Abstract
Stress response plays an important role on microbial adaptation under hostile environmental conditions. It is generally unclear how the signaling transduction pathway mediates a stress response in planktonic and biofilm modes of microbial communities simultaneously. Here, we showed that metalloid tellurite (TeO32–) exposure induced the intracellular content of the secondary messenger cyclic di-GMP (c-di-GMP) of Pseudomonas aeruginosa. Two diguanylate cyclases (DGCs), SadC and SiaD, were responsible for the increased intracellular content of c-di-GMP. Enhanced c-di-GMP levels by TeO32– further increased P. aeruginosa biofilm formation and resistance to TeO32–. P. aeruginosa ΔsadCΔsiaD and PAO1/plac-yhjH mutants with low intracellular c-di-GMP content were more sensitive to TeO32– exposure and had low relative fitness compared to the wild-type PAO1 planktonic and biofilm cultures exposed to TeO32–. Our study provided evidence that c-di-GMP level can play an important role in mediating stress response in microbial communities during both planktonic and biofilm modes of growth.
Similar content being viewed by others
Introduction
Microorganisms display a striking ability to adapt to unfavorable conditions such as exposure to UV radiation, heavy metals and antibiotic treatments, by inducing stress responses and forming surface-attached biofilms1,2. Biofilms consist of microbial cells embedded in their self-produced extracellular polymeric substances (EPS). The EPS can account for up to 90% of the biofilm biomass and serve as physical barriers to protect biofilm cells3. Hence, biofilms dramatically increase the tolerance of bacterial cells towards environmental stress and immune attack during the course of infections4,5. Extensive intercellular communication and interactions have been observed within biofilms and cells with distinct physiology may play different roles under stress conditions6,7,8.
Bis-(3′-5′)-cyclic dimeric guanosine monophosphate (C-di-GMP) plays an important role in biofilm formation of a wide range of bacteria9. Bacterial intracellular c-di-GMP content is determined by diguanylate cyclases (DGCs) that catalyze the formation of c-di-GMP and phosphodiesterases (PDEs), which degrade c-di-GMP9. When intracellular c-di-GMP content is high, bacterial cells reduce motility and increase synthesis of EPS matrix, resulting in biofilm formation10,11. In contrast, biofilm cells increase their motility and disperse from biofilms when the intracellular c-di-GMP content is low12,13. C-di-GMP signaling can be induced by stress conditions such as antimicrobial exposure14,15. The impact of c-di-GMP on mediating stress response by microbial communities during both planktonic and biofilm modes of growth remains unclear.
Anthropogenic activities have resulted in serious metal(loid) pollution, especially in industrialized countries and regions. The natural ecosystems are often direct or indirect recipients of toxic metal(loid)s such as TeO32−. Many environmental bacteria including Pseudomonas aeruginosa are capable of surviving in the presence of TeO32− at low concentrations by reducing TeO32− to Te(0) nanomaterials, as a result of either detoxification, redox maintenance or respiration16,17,18,19. Although the toxic effects of metal(oild)s on environmental microorganisms at individual cell levels have been extensively studied20, little is known about the impacts of metal(loid)s on bacterial social behaviours21.
In the present study, we investigated the role of c-di-GMP in mediating stress responses by the opportunistic pathogen Pseudomonas aeruginosa to a toxic metalloid, tellurite (TeO32–). TeO32− is highly toxic to most microbes and had been previously described by Alexander Fleming as an antimicrobial agent22. Bacterial cells take up TeO32– and subsequently reduce it to tellurium nanoparticles, which can be easily tracked by the black precipitates on the bacterial cell surface. Quantification of intracellular c-di-GMP and proteomic analysis indicated that c-di-GMP levels were induced by TeO32– exposure, which enhanced P. aeruginosa TeO32– resistance and biofilm formation. SadC and SiaD were found to be important in the induction of c-di-GMP by TeO32– exposure. We showed that mutants with low intracellular c-di-GMP content could be outcompeted by the wild-type strain from biofilm and planktonic cultures under metalloid stress condition.
Results
Stress responses of P. aeruginosa to TeO32− induced c-di-GMP signaling
Cultivation of different bacterial species in the presence of sub-lethal concentrations of antimicrobial agents is a widely employed method to investigate their stress responses23,24,25. The MIC of P. aeruginosa to TeO32− is 100 μg/ml in ABTGC medium. Large aggregates (approximately 1-3 mm) were formed when P. aeruginosa was grown in ABTGC media containing 10 μg/ml TeO32− at 37 °C (Fig. 1a). Further analysis of the TeO32−-induced aggregates by field emission scanning electron microscopy (FE-SEM) and energy-dispersive X-ray spectroscopy (EDX) revealed the presence of tellurium-containing precipitates around the bacterial cells (Fig. 1b,c). No tellurium-containing precipitates were observed for P. aeruginosa cells growing in medium without TeO32−. Thus, the tellurium-containing precipitates might generate conditions of membrane-associated stress for P. aeruginosa cells.
Aggregates formed by P. aeruginosa wild-type PAO1 in ABTGC medium with and without 10 μg/ml TeO32− under shaking condition after 1 d (a). Aggregates formed in TeO32− containing medium were analyzed by FE-SEM (b) and energy-dispersive X-ray spectroscopy (c). Arrows in the FE-SEM image indicate the bacterial cell and nanoparticles on the cell surface. ROS generation by P. aeruginosa PAO1 cells after exposure to sub-lethal concentration of TeO32− , SeO32− and SeO42− (d). Relative intracellular c-di-GMP content of PAO1 cultures in ABTGC medium with and without 10 μg/ml TeO32− was quantified by HPLC (e). Means and standard deviations of three replicates are shown. Student’s t-test was performed for testing differences between groups. * P < 0.05.
