Abstract
X-ray diffraction technique using a laboratory radiation has generally shown limitation in detectability. In this work, we investigated the in situ high-temperature crystallization of a lithium disilicate glass-ceramic in the SiO2–Li2O–CaO–P2O5–ZrO2 system with the aid of synchrotron radiation. The formation of lithium metasilicate and other intermediate phases in trace amount was successfully observed by synchrotron X-ray diffraction (SXRD). The crystallization mechanism in this glass was thus intrinsically revised to be the co-nucleation of lithium metasilicate and disilicate, instead of the nucleation of lithium disilicate only. The phase content, crystallite size and crystallographic evolutions of Li2Si2O5 in the glass-ceramic as a function of annealing temperature were studied by performing Rietveld refinements. It is found that the growth of Li2Si2O5 is constrained by Li2SiO3 phase at 580–700°C. The relationship between the crystallographic evolution and phase transition was discussed, suggesting a common phenomenon of structural response of Li2Si2O5 along its c axis to other silicon-related phases during glass crystallization.
Similar content being viewed by others
Introduction
Glass science and technology has been fundamentally important to the development of glasses and glass-ceramics over decades. The kinetics and thermodynamics of nucleation are described by several theories including the classical nucleation theory (CNT) and Johnson–Mehl–Avrami–Kolmogorov (JMAK) theory1. Traditionally, thermoanalytic techniques, such as differential scanning calorimetry (DSC) or differential thermal analysis (DTA), have been used to study the non-isothermal crystallization kinetics2,3,4. Recently, we have successfully applied the state-of-the-art synchrotron radiation to study the mechanism, kinetics and crystallographic change during the crystallization of lithium disilicate glasses5,6,7.
It has been well known that there are three types of reaction sequence occurring in the fabrication of lithium disilicate glass-ceramics, depending on glass composition5. One (type I) is the simultaneous nucleation of lithium metasilicate (LS) and lithium disilicate (LS2) phases, followed by the transformation of LS to LS27,8,9. In the second type (type II), LS nucleates first and then transforms to LS2 at a higher temperature10. The third type (type III) of reaction sequence is that the LS2 phase directly forms in the glasses whereas no LS phase forms11,12. The LS phase has very attractive machinability which enables the utilization of CAD/CAM technology for the fabrication of dental products with complex shapes. Thus glasses with types I and II reaction sequences are preferred for dental applications13. Glasses of type III are however not very common in the literature, which is probably because that it is technologically not emphasized due to the absence of the intermediate phase.
In our previously study, we examined the phase transformation of a complex LS2 glass in the SiO2–Li2O–CaO–P2O5–ZrO2 glass system using both in situ and ex situ laboratory-based XRD techniques14. The glass-ceramics derived from this composition have an optimized flexural strength of 439 ± 93 MPa. The crystallization mechanism was deemed to be the direct precipitation of LS2 phase (type III) whereas no LS phase was detected during glass crystallization14. In this study, we revisited the high-temperature phase transformation of the same LS2 glass using synchrotron radiation. A trace amount of LS phase was identified at the initial nucleation stage as well as other intermediate phases during crystallization. The results from this study may shed insight on the understanding of crystallisation in glass-ceramics.
Results
Trace phase formation in the glass
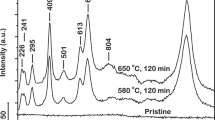
With the scheduled annealing profile, the in situ high-temperature XRD patterns of the complex lithium disilicate glass were acquired and depicted in a two-dimensional pattern in Figure 1a where the intensities are illustrated in color. No diffraction peaks were observed at temperatures below 580°C. There are five crystalline phases identified from the synchrotron diffraction patterns: LS, LS2, Li3PO4 (LP), β-cristobalite (CR) and β-quartz (QZ). The diffraction peaks initiated at 580°C can be assigned to LS and LS2 phases. The intensity of LS phase (~500 counts) is considerably lower than that of LS2 phase (~160,000 counts), as shown in Figure 1b. Such a high intensity ratio of LS2 to LS (over 300 times) may explain why the precipitation of LS in this glass was hardly detected by the conventional XRD technique14. Due to the distortion of the sample, data acquisition of synchrotron powder diffraction of this glass failed at a temperature ≥920°C where melting was accelerated.
