Abstract
Parents of many bird species produce alarm calls when they approach and deter a nest predator in order to defend their offspring. Alarm calls have been shown to warn nestlings about predatory threats, but parents also face a similar risk of predation when incubating eggs in their nests. Here, I show that incubating female Japanese great tits, Parus minor, assess predation risk by conspecific alarm calls given outside the nest cavity. Tits produce acoustically discrete alarm calls for different nest predators: “jar” calls for snakes and “chicka” calls for other predators such as crows and martens. Playback experiments revealed that incubating females responded to “jar” calls by leaving their nest, whereas they responded to “chicka” calls by looking out of the nest entrance. Since snakes invade the nest cavity, escaping from the nest helps females avoid snake predation. In contrast, “chicka” calls are used for a variety of predator types and therefore, looking out of the nest entrance helps females gather information about the type and location of approaching predators. These results show that incubating females derive information about predator type from different types of alarm calls, providing a novel example of functionally referential communication.
Similar content being viewed by others
Introduction
Predation is a significant and unpredictable event that reduces the breeding success of most birds1,2, many of which produce alarm calls in response to approaching nest predators in an attempt to defend their offspring3,4. Previous research has primarily focused on parent-nestling communication in which parent birds use alarm calls when predators are present to communicate this threat to nestlings4. Nestlings typically respond to alarm calls by suppressing their begging calls5,6,7 and this prevents predators from detecting the exact location of the nest8. In several bird species, the acoustic structure of alarm calls varies with the type of nest predator9,10 and this variation may be used to elicit different anti-predator behaviours in nestlings11. These calls are considered functionally referential signals12,13, because they convey information about predator type to conspecific receivers.
Functionally referential communication may also be advantageous for breeding parents during the egg-laying and incubation stages. For example, yellow warblers, Dendroica petechia, produce “seet” calls when they detect a brood-parasitic cowbird during the egg-laying period14 and females react with a unique response that prevents brood parasitism, i.e., they rush back to and sit tightly on their nests15. Since other types of alarm calls do not elicit such nest protection behaviours in females14,15, the “seet” calls are considered functionally referential signals that warn females of the presence of a cowbird. During incubation periods, alarm calls are commonly produced in response to nest predators that prey on eggs9,16, but it is not clear whether these calls provide specific information about predator type to parents.
If nests face multiple nest predators that differ in their ability to access and prey on eggs, selection may favour incubating parents that use distinct anti-predator behaviours to defend their eggs. While nest predators mainly prey on eggs during the incubation stage, they often attack and prey on incubating adults that stay in their nests17,18,19. Therefore, nest predators may also select for different avoidance behaviours in incubating birds. One species whose nests face a variety of predators is the Japanese great tit, Parus minor. The tits are socially monogamous passerines that nest in cavities and only females incubate eggs. In contrast to open-nesting species, the tits are limited by their visual fields while incubating their eggs inside their nest cavities. Males often produce alarm calls in response to nest predators during the incubation stage9,16, which may allow the incubating females to use alarm calls to assess the type of nest predator outside the nest cavities.
Previous studies have shown that Japanese great tits produce functionally referential alarm calls for different nest predators, i.e., “jar” calls for Japanese rat snakes, Elaphe climacophora and “chicka” calls for other predators such as jungle crows, Corvus macrorhynchos and Japanese martens, Martes melampus10,11,20 (Fig. 1). Incubating females may show distinct anti-predator responses to the two types of alarm calls, since snakes and other predators use different methods to access eggs and nests; snakes invade the nest cavity, whereas crows and martens attack the nestlings from outside the nest entrance using their beaks or forelimbs21,22. I previously showed that seventeen-day-old nestlings (one day before the typical age of fledging), which respond to the two types of alarm calls with adaptive behaviours to avoid predation, jump out of the nest cavity in response to “jar” calls and crouch down inside the cavity in response to “chicka” calls11.
I hypothesized that incubating female Japanese great tits assess predation risk by deriving referential information from conspecific alarm calls given outside the nest cavity. I conducted a playback experiment that examined the response of incubating females to the two types of alarm calls in the absence of actual predators. Eighteen females bred in nestboxes were used for the experiment. After a female returned to her nestbox and sat on eggs, it was subjected to 1-min of “jar” calls, “chicka” calls, or background noise (control) that were played back from a speaker set up in front of the nestbox. I observed behavioural responses of females to playbacks by using a CCD camera attached to the ceiling of the nestboxes. All three treatments were tested in each female and the order of trials was counter-balanced.