TeO32− and oxyanions such as selenate/selenite are well known to exert their toxic effects on microorganisms via generation of reactive oxygen species (ROS)26,27. We measured the generation of ROS by P. aeruginosa cells exposed to sub-lethal concentrations of TeO32− as well as SeO32− and SeO42− by using the OxiSelect™ in vitro ROS/RNS assay kit. As anticipated, exposure of P. aeruginosa cells to the TeO32−, SeO32− and SeO42− significantly increased their cytoplasmic ROS content regardless of the nutrient conditions (Fig. 1d).
Proteomic analysis of TeO32− stressed P. aeruginosa cells
Oxidative stress response by P. aeruginosa leading to aggregate formation, recently reported to resemble the biofilm physiology28 has not been documented. We thus investigated the overall impact of TeO32− on P. aeruginosa cells using a comparative proteomic approach for cells cultivated with and without 10 μg/ml TeO32−.
Using a p-value cut-off of 0.05 and a fold change cut-off of 5 (as described in the Materials and Methods), 129 proteins were significantly affected by TeO32− exposure with 64 proteins upregulated (Supplementary table 1) and 65 proteins being down-regulated (Supplementary table 2).
The expression of several of outer membrane associated proteins was induced by TeO32− treatment, including OprQ (PA2760, 28.8-fold), OprI precursor (PA2853, 15-fold), probable outer membrane protein precursor (PA2391, 10.9-fold), OprM (PA0427, 10.5-fold), OprL precursor (PA0973, 9.8-fold), OprD precursor (PA0958, 9.8-fold), OprB (PA3186, 9.7-fold) and OprC (PA3790, 8.1-fold) (Supplementary table 1). The membrane transporter CdrB of the large extracellular protein CdrA29 was induced 25.8-fold by exposure to TeO32− (Supplementary table 1). CdrAB expression has been used as a c-di-GMP indicator30 and reported to promote biofilm formation and auto-aggregation in a Psl polysaccharide dependent manner29 and co-immunoprecipitation experiments have clearly shown that CdrA binds to Psl29. HPLC analysis showed that P. aeruginosa PAO1 cultivated in ABTGC medium with 10 μg/ml TeO32− treatment had a higher relative intracellular c-di-GMP concentration compared to untreated control samples (approximately 2.5-fold) (Fig. 1e).
SadC and SiaD contribute to c-di-GMP induction by TeO32−
CdrAB belongs to a family of bacterial proteins secreted by the two-partner secretion system31. Recently, two other members of this family, XacFhaB from Xanthomonas axonopodis pv. Citri and FHA from Bordetella pertussis have also been implicated in biofilm formation32,33. These large inter-bacterial adhesins may play a key role in establishing structured biofilm communities under stress conditions. The cdrA promoter is positively regulated by the c-di-GMP concentration and the expression of PcdrA-gfp has been recently used as a biosensor of the intracellular content of c-di-GMP in P. aeruginosa30. We tested the expression of the PcdrA-gfp reporter in P. aeruginosa cultures with and without the presence of TeO32− and found that TeO32− exposure significantly increased the expression of fluorescence in a dose dependent manner (Fig. 2a). This result is in accordance with our HPLC quantification and indicates that TeO32− exposure increases the intracellular content of c-di-GMP and that TeO32− induced aggregates might carry physiological traits similar to those of biofilms.
Expression of biosensor PcdrA-gfp (a) and Ppel-lacZ (b) by P. aeruginosa strains in ABTGC medium with and without the presence of 10 μg/ml TeO32−. The PcdrA-gfp expression was shown as relative fluorescence units (RFU) per OD600. The Ppel-lacZ expression was shown as Miller Unit. Means and standard deviations of three replicates are shown. Student’s t-test was performed for testing differences between groups. * P < 0.05.
Recently, both SadC and SiaD, were shown to be able to transduce an extracellular signal generated by the toxic detergent SDS and catalyze synthesis of c-di-GMP for facilitating biofilm formation by P. aeruginosa34,35. The defect environmental signaling ΔsadC and ΔsiaD mutants were severely impaired in expression of the PcdrA-gfp reporter in the presence of TeO32− (Fig. 2a). SiaD appears to be more important than SadC for PcdrA-gfp induction by TeO32− since the ΔsadC mutant still displayed a slight induction of PcdrA-gfp by TeO32− (Fig. 2a).
Exopolysaccharides are the major EPS components of P. aeruginosa biofilms and are well known to be induced by high intracellular c-di-GMP content in P. aeruginosa. We examined the expression of a lacZ-based biosensor of the Pel synthesis operon (mini-CTX-pel-lacZ36) in P. aeruginosa strains under TeO32− stress. As with PcdrA-gfp fusion, the expression of the pel-lacZ fusion was induced by TeO32− treatment, with SiaD essential for this induction (Fig. 2b). However, there was a slight induction of the pel-lacZ fusion by tellurite even in the ΔsadCΔsiaCD double mutant (Fig. 2b).
Consistent with our observation of TeO32−-induced aggregation, P. aeruginosa grown in the presence of TeO32− formed more biofilms than cells grown without TeO32− (Fig. 3). The induction of biofilm formation was dependent on the presence of Pel and Psl polysaccharides (Fig. 3).