(a) In situ high-temperature XRD patterns (in color contour) of the complex lithium disilicate glass, (b) the line patterns of partial data to show the peaks of LS phase (directed by arrows) and peaks of other unidentified phase (directed by stars). D: lithium disilicate (LS2); M: lithium metasilicate (LS); P: lithium phosphate (LP); C: β-cristobalite (CR); Q: β-quartz (QZ); U: unidentified intermediate phase.
Kinetics of glass crystallization
The crystallographic parameters of LS2 and weight fraction of each phase are determined by applying Rietveld refinement using the full patterns. The change in weight fraction of involved phases during the glass crystallization is presented in Figure 2. Both lithium metasilicate and disilicate phases were detected at 580°C, with a limited quantity of 0.09 wt% and 0.08 wt% respectively as determined by quantitative phase analysis. The LS2 phase dominated the glass-ceramic during crystallization, which reached a fraction of above 50 wt% at 660°C. The crystallization of LS2 reached a maximum of ~63 wt% at ~760°C. In this glass, LS with a maximum amount of ~2 wt% formed and completely disappeared at 700°C.
With the P2O5 component added as a nucleating agent in the base glass, lithium phosphate (Li3PO4, LP) nuclei with short range order form in the glass at the early stage, which then trigger the compositional gradients of parent glass and lead to the nucleation of silicate phases7,15. In the current glass, the LP phase was observed at temperatures of 700°C and above. The silica phases including β-cristobalite and β-quartz crystallized when the temperature reached 780 and 820°C, respectively. As indicated in Figure 2, LP, CR and QZ showed a linear increasing trend after their precipitation. The complete crystallization of LP gave a maximum fraction of ~4 wt%, whereas the maximum fraction of CR and QZ was about 2 wt% and less than 1 wt%, respectively.
The evolution of crystallite size of LS2 with temperature is shown in Figure 3. The size of LS2 crystals was refined to be about 200 nm at 600°C. Very interestingly, the crystallite size of LS2 remained in the same level at the temperature range of 600–700°C where the LS phase co-existed with LS2. Right after the disappearance of LS at 700°C, the LS2 phase showed a gradual crystal growth.
Crystallographic change of LS2 in the glass
The evolution of lattice parameters of LS2 as a function of temperature is presented in Figure 4a. A complex trend was observed in the lattice parameter c of LS2. Specifically, it started with a dramatic decrease at 600–700°C, followed by a slight increase until the LS phase disappeared. The inflection point at 660°C was found to hold a precipitation event of an unidentified intermediate phase. After the completion of LS-to-LS2 transformation at 700°C, the parameter c showed a reversed trend in that it decreased again until 780°C where a silica phase of β-cristobalite precipitated. From 800°C where another silica phase (β-quartz) crystallized, it began to increase instead. Such a complex trend of crystallographic evolution has been interpreted in the previous findings5. The events at transition points of the evolution trend are highlighted by the selected peaks in Figure 5.
The crystallographic a and b axes do not have similar trend as in the c axis, which was observed previously as well5. They basically had an increasing trend. As the small amounts of additives (other than SiO2, Li2O, P2O5) was not detected to crystallize from the glass matrix, the influence of them on the change of the c-axis of LS2 is unclear at the time being. The as-measured unit cell volume (V) of the LS2 phase, which can be calculated by multiplying the parameters, i.e. V = a × b × c, shows a near linear increasing trend as a function of temperature (Figure 4b).