Results
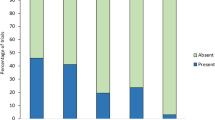
Incubating female Japanese great tits responded differently to playbacks of “jar” calls, “chicka” calls and background noise (Fig. 2). The response scores (see Methods) of females to playbacks varied across the three experimental treatments (CLMM, χ2 = 33.59, df = 2, P < 0.0001). In response to playback of “jar” calls, most females stepped onto the nest entrances (15/18) and many left the nestboxes (13/15) immediately after playback wa s commenced (median = 15 s, range: 1–33 s). In contrast, they rarely left the nestboxes during playbacks of “chicka” calls (2/18) and background noise (1/18). The response scores of females to “jar” calls were significantly higher than they were to “chicka” calls (post-hoc CLMM, χ2 = 16.34, df = 1, P < 0.0001, α = 0.025) and to background noise (post-hoc CLMM, χ2 = 46.58, df = 1, P < 0.0001, α = 0.017). Females always searched for and gazed at the ground near to the nestboxes after they left in response to playback of “jar” calls (13/13), but such behaviour was not observed when females left the nests during playbacks of “chicka” calls (n = 2) and background noise (n = 1). Females responded differently to “chicka” calls; three females stood on their nest materials and looked toward the nest entrances, four females stepped onto and looked out of the nest entrances and two females left the nestboxes. Nine females did not respond to playback of “chicka” calls. During playback of background noise, most of the tits stayed inside their nestboxes (17/18), rarely stood up (1/18), looked the outside (1/18) and left the nest (1/18). The response scores of females to “chicka” calls were significantly higher than they were to background noise (post-hoc CLMM, χ2 = 4.80, df = 1, P = 0.028, α = 0.05).
Discussion
The different responses to two types of alarm calls would provide adaptive value for incubating females. The females left the nestboxes immediately after hearing playback of “jar” calls. Since snakes invade the nestboxes and may prey on adults as well as on eggs and nestlings22, escaping from nests before their invasion is the only way to avoid snake attack. After leaving the nests, all of the females gazed at the ground, a behaviour that would help them to detect snakes approaching the nest from the ground20. Once a snake is detected, females may approach it closely, spread out their wings and tails and hover over it, which would help to disturb its invasion22. The tits looked out of the nest entrance in response to playback of “chicka” calls. This response may help the females gather information about the type and location of predators, since “chicka” calls are used for a variety of predator types, such as crows and martens10, that approach nests from different spatial locations (airs vs ground)21,22. Most females stayed in the nestboxes during playback of “chicka” calls; this might serve to prevent visually hunting predators from detecting the exact location of a nest by using parental activity near the nest19. Crows and martens cannot invade the nestboxes and may attack eggs from outside the nest entrances21,22. Nestlings of the tits often avoid predation by crows22 and martens21 by staying inside nestboxes. Therefore, incubating females may not be required to escape from the nest cavities when hearing “chicka” calls.
These results show that incubating female Japanese great tits derive information about predator type (i.e., snakes or non-snake predators) from conspecific alarm calls. Gathering information is crucial for animals to survive predation hazards and to defend their offspring4,23. Parents are limited by their visual fields when incubating eggs, particularly in nestboxes or in covered nests and therefore the ability to gather information from auditory cues and signals that are associated with predators is required. It has been shown in other cavity-breeding or dome-nesting species that breeders sample predator information in the nest surroundings and react adaptively to risk outside the nest. For example, incubating female brown thornbills, Acanthiza pusilla, look out of their dome nests when hearing predators’ vocalizations, which may increase the amount of predator information gathered24. Incubating female house sparrows, Passer domesticus, use the sound made by an approaching predator to detect an approaching attack25. Alarm calls may provide more detailed information about predators (e.g., predator distance and behaviour) to incubating parents, as described in intra-group communication in several birds26,27 and mammals28,29,30. Therefore, further study is required to elucidate how finely incubating parents can extract information about predators from conspecific calls or other auditory cues from predators and how they respond to the perceived risk.
Since the type of threat that causes breeding failure may vary during the breeding cycle of birds3, the function of alarm calls may be different between different breeding stages. For example, females of the yellow warbler respond to brood parasite specific alarm calls by nest protection behaviour and this response is more intense at egg-laying stage than at nestling stage15. In the case of Japanese great tits, the response of parents to alarm calls is different between incubating and nestling periods. Whereas incubating females showed predator avoidance or information gathering behaviour in response to the two types of alarm calls, they respond to these calls by predator-searching behaviours during the nestling period; they gaze at the ground in response to “jar” calls (i.e., searching for snakes) and scan the horizon in response to “chicka” calls (i.e., searching for non-snake predators)20. Older nestlings also respond to the different alarm calls by appropriate predator avoidance behaviours11. The intensity of alarm calls varies throughout the breeding cycle: the calling rate is typically lower during incubation stage and increases as nestlings grow up3,9,16. While variation in the intensity of alarm calls has been explained in terms of parental investment theory3, it might also reflect changes in the intended targets of alarm calls as well as the type of risk that they face16,31.