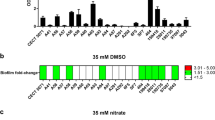
Biofilm formation by P. aeruginosa PAO1, ΔpelA, ΔpslBCD and ΔpelAΔpslBCD in medium containing 0, 10, 25 and 50 μg/ml TeO32− under static conditions after 1 d incubation. Biofilms were firstly stained with 0.01% (w/v) crystal violet (a) and then quantified by dissolving in 96% ethanol and measuring absorbance at 590 nm (b). Means and standard deviations of three replicates are shown. Student’s t-test was performed for testing differences between groups. * P < 0.05.
Induction of c-di-GMP confers a growth advantage under tellurite exposure during planktonic cultures
Since c-di-GMP signaling was induced by TeO32− exposure, we examined whether induction of c-di-GMP signaling would confer a growth advantage of P. aeruginosa under TeO32− exposure. There was no growth defect of ΔsadC, ΔsiaD and ΔsadCΔsiaD mutants under normal growth condition as compared to PAO1 control (Fig. 4a). However, the P. aeruginosa ΔsadC, ΔsiaD single or double mutants were more sensitive to TeO32− (Fig. 4b). Similarly, the PAO1/plac-yhjH mutant, which contains a PBBRMCS-2 plasmid with a constitutively expressed phosphodiesterase gene yhjH fused to and expressed by the lac promoter and thus has a low intracellular of c-di-GMP content12, was also more sensitive to TeO32− (Fig. 4). These results showed that intracellular c-di-GMP content determines the tolerance of P. aeruginosa to TeO32− exposure during planktonic cultures.
Growth curve (a) and TeO32− tolerance assay (b). P. aeruginosa PAO1, ΔsadC, ΔsiaD, ΔsadCΔsiaD and PAO1/plac-yhjH strains were cultivated in ABTGC medium at 37 °C with shaking for growth measurement. For TeO32− tolerance assay, P. aeruginosa PAO1, ΔsadC, ΔsiaD, ΔsadCΔsiaD and PAO1/plac-yhjH strains were cultivated in ABTGC medium with the presence of 20 μg/ml TeO32− overnight followed by CFU determination. Means and standard deviations of three replicates are shown.
Low intracellular c-di-GMP mutants lose fitness under stress during both planktonic and biofilm modes of growth
When cfp-tagged PAO1 and yfp-tagged ΔsadCΔsiaD mutant strains were combined 1:1 (vol/vol) for planktonic co-cultivation experiments, the wild-type showed higher survival rates and gained a higher level of relative fitness than the ΔsadCΔsiaD mutant in the presence of TeO32− than without TeO32− (Fig. 5a). Since diverse phenotypic and genotypic variants coexist in bacterial biofilms37,38, we tested whether TeO32− exposure-induced biofilm formation by high c-di-GMP containing cells would lead to protection of mutants with low intracellular c-di-GMP content in co-cultures. Here, PAO1 displayed a higher relative fitness than the ΔsadCΔsiaD mutant in biofilm co-cultures with and without the presence of TeO32− (Fig. 5b). However, the relative fitness of ΔsadCΔsiaD compared to PAO1 in biofilm co-cultures was slightly higher with the presence of TeO32− than in its absence (Fig. 5b). This suggests TeO32− could potentially induce expression of other DGC harboring proteins in the ΔsadCΔsiaD mutant and partly restore the intracellular c-di-GMP levels and biofilm formation.
Relative fitness of ΔsadCΔsiaD mutant to PAO1 in planktonic co-cultures and biofilm co-cultures in ABTGC medium with and without the presence of 10 μg/ml TeO32− (a). Means and standard deviations of three replicates are shown. Student’s t-test was performed for testing differences between groups. * P < 0.05. CLSM images of biofilm co-cultures formed by cfp-tagged P. aeruginosa PAO1 and yfp-tagged ΔsadCΔsiaD mutant in ABTGC medium with and without the presence of 10 μg/ml TeO32− (b). Representative image from triplicate experiments was shown for each condition. Bars, 50 μm.
When we mixed cfp-tagged PAO1 and yfp-tagged PAO1/plac-yhjH strains 1:1 (vol:vol) for planktonic co-cultivation experiments, the wild-type PAO1 strain gained a higher level of relative fitness than the c-di-GMP depleted PAO1/plac-yhjH strain with and without exposure to TeO32− (Fig. 6a). Moreover, PAO1/plac-yhjH was fully outcompeted by PAO1 in biofilm co-cultures supplemented with TeO32− (Fig. 6b). These results suggest that variants with low intracellular c-di-GMP content are unlikely to be protected and maintained by both P. aeruginosa planktonic and biofilm communities when c-di-GMP is required for stress response.
Relative fitness of PAO1/plac-yhjH mutant to PAO1 in planktonic co-cultures and biofilm co-cultures in ABTGC medium with and without the presence of 10 μg/ml TeO32− (a). Means and standard deviations of three replicates are shown. Student’s t-test was performed for testing differences between groups. * P < 0.05. CLSM images of biofilm co-cultures formed by cfp-tagged P. aeruginosa PAO1 and yfp-tagged PAO1/plac-yhjH mutant in ABTGC medium with and without the presence of 10 μg/ml TeO32− (b). Representative image from triplicate experiments was shown for each condition. Bars, 50 μm.