Discussion
It is noteworthy that the LS phase in this glass was not detected by the conventional XRD technique, either in situ or ex situ14. With the laboratory data, the as-investigated glass herein should be regarded as a glass with type III reaction sequence. Then, it could lead to a “LS-free” nucleation mechanism as the LS phase was blindly neglected due to the shortness of laboratory technique. With the aid of the advanced synchrotron radiation, we managed to track the trace phase formation of LS, from a trace amount as low as 0.08 wt% to the maximum fraction of ~2 wt% (Figure 2), which nucleated simultaneously with LS2 at 580°C. Correspondingly, the involved mechanism is revised to be that the crystallization of this glass follows the type I sequence5.
The formation of limited fraction of LS phase has also been reported by Soares Jr et al. during the investigation of crystallization of binary Li2O-SiO2 glasses16. The LS phase coexisted with LS2 up to 120 hours at the glass transition temperature, suggesting the same reaction sequence in the glasses involving the formation of trace LS phase.
From the crystallographic aspect, it has been found that the initial evolution trend of the c axis of LS2 was opposite in the glasses of type I and III sequences, i.e., decreasing and increasing respectively5,6. Thus, crystallographic interaction within silicates is indicated. The observed mode of crystallographic evolution of LS2 in this glass is consistent with that in the glasses of type I sequence5,6, rather than those of type III sequence. A slight change in the evolution trend of lattice parameter a was observed at 700°C (Figure 4a), which probably indicates the effect of the disappearance of LS phase on this crystallographic axis.
From this study and the recent observations5,6, we believe that a common phenomenon of structural response takes place during the crystallization of lithium disilicate glasses. To be specific, the crystallographic change of LS2 in c axis was largely affected from a normal increasing trend (if no LS precipitated, i.e., the case in type III sequence) to the opposite with the existence of LS phase (i.e., the cases in types I and II sequences). In addition, the silicon-related phase formation will also affect the evolution trend. The reversed trend has also been observed in other glasses5 upon the precipitation of the crystalline phases that share the [O–Si–O] source from glass matrix, such as silicates (LS, LS2), silica (CR, QZ, etc.) and other Si-related phases. Unsurprisingly, the unidentified intermediate phases also have an effect on such a trend (Figure 4a), indicating that it may be silicon-containing in composition.
The crystallite size of LS2 at the temperature range where LS crystallizes remains at the same level of around 200 nm and then it gradually increases (Figure 3). A similar trend was reported in other lithium disilicate glasses7. This may imply that the existence of LS in the glass not only affects the crystallographic structure of LS2 but also hampers its crystal growth capability.
In summary, the state-of-the-art synchrotron radiation enables the detection of the trace phase formation of Li2SiO3 in a complex lithium disilicate glass by in situ X-ray diffraction investigation. The peaks of Li2SiO3 in very weak intensity was successfully observed at 580–700°C. The kinetics study indicates that the crystallite size of Li2Si2O5 is hampered by the coexistence of LS phase. The crystallographic evolution of Li2Si2O5 showed a complex trend. Particularly in c axis, it demonstrates a phenomenon of structural response to other silicon-related phases, such as Li2SiO3 and silica phases. This phenomenon has been also observed in other glasses, suggesting the generalization of crystallographic interaction phenomenon during glass crystallization.
Methods
The composition of the complex lithium disilicate glass was reported in our previous paper14. The main composition of this glass were 63.3SiO2, 26.4Li2O, 2.6CaO, 1.7P2O5, 0.9ZrO2, 1.3Na2O, 1.1K2O, 2.7MgO (in mol.%). The glass was melted in a Pt crucible at 1500°C for 3 hrs. The glass samples were mechanically ground to the dimensions of about 5 mm × 5 mm × 0.4 mm. Then they were subjected to synchrotron X-ray diffraction (SXRD) at elevated temperatures on the Powder Diffraction Beamline in the Australian Synchrotron. The energy of the X-ray beam was 11 keV (corresponding wavelength of 1.1273 Å). The detector setup is the same with that described previously5,6,7. The monolithic glass samples were heated up in an Anton Paar HTK-2000 furnace with a platinum (Pt) resistance strip heater at a ramp rate of 400 K·min−1. There were 22 holding stages within the temperature range of 500–920°C, with an interval of 20°C (Figure 6), for recording the diffraction patterns. At each stage, X-ray was switched on for 4 min to detect the phases at corresponding temperatures. During the in-situ measurements, no external reference materials were used.
