lett. 103, 093720 (2008).
Dediu, V. et al. Room-temperature spintronic effects in Alq3-based hybrid devices. Phys. Rev. B 78, 115203 (2008).
Barraud, C. et al. Unravelling the role of the interface for spin injection into organic semiconductors. Nat. Phys. 6, 615–620 (2010).
Steil, S. et al. Spin-dependent trapping of electronics at spinterfaces. Nat. Phys. 9, 242–247 (2013).
Dediu, V. A. Organic spintronics: Inside the interface. Nat. Phys. 9, 210–211 (2013).
Santos, T. S. et al. Room-temperature tunnel magnetoresistance and spin-polarized tunneling through an organic semiconductor barrier. Phys. Rev. Lett. 98, 016601 (2007).
Jiang, J.S., Pearson, J.E. & Bader, S. D. Absence of spin transport in the organic semiconductor Alq3 . Phys. Rev. B 77, 035303 (2008).
Davis, A. H. & Bussmann, K. Organic luminescent devices and magnetoelectronics. J. Appl. Phys. 93, 7358–7360 (2003).
Rocha, A. R. et al. Towards molecular spintronics. Nat. Mater. 4, 335–339 (2005).
Atodiresei, N., Caciuc, V., Lazić, P. & Blügel, S. Engineering the magnetic properties of hybrid organic-ferromagnetic interfaces by molecular chemical functionalizaton. Phys. Rev. B 84, 172402 (2011).
Friedrich, R. et al. Electronic states and the influence of oxygen addition on the optical absorption behaviour of manganese phthalocyanine. J. Chem. Phys. 136, 064704 (2012).
Friedrich, R., Kersting, B. & Kortus, J. Fermi level engineering in organic semiconductors for controlled manufacturing of charge and spin transfer materials. Phys. Rev. B 88, 155327 (2013).
Wang, Y., Che, J. G., Fry, J. N. & Cheng, H. P. Reversible spin polarization at hybrid organic-Ferromagnetic interfaces. J. Phys. Chem. Lett. 4, 3508–3512 (2013).
Sanvito, S. Molecular spintronics: The rise of spinterface science. Nat. Phys. 6, 562–564 (2010).
Qi, S. F., Ning, W. W. & Xu, X. H. The spin-filter role of the ill-defined layer in FM/Alq3/FM organic spin valve: A first-principle study. Synth. Met. 177, 82–88 (2013).
Atodiresei, N. et al. Design of local spin polarization at the organic-ferromagnetic interface. Phys. Rev. Lett. 105, 066601 (2010).
Wang, X., Zhu, Z., Manchon, A. & Schwingenschlögl, U. Peculiarities of spin porlarization inversion at a thiophene/cobalt interface. Appl. Phys. Lett. 102, 111604 (2013).
Caffrey, N. M., Ferriani, P., Marocchi, S. & Heinze, S. Atomic-scale inversion of spin polarization at an organic-antiferromagnetic interface. Phys. Rev. B 88, 155403 (2013).
Sun, X., Wang, B. & Yamauchi, Y. Electronic structure and spin polarization of metal (Mn, Fe, Cu) phthalocyanines on an Fe(100) surface by first-principles calculations. J. Phys. Chem. 116, 18752–18758 (2012).
Amin, B., Nazir, S. & Schwingenschlögl, U. Molecular distortion and charge transfer effects in ZnPc/Cu(111). Sci. Rep. 3, 1705 (2013).
Sun, X., Wang, B., Pratt, A. & Yamauchi, Y. Magnetic moment enhancement and spin polarization switch of the manganese phthalocyanine molecule on an IrMn(100) surface. J. Chem. Phys. 141, 034703 (2014).
Jang, Y. R., Kim, I. G. & Lee, J. I. Electronic structure and magnetic properties of Fe4N(001). J. Magn. Magn. Mater. 263, 366–372 (2003).
Shirane, G., Takei, W. J. & Ruby, S. L. Mössbauer study of hyperfine fields ans isomer shifts in Fe4N and (Fe, Ni)4N. Phys. Rev. 126, 49 (1962).
Lee, T. H. D., Hu, S. & Madulid, N. Stablity studies of iron particle. IEEE Trans. Magn. 23, 2880–2882 (1987).
Tagawa, K., Kita, E. & Tasaki, A. Synthesis of fine Fe4N powder and its magnetic characteristics. Jpn. J. Appl. Phys. 21, 1596–1598 (1982).
Mi, W. B., Guo, Z. B., Feng, X. P. & Bai, H. L. Reactively sputtered epitaxial γ´-Fe4N films: Surface morphology, microstructure, magnetic and electrical transport properties. Acta Mater. 61, 6387–6395 (2013).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169 (1996).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758 (1999).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865 (1996).
Wortmann, D., Heinze, S., Kurz, Ph., Bihlmayer, G. & Blügel, S. Resolving complex atomic-scale spin structures by spin-polarized scanning tunneling microscopy. Phys. Rev. Lett. 86, 4132 (2001).
Henkelman, G., Arnaldsson, A. & Jósson, H. A fast and robust algorithm for Bader decomposition of charge density. Comput. Mater. Sci. 36, 354–360 (2006).
Sanville, E., Kenny, S. D., Smith, R. & Henkelman, G. Improved grid-based algorithm for bader charge allocation. J. Comput. Chem. 28, 899–908 (2007).
Tang, W., Sanville, E. & Henkelman, G. A grid-based bader analysis algorithm without lattice bias. J. Phys.: Condens. Matter 21, 084204 (2009).
Dunn, J. H., Arvanitis, D. & Martensson, N. Magnetism of thin Fe films on Cu(100). Phys. Rev. B 54, R11157 (1996).
Nakagawa, T., Takagi, Y., Matsumoto, Y. & Yokoyama, T. Enhancements of spin and orbital magnetic moments of submonolayer Co on Cu(001) studied by X-ray magnetic circular dichroism using superconducting magnet and liquid He cryostat. Jpn. J. Appl. Phys. 47, 2132–2136 (2008).
Shi, Y. J., Du, Y. L. & Chen, G. Ab initio study of structure and magnetic properties of cubic Fe4N(001) surface. Solid State Commun. 152, 1581–1584 (2012).
Acknowledgements
This work is supported by the National Natural Science Foundation of China (51171126), Key Project of Natural Science Foundation of Tianjin City (12JCZDJC27100 and 14JCZDJC37800), Program for New Century Excellent Talents in University (NCET-13-0409), Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry. It is also supported by High Performance Computing Center of Tianjin University, China.
Author information
Authors and Affiliations
Contributions
All authors designed the outline of the manuscript. Q.Z. and W.B.M. wrote the main text; X.C.W. and X.H.W. contributed detailed discussions and revisions; All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Zhang, Q., Mi, W., Wang, X. et al. Spin Polarization Inversion at Benzene-Absorbed Fe4N Surface. Sci Rep 5, 10602 (2015). https://doi.org/10.1038/srep10602
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep10602
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.