Discussion
Bacterial cells face various types of stress during the colonization of natural environments and hosts. A series of stress response mechanisms has evolved in bacteria to cope with these harmful conditions. One well characterized stringent stress response mechanism is SpoT-mediated ppGpp accumulation, which can be provoked by nutritional stress caused by harmful conditions such as antibiotic treatment and UV irradiation39. ppGpp is able to bind directly to the bacterial RNA polymerase and further regulate transcriptional activity of many genes.
In addition to the stringent stress response, bacteria employ a wide range of social behaviors for surviving under unfavorable environmental conditions and these responses also contribute to bacterial pathogenesis40. For example, the Staphylococcus aureus agr quorum-sensing system is involved in the oxidative stress response41. Biofilm formation is evoked as a stress response mechanism by a wide range of bacteria42. It involves encasing bacterial cells inside the densely packed EPS matrix components and attaching firmly to biotic and abiotic surfaces. Biofilms are up to 1,000 times more resistant to antimicrobial agents compared to their planktonic counterparts43.
Recently, bacteria were found to form floating biofilm-resembling aggregates that are resistant to antimicrobials and phagocytosis28. Our work here showed that TeO32− exposure can elevate the c-di-GMP level in P. aeruginosa and lead to the formation of floating aggregates. TeO32−-induced floating aggregate formation requires Pel and Psl polysaccharides as well as extracellular DNA (eDNA) (Fig. S1), in accordance with the Psl polysaccharide-eDNA interaction enabling the formation of skeleton of P. aeruginosa biofilms44. In addition to serving as matrix scaffolds, the polysaccharides could also induce synthesis of iron siderophore pyoverdine via the Gac/Rsm pathway in the floating aggregates, as we had previously demonstrated45. The formation of stress-induced biofilm-resembling aggregates might contribute to the dissemination of infection in the host.
The results presented here demonstrate that P. aeruginosa mutants with low c-di-GMP content were more sensitive to TeO32− exposure in planktonic cultures and thus their growth was negatively affected by TeO32− exposure, as compared to c-di-GMP containing wild-type strain (Fig. 4). Consistent with this finding, a recent study on biodegradation of 3-chloroaniline by Comamonas testosteroni reported that, compared with the wild type, the strain with an elevated c-di-GMP level exhibited a better growth on the toxic substrate at high concentrations46. In addition to TeO32−, the detergent Na-dodecylsulfate (SDS)35 also raised the c-di-GMP levels and caused aggregation of P. aeruginosa. In accordance with the TeO32− findings, the ΔsiaD mutant with low intracellular c-di-GMP content was more sensitive to SDS during planktonic growth35. Together, these studies highlight that c-di-GMP signaling is involved in multiple stress response mechanisms, which might due to multiple DGCs and PDEs being encoded by many bacterial species.
Finally, we found that wild-type PAO1 strain biofilms prevented the attachment of mutants with low intracellular c-di-GMP content in both normal and TeO32− stress co-cultures. Our previous study revealed that the polysaccharides in P. aeruginosa biofilms could not be shared, for structural or functional benefits, by mutants that are defective in their synthesis38. These latter findings corroborate with the results presented here and c-di-GMP mediated synthesis of polysaccharides may form another strategy to repress the proliferation and maintenance of c-di-GMP defective variants in biofilms. Considering that polysaccharides with similar structure to the P. aeruginosa polysaccharides are widely distributed in natural bacterial species, our results might reflect a conserved strategy employed by a range of bacterial species to repress the spreading of variants which cannot respond to environmental conditions by moderating their own c-di-GMP levels.
Methods
Bacterial strains and growth medium
The bacterial strains, plasmids and primers used in this study are listed in Table 1. Escherichia coli DH5α strain was used for standard DNA manipulations. LB medium47 was used to cultivate E. coli strains. Batch cultivation of P. aeruginosa was carried out at 37 °C in ABT minimal medium7 supplemented with 5 g glucose l–1 (ABTG) or 2 g glucose l–1 and 2 g casamino acids l–1 (ABTGC). For plasmid maintenance in E. coli, the medium was supplemented with 100 μg ampicillin (Ap) ml−1, 15 μg gentamicin (Gm) ml−1, 15 μg tetracycline (Tc) ml−1, or 8 μg chloramphenicol (Cm) ml−1. For marker selection in P. aeruginosa, 30 μg Gm ml−1, 50 μg Tc ml−1 and 200 μg carbenicillin (Cb) ml−1 were used, when appropriate. Antibiotics were not added to P. aeruginosa cultures for c-di-GMP, stress response and biofilm assays as the plasmids we used were highly stable for these short-term experiments.
Construction of P. aeruginosa mutants.
The ΔpelA, ΔpslBCD and ΔpelAΔpslBCD mutants defective for Pel and/or Psl polysaccharide biogenesis were constructed by allelic displacement as previously described48. The ΔsadC, ΔsiaD and ΔsadCΔsiaD mutants defective for SadC and/or SiaD diguanylate cyclase were constructed by allelic displacement as previously described34.
Quantification of static biofilms
The microtitre tray biofilm formation assay was performed as described by O’Toole & Kolter49. Briefly, overnight cultures grown in ABTG medium were diluted to OD600 = ~0.001 with fresh ABTG medium and transferred to the wells of polystyrene 96-well microtitre trays (200 μl per well) and incubated for 24 h at 37 °C. Liquid culture was removed from each well and the wells were washed twice with 0.9% NaCl followed by staining with 0.1% crystal violet and washing twice with 0.9% NaCl. The crystal violet-stained biofilms were then resuspended in 96% ethanol and the absorbance of biofilm-associated dye was measured at 600 nm. Experiments were performed in triplicate and the results are shown as the mean ± sd.