The present findings may represent a novel example of functionally referential communication, which may shed new light on the evolution of communication12,13. Snakes pose a unique threat because they use specific hunting tactics in comparison to other predator types, which require a specific escape strategy for females and nestlings. Therefore, divergence of nest predator types may provide a selective force for the evolution of multiple types of alarm calls in breeding birds. In addition, nest architecture may also be an important ecological factor determining the complexity in communication. Incubating parents and nestlings of cavity-nesting species may have selected to use two contrasting behaviours to avoid snakes and other predators, i.e., leaving the nest or staying at the nest. In contrast, for open-nesting species, it would be sufficient for both incubating parents and nestlings to use the same escape strategy because leaving the nest is the best avoidance strategy for all predator types. Further comparative work with regard to predator diversity and nest architecture may help in our understanding of evolutionary pathway of functionally referential communication in animals.
Methods
Study site and subjects
I collected data from 15 May to 4 June 2014 on 18 female Japanese great tits inhabiting in a mixed deciduous–coniferous forest in Karuizawa, Nagano, Japan (36°21–22’N, 138°35–36’E). I attached nest boxes to tree trunks at 1.8 m from the ground and the females used these boxes for their first broods of the season. The average clutch size was 9 ±1 (mean ± SD, n = 18). During nine breeding seasons from 2006 to 2014, up to 8% of females were depredated during incubation and the predation rate on eggs ranged from 4% to 17% of nests (unpublished data).
Playback preparation
I constructed playback stimuli from alarm calls that were previously recorded from Japanese great tits of the study population in the 2009 and 2010 breeding seasons. The “jar” calls were elicited by exposure to a live Japanese rat snake near the nestboxes, whereas the “chicka” calls were elicited using a stuffed jungle crow10,11. Whereas “jar” calls are specifically produced for snakes, “chicka” calls are used for predators other than snakes and their note composition subtly varies with the type of predator (e.g., crows vs martens)10. In this study, I only used the “chicka” calls given for crows in “chicka” call playbacks to control for the possibility that females respond differently to the subtle variation in these calls.
I used Adobe Audition 3.0 software (Adobe Corporation, San Jose, CA, U.S.A.) to construct the playback stimuli. I chose 90 “jar” calls from the recordings of nine individuals (five males and four females) and 90 “chicka” calls from the recordings of 10 individuals (six males and four females) on the basis of the sound quality (i.e., when the signaller was close to the microphone when it called and there was low background noise). Since tits seem not to discriminate between alarm calls of males and females20, calls from both sexes were used for the experiment. I chose five calls from the same individual and recorded them onto a sound file at a rate of 5 calls/12 s (roughly one call every 2.4 s) and repeated the 12-s file to fill a 1-min sound file. I filtered out low-frequency (<1 kHz) noise and amplified the calls to the same loudness level on a computer in order to equalize the amplitude across call playbacks. I created background noise files in the same way as the call files. Thus, I obtained 18 unique exemplars for each treatment. I saved all the sound files in WAV format (16-bit accuracy, 48.0 kHz sampling rate) on an SD memory card. I used unique exemplars for each focal female to avoid pseudoreplication32.
Experimental procedure
I tested the response of female Japanese great tits (n = 18) to playbacks of “jar” calls, “chicka” calls and background noise (control). An AT-SPG50 speaker (Audio-Technica Corporation, Tokyo, Japan) was attached atop a tripod at a height of 1.25 m from the ground and 2.0 m from the nest and was directed toward the nestbox. The playback speaker was connected to an R-09HR digital audio recorder using EXC-12A extension cords (Victor Company of Japan, Kanagawa, Japan), which enabled the control of playbacks from an observation position 15 m away from the nest. I played back alarm calls at a constant volume (75 dB at 1 m from the speaker measured using an SM-325 sound level meter; AS ONE Corporation, Osaka, Japan) and background noise at the same amplitude as the background noise level of the call playbacks (50 dB at 1 m). All three stimuli (“jar” calls, “chicka” calls and background noise) were tested for each female. Females were subject to playback of calls that were recorded from individuals other than their mates or neighbours to control for the effect of familiarity. Playbacks began at least 3 min after females returned to the nestbox, in the absence of birds calling outside of the nestbox. No males visited the nests during 1-min of playbacks. Each trial was separated by at least 1.5 h to reduce habituation. The order of playbacks was counter-balanced. Trials took place under calm and dry weather conditions between 08:30 and 16:00 h (Japan Standard Time).
I recorded female responses to alarm calls by using a KPC-EX20B CCD camera (KT&C Corporation, Seoul, Korea) connected to a GZ-MG880 digital video camera (Victor Corporation, Kanagawa, Japan). Behavioural responses of females during 1-min playbacks were categorized and scored as follows: (i) No response (0 points): females continued to incubate eggs and did not show any marked responses. (ii) Standing up (1 point): females stood up on their nest materials and looked at the nest entrances. (iii) Looking out of the nest entrance (2 points): females stood up on their nests, stepped on the nest entrances and looked out of the nest entrances. (iv) Leaving the nest (3 points): females stepped on the nest entrances and left the nestboxes. I also recorded the time at which females left the nestboxes. When females left the nestboxes during the 1-min playbacks, I continued the observation of the behaviour of females until the playbacks were finished. I noted whether females gazed at the ground after leaving the nests because this response has been known as a specific behaviour to search for and detect snakes20.
Statistical analysis
I used cumulative link mixed models (CLMMs) to analyse the effect of playback treatments on the female response scores. Primary analysis was conducted by including treatment (“jar”, “chicka” and background noise) as a fixed term and individual identity of focal birds as a random term. In the event of a significant effect of treatment, I further performed post-hoc pairwise comparison between treatments (“jar” versus “chicka”, “chicka” versus background noise and “jar” versus background noise). In the same way as the primary analysis, the models for the pairwise comparisons included treatment as a fixed term and individual identity of focal birds as a random term. CLMMs were run using R for Mac version 3.1.133 with the clmm function in the package ordinal. I used two-tailed, log likelihood ratio tests to calculate P values. The level of significance was first set at α = 0.05, but the α value was adjusted according to the sequential Bonferroni method34 when making multiple comparisons.
Ethical statement
All experiments were performed in accordance with relevant guidelines and regulations. All experimental protocols were approved by the Animal Care and Use Committees at the Graduate University for Advanced Studies and adhered to the Guidelines for the Use of Animals in Research of the Animal Behavior Society/Association for the Study of Animal Behaviour. This research was performed under permission from the Ministry of the Environment and the Forestry Agency of Japan.
Additional Information
How to cite this article: Suzuki, T. N. Assessment of predation risk through referential communication in incubating birds. Sci. Rep. 5, 10239; doi: 10.1038/srep10239 (2015).
References
Ricklefs, R. E. An analysis of nesting mortality in birds. Smith. Contr. Zool. 9, 1–48 (1969).
Martin, T. E. Avian life history evolution in relation to nest sites, nest predation and food. Ecol. Monogr. 65, 101–127 (1995).
Caro, T. Antipredator Defenses in Birds and Mammals . (University of Chicago Press, Chicago, 2005).
Magrath, R. D., Haff, T. M., Horn, A. G. & Leonard, M. L. Calling in the face of danger: predation risk and acoustic communication by parent birds and their offspring. Adv. Study Behav . 41, 187–253 (2010).
Platzen, D. & Magrath, R. D. Parental alarm calls suppress nestling vocalization. Proc. R. Soc. Lond. B 271, 1271–1276 (2004).
Platzen, D. & Magrath, R. D. Adaptive differences in response to two types of parental alarm call in altricial nestlings. Proc. R. Soc. Lond. B 272, 1101–1106 (2005).
Serra, C. & Fernández, G. J. Reduction of nestlings’ vocalizations in response to parental alarm calls in the southern house wren, Troglodytes musculus. J. Ornithol. 152, 331–336 (2010).
Haff, T. M. & Magrath, R. D. Calling at a cost: elevated nestling calling attracts predators to active nests. Biol. Lett . 7, 493–495 (2011).
Fasanella, M. & Fernández, G. J. Alarm calls of the southern house wren Troglodytes musculus: variation with nesting stage and predator model. J. Ornithol. 150, 853–863 (2009).
Suzuki, T. N. Communication about predator type by a bird using discrete, graded and combinatorial variation in alarm calls. Anim. Behav. 87, 59–65 (2014).