Field emission scanning electron microscopy (FE-SEM) and energy-dispersive X-ray spectroscopy (EDX)
The aggregates were dried and coated with platinum (Pt) using a vacuum electric sputter coater (JEOL JFC-1300, JEOL Asia Pte Ltd, Singapore). SEM images were taken using a field emission scanning electron microscope (FE-SEM, JSM-7600, JEOL Asia Pte Ltd, Singapore) at a voltage of 2.0-5.0 kV and EDX spectra were obtained using an energy-dispersive X-ray spectroscope (AZtecEnergy, Oxford Instruments, Oxfordshire, UK) as previously described50. Experiments were performed in triplicate and representative images were shown.
Reactive oxygen species (ROS) assay
PAO1 cultures were grown in ABTGC or LB medium controls and media with 10 μg ml−1 TeO32−, SeO32− and SeO42−, respectively. The ROS content of 1 ml stationary phase bacterial cells were then measured by using the OxiSelect™ in vitro ROS/RNS assay kit (Green Fluorescence), accordingly to manufacturer’s instructions. 2’, 7’-dichlorodihydrofluorescein (DCF) was used as a standard and the concentrations of ROS from PAO1 cultures were estimated according to the DCF standard curve. The fluorescence of the samples was read by the Tecan Infinite 2000 Microplate Reader at 480 nm excitation/530 nm emission. Experiments were performed in triplicate and the results are shown as the mean ± sd. Student’s t-test was performed for testing differences between groups.
iTRAQ-based proteomics analyses
P. aeruginosa PAO1 was grown in ABTG medium with and without 10 μg/ml TeO32− at 37 °C with shaking until stationary phase was reached. Cells were harvested and iTRAQ-based proteomics analyses were carried out as previously described12.
Determination of minimal inhibitory concentration (MIC)
The MIC assays employed a microtiter broth dilution method as previously described in the NCSLA guidelines51. Briefly, fresh ~16 h cultures of P. aeruginosa were diluted in ABTG medium. For determination of MIC, potassium tellurite was dissolved in water at a concentration 10 times higher than the required range by serial dilutions from a stock solution. 10 μl of each concentration were added to each corresponding well of a 96-well microtiter plate (polypropylene, Costar Corp.) and 90 μl of bacterial culture (~1 × 105 cells) in ABTG medium were added. The plate was incubated at 37 °C for 16-18 h. MIC was taken as the lowest concentration where no visual growth (based on OD600) of bacteria was detected. Experiments were performed in triplicate and representative results were shown.
TeO32− tolerance assay
Overnight cultures of different P. aeruginosa strains were inoculated into ABTGC medium containing 20 μg/ml TeO32− and cultivated overnight (24 h). Overnight cultures were serially diluted and plated onto LB agar media. LB plates were incubated at 37 °C overnight before CFU calculation. Experiments were performed in triplicate and the results are shown as the mean ± sd.
Beta-galactosidase activity assay
A classical β-galactosidase assay52 was used to measure expression of the Ppel-lacZ fusion in P. aeruginosa strains transformed with the mini-CTX-pel-lacZ fusion36, which carries the pel promoter fused to the E. coli lacZ gene. Experiments were performed in triplicate and the results are shown as the mean ± sd. Student’s t-test was performed for testing differences between groups.
Gfp reporter fusion assay
The expression of the c-di-GMP PcdrA-gfp biosensor30 in P. aeruginosa strains in the presence and absence of TeO32− was monitored by using a Tecan Infinite 2000 Microplate Reader. Monitoring strains were cultivated in 24-well microtiter plate with ABTGC medium with different concentrations of TeO32− at 37 °C with shaking. OD600 and GFP fluorescence (in relative fluorescence units, rfu) were measured every hour until the culture reach stationary growth phase. Experiments were performed in triplicate and the results are shown as the mean ± sd. Student’s t-test was performed for testing differences between groups.
Quantification of c-di-GMP concentration
Extraction of c-di-GMP was conducted as previously described45. 10 ml of P. aeruginosa cells in the early stationary phase from the ABTGC medium with and without 10 μg/ml TeO32− were washed twice with 1 mM ammonium acetate. Cells were lysed with 0.6 M HClO4 on ice for 30 min. Cell debris was removed by centrifugation and supernatant was neutralized to pH 6.0 with the addition of 2.5 M KHCO3. The precipitated KClO4 was removed by centrifugation and the supernatant was used for relative quantification of c-di-GMP. The concentration was measured by High Performance Liquid Chromatography (HPLC), the injection volume is 20 µl with 254 nm as detection wavelength. Reverse-phase C18 Targa column (2.1 x 40 mm, 5 μm) (catalog number: TR-0421-C185) was used with solvent A (10 mM ammonium acetate in water) and solvent B (10 mM ammonium acetate in methanol) at a flow rate of 0.2 ml min-1. Eluent gradient is as follows: 0 to 8 min, 1% B; 8 to 14 min, 15% B; 14 to 16 min, 19% B; 16 to 24 min, 100% B; 24 to 32 min, 100% B; 32 to 40 min, 1% B; 40 to 42 min, 1% B. The retention time of c-di-GMP is around 14.0 min. The c-di-GMP concentration was normalized by total protein concentration. The relative c-di-GMP concentrations of cells treated with 10 μg ml−1 tellurite against cells in ABTGC only were shown. Experiments were performed in triplicate and the results are shown as the mean ± sd. Student’s t-test was performed for testing differences between groups.
Competition assay
Competition assays were performed in both planktonic and biofilm co-cultures. In planktonic co-cultures, cfp-tagged wild-type PAO1 was mixed 1:1 (vol/vol) with yfp-tagged PAO1/plac-yhjH (or yfp-tagged ΔsadCΔsiaD) and the mixtures inoculated into fresh ABTGC medium with and without the presence of 10 μg/ml TeO32−. For relative fitness calculation, co-cultures were plated in LB agar plates after 24 h cultivation at 37 °C with shaking. Colony-forming units (CFUs) Ni were determined from three individual experiments and the number of PAO1 and PAO1/plac-yhjH (or ΔsadCΔsiaD) colonies were determined based on their specific fluorescence at times t = 0 and at t = T. Relative fitness was determined as rij = [Ni(T)-Ni(0)]/[Nj(T)-Nj(0)] as previously described with modification53, resulting in a fitness of ‘1’ when competing organisms are equally fit. Experiments were performed in triplicate and the results are shown as the mean ± sd. Student’s t-test was performed for testing differences between groups.
In biofilm co-cultures, cfp-tagged wild-type PAO1 cells were mixed with yfp-tagged PAO1/plac-yhjH (or yfp-tagged ΔsadCΔsiaD) cells at 1:1 (vol/vol) and the mixtures were inoculated into fresh ABTGC medium with and without the presence of 10 μg/ml TeO32−. Static biofilms were cultivated on cover slides at 37 °C for 24 h as previously described54. Biofilms were imaged with a Zeiss LSM780 confocal laser scanning microscope (CLSM) equipped with detectors and filter sets for monitoring of Cfp and Yfp fluorescence. Images were obtained using a 40 × /1.4 objective. Simulated three-dimensional images and sections as well as biovolumes were generated using the Imaris software package (Bitplane AG)8. The biovolume Vi of each strain in the biofilm mode was determined from three individual experiments based on their fluorescence at times t = 0 and at t = T. Relative fitness was determined as rij = [Vi(T)-Vi(0)]/[Vj(T)-Vj(0)] as previously described with modification53, resulting in a fitness of ‘1’ when competing organisms are equally fit. Experiments were performed in triplicate and the results are shown as the mean ± sd. Student’s t-test was performed for testing differences between groups.
Additional Information
How to cite this article: Chua, S. L. et al. C-di-GMP regulates Pseudomonas aeruginosa stress response to tellurite during both planktonic and biofilm modes of growth. Sci. Rep. 5, 10052; doi: 10.1038/srep10052 (2015).
References
Schwartz, T., Hoffmann, S. & Obst, U. Formation of natural biofilms during chlorine dioxide and u.v. disinfection in a public drinking water distribution system. J Appl Microbiol. 95, 591–601 (2003).
Baker-Austin, C., Wright, M. S., Stepanauskas, R. & McArthur, J. V. Co-selection of antibiotic and metal resistance. Trends Microbiol. 14, 176–182 (2006).
Flemming, H. C., Neu, T. R. & Wozniak, D. J. The EPS matrix: the “house of biofilm cells”. J Bacteriol 189, 7945–7947 (2007).
Stewart, P. S. & Costerton, J. W. Antibiotic resistance of bacteria in biofilms. Lancet 358, 135–138 (2001).
Yang, L. et al. Combating biofilms. FEMS Immunol Med Microbiol 65, 146–157 (2012).
Herrmann, G. et al. Colistin-tobramycin combinations are superior to monotherapy concerning the killing of biofilm Pseudomonas aeruginosa. J Infect Dis. 202, 1585–1592, 10.1086/656788 (2010).
Yang, L. et al. Effects of iron on DNA release and biofilm development by Pseudomonas aeruginosa. Microbiology 153, 1318–1328 (2007).
Chua, S. L. et al. Dispersed cells represent a distinct stage in the transition from bacterial biofilm to planktonic lifestyles. Nature communications 5, 4462 (2014).
Hengge, R. Principles of c-di-GMP signalling in bacteria. Nat Rev Microbiol. 7, 263–273 (2009).
Lory, S., Merighi, M. & Hyodo, M. Multiple activities of c-di-GMP in Pseudomonas aeruginosa. Nucleic Acids Symp Ser (Oxf) 53, 51–52 (2009).
Yang, L. et al. Pattern differentiation in co-culture biofilms formed by Staphylococcus aureus and Pseudomonas aeruginosa. FEMS Immunol Med Microbiol. 62, 339–347 (2011).
Chua, S. L. et al. Bis-(3’-5’)-cyclic dimeric GMP regulates antimicrobial peptide resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother 57, 2066–2075 (2013).
Gjermansen, M., Nilsson, M., Yang, L. & Tolker-Nielsen, T. Characterization of starvation-induced dispersion in Pseudomonas putida biofilms: genetic elements and molecular mechanisms. Mol Microbiol. 75, 815–826 (2010).
Kaplan, J. B. Antibiotic-induced biofilm formation. Int J Artif Organs 34, 737–751 (2011).
Klebensberger, J., Lautenschlager, K., Bressler, D., Wingender, J. & Philipp, B. Detergent-induced cell aggregation in subpopulations of Pseudomonas aeruginosa as a preadaptive survival strategy. Environ Microbiol. 9, 2247–2259 (2007).
Klonowska, A., Heulin, T. & Vermeglio, A. Selenite and tellurite reduction by Shewanella oneidensis. Appl Environ Microbiol. 71, 5607–5609 (2005).