Suzuki, T. N. Parental alarm calls warn nestlings about different predatory threats. Curr. Biol. 21, R15–R16 (2011).
Gill, S. A. & Bierema, A. M. K. On the meaning of alarm calls: a review of functional reference in avian alarm calling. Ethology 119, 449–461 (2013).
Townsend, S. W. & Manser, M. B. Functionally referential communication in mammals: the past, present and the future. Ethology 119, 1–11 (2013).
Gill, S. A. & Sealy, S. G. Nest defence by yellow warblers: recognition of a brood parasite and an avian nest predator. Behaviour 133, 263–282 (1996).
Gill, S. A. & Sealy, S. G. Functional reference in an alarm signal given during nest defence: seet calls of yellow warblers denote brood-parasitic brown-headed cowbirds. Behav. Ecol. Sociobiol. 56, 71–80 (2004).
Randler, C. Alarm calls of the cyprus wheatear Oenanthe cypriaca—one for nest defence, one for parent–offspring communication? Acta Ethol. 16, 91–96 (2012).
Albrecht, T. & Klvaňa, P. Nest crypsis, reproductive value of a clutch and escape decisions in incubating female mallards Anas platyrhynchos. Ethology 110, 603–613 (2004).
Amat, J. A. & Masero, J. A. Predation risk on incubating adults constrains the choice of thermally favourable nest sites in a plover. Anim. Behav. 67, 293–300 (2004).
Martin, T. E., Scott, J. & Menge, C. Nest predation increases with parental activity: separating nest site and parental activity effects. Proc. R. Soc. Lond. B 267, 2287–2293 (2000).
Suzuki, T. N. Referential mobbing calls elicit different predator-searching behaviours in Japanese great tits. Anim. Behav. 84, 53–57 (2012).
Barnett, C. A., Sugita, N. & Suzuki, T. N. Observations of predation attempts on avian nest boxes by Japanese martens (Martes melampus). Mamm. Study 38, 269–274 (2013).
Suzuki, T. N. & Ueda, K. Mobbing calls of Japanese tits signal predator type: field observations of natural predator encounters. Wilson J. Ornithol. 125, 412–415 (2013).
Dall, S., Giraldeau, L., Olsson, O., McNamara, J. & Stephens, D. Information and its use by animals in evolutionary ecology. Trends Ecol. Evol. 20, 187–193 (2005).
Schneider, N. A. & Griesser, M. Incubating females use dynamic risk assessment to evaluate the risk posed by different predators. Behav. Ecol. 24, 47–52 (2013).
Meillère, A., Brischoux F. & Angelier, F. Impact of chronic noise exposure on antipredator behavior: an experiment in breeding house sparrows. Behav. Ecol. in press (2015).
Templeton, C. N., Greene, E. & Davis, K. Allometry of alarm calls: black-capped chickadees encode information about predator size. Science 308, 1934–1937 (2005).
Griesser, M. Referential calls signal predator behavior in a group-living bird species. Curr. Biol. 18, 69–73 (2008).
Manser, M. B. The acoustic structure of suricates’ alarm calls varies with predator type and the level of response urgency. Proc. R. Soc. Lond. B 268, 2315–2324 (2001).
Manser, M. B., Bell, M. B. & Fletcher, L. B. The information that receivers extract from alarm calls in suricates. Proc. R. Soc. Lond. B 268, 2485–2491 (2001).
Furrer, R. D. & Manser, M. B. Banded mongoose recruitment calls convey information about risk and not stimulus type. Anim. Behav. 78, 195–201 (2009).
Magrath, R. D., Platzen, D. & Kondo, J. From nestling calls to fledgling silence: adaptive timing of change in response to aerial alarm calls. Proc. R. Soc. Lond. B 273, 2335–2341 (2006).
Kroodsma, D. E., Byers, B. E., Goodale, E. & Johnson, S. Pseudoreplication in playback experiments, revisited a decade later. Anim. Behav. 61, 1029–1033 (2001).
R Core Team R: a language and environment for statistical computing . R Foundation for Statistical Computing, Vienna, Austria (2014). Available at: http://www.R-project.org/. (Accessed: 17th November 2014)
Holm, S. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 6, 65–70 (1979).
Acknowledgements
This study was supported by JSPS KAKENHI Grant Number 25-3391.
Author information
Authors and Affiliations
Contributions
T. N. S. designed the experiment, collected the data, analysed the data and wrote the paper.
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Suzuki, T. Assessment of predation risk through referential communication in incubating birds. Sci Rep 5, 10239 (2015). https://doi.org/10.1038/srep10239
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep10239
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.