Yurkov, V., Jappé, J. & Verméglio, A. Tellurite resistance and reduction by obligately aerobic photosynthetic bacteria. Appl Environ Microbiol. 62, 4195–4198 (1996).
Moscoso, H., Saavedra, C., Loyola, C., Pichuantes, S. & Vasquez, C. Biochemical characterization of tellurite-reducing activities of Bacillus stearothermophilus V. Res Microbiol. 149, 389–397 (1998).
Trutko, S. M. et al. Involvement of the respiratory chain of gram-negative bacteria in the reduction of tellurite. Arch Microbiol. 173, 178–186 (2000).
Zannoni, D. Bacterial processing of metalloids as a tool in Biotechnology. J Biotechnol. 150, S52–S53 (2010).
Mohanty, A., Liu, Y., Yang, L. & Cao, B. Extracellular biogenic nanomaterials inhibit pyoverdine production in Pseudomonas aeruginosa: A novel insight into impacts of metal(loid)s on environmental bacteria. Applied Microbiology and Biotechnology. 99, 1957–1966 (2015).
Fleming, A. & Young, M. Y. The inhibitory action of potassium tellurite on coliform bacteria. J Pathol Bacteriol 51, 29–35 (1940).
Cummins, J., Reen, F. J., Baysse, C., Mooij, M. J. & O’Gara, F. Subinhibitory concentrations of the cationic antimicrobial peptide colistin induce the pseudomonas quinolone signal in Pseudomonas aeruginosa. Microbiology 155, 2826–2837 (2009).
Brazas, M. D. & Hancock, R. E. Ciprofloxacin induction of a susceptibility determinant in Pseudomonas aeruginosa. Antimicrob Agents Chemother 49, 3222–3227 (2005).
Thompson, D. K. et al. Proteomics reveals a core molecular response of Pseudomonas putida F1 to acute chromate challenge. BMC Genomics 11, 311 (2010).
Perez, J. M. et al. Bacterial toxicity of potassium tellurite: unveiling an ancient enigma. PLoS One 2, e211 (2007).
Bebien, M., Chauvin, J. P., Adriano, J. M., Grosse, S. & Vermeglio, A. Effect of selenite on growth and protein synthesis in the phototrophic bacterium Rhodobacter sphaeroides. Appl Environ Microbiol. 67, 4440–4447 (2001).
Alhede, M. et al. Phenotypes of non-attached Pseudomonas aeruginosa aggregates resemble surface attached biofilm. PLoS One 6, e27943 (2011).
Borlee, B. R. et al. Pseudomonas aeruginosa uses a cyclic-di-GMP-regulated adhesin to reinforce the biofilm extracellular matrix. Mol Microbiol. 75, 827–842 (2010).
Rybtke, M. T. et al. Fluorescence-Based Reporter for Gauging Cyclic Di-GMP Levels in Pseudomonas aeruginosa. Appl Environ Microbiol. 78, 5060–5069 (2012).
Jacob-Dubuisson, F., Locht, C. & Antoine, R. Two-partner secretion in Gram-negative bacteria: a thrifty, specific pathway for large virulence proteins. Mol Microbiol. 40, 306–313 (2001).
Gottig, N., Garavaglia, B. S., Garofalo, C. G., Orellano, E. G. & Ottado, J. A filamentous hemagglutinin-like protein of Xanthomonas axonopodis pv. citri, the phytopathogen responsible for citrus canker, is involved in bacterial virulence. PLoS One 4, e4358 (2009).
Serra, D. O. et al. FHA-mediated cell-substrate and cell-cell adhesions are critical for Bordetella pertussis biofilm formation on abiotic surfaces and in the mouse nose and the trachea. PLoS One 6, e28811 (2011).
Irie, Y. et al. Self-produced exopolysaccharide is a signal that stimulates biofilm formation in Pseudomonas aeruginosa. P Natl Acad Sci USA 109, 20632–20636 (2012).
Klebensberger, J., Birkenmaier, A., Geffers, R., Kjelleberg, S. & Philipp, B. SiaA and SiaD are essential for inducing autoaggregation as a specific response to detergent stress in Pseudomonas aeruginosa. Environ Microbiol. 11, 3073–3086 (2009).
Sakuragi, Y. & Kolter, R. Quorum-sensing regulation of the biofilm matrix genes (pel) of Pseudomonas aeruginosa. Journal of bacteriology 189, 5383–5386 (2007).
Chew, S. C. et al. Dynamic remodeling of microbial biofilms by functionally distinct exopolysaccharides. mBio. 5, e01536–01514 (2014).
Yang, L. et al. Distinct roles of extracellular polymeric substances in Pseudomonas aeruginosa biofilm development. Environ Microbiol 13, 1705–1717 (2011).
Potrykus, K. & Cashel, M. (p)ppGpp: still magical? Annu Rev Microbiol. 62, 35–51 (2008).
West, S. A., Diggle, S. P., Buckling, A., Gardner, A. & Griffins, A. S. The social lives of microbes. Annu Rev Ecol Evol S 38, 53–77 (2007).
Sun, F. et al. Quorum-sensing agr mediates bacterial oxidation response via an intramolecular disulfide redox switch in the response regulator AgrA. Proc Natl Acad Sci U S A 109, 9095–9100 (2012).
O’Toole, G. A. & Stewart, P. S. Biofilms strike back. Nature biotechnology 23, 1378–1379 (2005).
Hoiby, N., Bjarnsholt, T., Givskov, M., Molin, S. & Ciofu, O. Antibiotic resistance of bacterial biofilms. International journal of antimicrobial agents 35, 322–332 (2010).
Wang, S. et al. The exopolysaccharide Psl-eDNA interaction enables the formation of a biofilm skeleton in Pseudomonas aeruginosa. Environ Microbiol Rep, 7, 330–340 (2015).
Chen, Y. et al. Multiple diguanylate cyclase-coordinated regulation of pyoverdine synthesis in Pseudomonas aeruginosa. Environ Microbiol Rep, In Press (2015).
Wu, Y., Ding, Y., Cohen, Y. & Cao, B. Elevated level of the second messenger c-di-GMP in Comamonas testosteroni enhances biofilm formation and biofilm-based biodegradation of 3-chloroaniline. Appl Microbiol Biotechnol. 99, 1967–1976 (2015)
Bertani, G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol 62, 293–300 (1951).
Starkey, M. et al. Pseudomonas aeruginosa rugose small-colony variants have adaptations that likely promote persistence in the cystic fibrosis lung. J Bacteriol 191, 3492–3503 (2009).
O’Toole, G. A. & Kolter, R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol. 30, 295–304 (1998).
Ng, C. K. et al. Influence of outer membrane c-type cytochromes on particle size and activity of extracellular nanoparticles produced by Shewanella oneidensis. Biotechnol Bioeng. 110, 1831–1837 (2013).
Wiegand, I., Hilpert, K. & Hancock, R. E. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc. 3, 163–175 (2008).
Smale, S. T. Beta-galactosidase assay. Cold Spring Harbor protocols 2010, pdb prot5423 (2010).
Flohr, R. C., Blom, C. J., Rainey, P. B. & Beaumont, H. J. Founder niche constrains evolutionary adaptive radiation. Proceedings of the National Academy of Sciences of the United States of America 110, 20663–20668 (2013).
Liu, Y., Yang, L. & Molin, S. Synergistic activities of an efflux pump inhibitor and iron chelators against Pseudomonas aeruginosa growth and biofilm formation. Antimicrob Agents Chemother 54, 3960–3963 (2010).
Holloway, B. W. & Morgan, A. F. Genome organization in Pseudomonas. Annu Rev Microbiol. 40, 79–105 (1986).
West, S. E., Schweizer, H. P., Dall, C., Sample, A. K. & Runyen-Janecky, L. J . Construction of improved Escherichia-Pseudomonas shuttle vectors derived from pUC18/19 and sequence of the region required for their replication in Pseudomonas aeruginosa. Gene 148, 81–86 (1994).
Starkey, M. et al. Pseudomonas aeruginosa Rugose Small-Colony Variants Have Adaptations That Likely Promote Persistence in the Cystic Fibrosis Lung. J Bacteriol 191, 3492–3503 (2009).
Kirisits, M. J., Prost, L., Starkey, M. & Parsek, M. R. Characterization of colony morphology variants isolated from Pseudomonas aeruginosa biofilms. Appl Environ Microbiol. 71, 4809–4821 (2005).
Hoang, T. T., Karkhoff-Schweizer, R. R., Kutchma, A. J. & Schweizer, H. P. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212, 77–86 (1998).
Kessler, B., de Lorenzo, V. & Timmis, K. N. A general system to integrate lacZ fusions into the chromosomes of gram-negative eubacteria: regulation of the Pm promoter of the TOL plasmid studied with all controlling elements in monocopy. Mol Gen Genet 233, 293–301 (1992).
Acknowledgements
We thank Dr. Matthew R. Parsek (University of Washington, Seattle, USA) for providing the pEX18Gm::ΔsadC and pEX18Gm::ΔsiaD plasmids for constructing the ΔsadC and ΔsiaD mutants. This research was supported by the National Research Foundation and Ministry of Education Singapore under its Research Centre of Excellence Programme and the Start-up Grants (M4080847.030) and (M4330002.C70) from Nanyang Technological University, Singapore. A portion of this work was supported by grants from the Danish Council for Strategic Research (M.G.) and AcRF Tier 2 (MOE2014-T2-2-172) from Ministry of Education, Singapore.
Author information
Authors and Affiliations
Contributions
T.T.N., B.C., S.K. and L.Y. designed the project. S.L.C., M.T.R., J.B.A., M.J.Y. and K.S. performed the experiments. T.E.N., M.G., B.C. and L.Y. interpreted data. B.C. and L.Y. wrote the main manuscript text. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Chua, S., Sivakumar, K., Rybtke, M. et al. C-di-GMP regulates Pseudomonas aeruginosa stress response to tellurite during both planktonic and biofilm modes of growth. Sci Rep 5, 10052 (2015). https://doi.org/10.1038/srep10052
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep10052
This article is cited by
-
A review on microplastic pollution in the mangrove wetlands and microbial strategies for its remediation
Environmental Science and Pollution Research (2022)
-
rpoS-mutation variants are selected in Pseudomonas aeruginosa biofilms under imipenem pressure
Cell & Bioscience (2021)
-
Dynamic swimming pattern of Pseudomonas aeruginosa near a vertical wall during initial attachment stages of biofilm formation
Scientific Reports (2021)
-
Regulation of c-di-GMP in Biofilm Formation of Klebsiella pneumoniae in Response to Antibiotics and Probiotic Supernatant in a Chemostat System
Current Microbiology (2021)
-
Role of c-di-GMP in improving stress resistance of alginate-chitosan microencapsulated Bacillus subtilis cells in simulated digestive fluids
Biotechnology Letters (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.