After acquiring the data, the structure refinement was conducted with the MAUD software on the basis of the full-pattern analysis using the Rietveld method17. The data sets acquired at 580–900°C were refined sequentially, where a Delf line broadening model was employed and an iterative least-square procedure by minimizing the residual parameters Rwp, RB and Rexp was adopted. The crystallite size and the microstrain (root mean square, r.m.s. strain, <ε2>1/2) were evaluated using an isotropic size-strain model. Those patterns without the peaks of crystalline phase(s) (i.e., at 500–560°C) and those collected after sample distortion (i.e., at 920°C) were not used for the analyses. During Rietveld analyses, the CIF files ICSD-64980 (Inorganic Crystal Structure Database), ICSD-44095, ICSD-100402, ICSD-280481 and ICSD-79427 were referenced for the profile fitting of β-quartz, β-cristobalite, lithium metasilicate, lithium disilicate and lithium orthophosphate phase, respectively.
References
Fokin, V. M., Zanotto, E. D., Yuritsyn, N. S. & Schmelzer, J. W. P. Homogeneous crystal nucleation in silicate glasses: A 40 years perspective. J. Non-Cryst. Solids 352, 2681–2714 (2006).
Kemény, T. & Šesták, J. Comparison of crystallization kinetics determined by isothermal and non-isothermal methods. Thermochim. Acta 110, 113–129 (1987).
Henderson, D. W. Thermal analysis of non-isothermal crystallization kinetics in glass forming liquids. J. Non-Cryst. Solids 30, 301–315 (1979).
Henderson, D. W. Experimental analysis of non-isothermal transformations involving nucleation and growth. J. Therm. Anal. Calorim. 15, 325–331 (1979).
Huang, S., Huang, Z., Gao, W. & Cao, P. Structural Response of Lithium Disilicate in Glass Crystallization. Cryst. Growth Des. 14, 5144–5151 (2014).
Huang, S., Huang, Z., Gao, W. & Cao, P. In Situ High-Temperature Crystallographic Evolution of a Nonstoichiometric Li2O·2SiO2 Glass. Inorg. Chem. 52, 14188–14195 (2013).
Huang, S., Cao, P., Li, Y., Huang, Z. & Gao, W. Nucleation and crystallization kinetics of a multi-component lithium disilicate glass by in situ and real-time synchrotron X-ray diffraction. Cryst. Growth Des. 13, 4031–4038 (2013).
von Clausbruch, S. C., Schweiger, M., Höland, W. & Rheinberger, V. The effect of P2O5 on the crystallization and microstructure of glass-ceramics in the SiO2-Li2O-K2O-ZnO-P2O5 system. J. Non-Cryst. Solids 263–264, 388–394 (2000).
Höland, W., Apel, E., van t Hoen, C. & Rheinberger, V. Studies of crystal phase formations in high-strength lithium disilicate glass-ceramics. J. Non-Cryst. Solids 352, 4041–4050 (2006).
Hammetter, W. F. & Loehman, R. E. Crystallization Kinetics of a Complex Lithium Silicate Glass-Ceramic. J. Am. Ceram. Soc. 70, 577–582 (1987).
Barker, M. F., Wang, T.-H. & James, P. F. Nucleation and growth kinetics of lithium disilicate and lithium metasilicate in lithia-silica glasses. Phys. Chem. Glasses 29, 240–248 (1988).
Apel, E. et al. Inventors; Ivoclar Vivadent AG, assignee. Lithium disilicate glass ceramic. United States patent US 7,871,948 B2. 2011 Jan 18.
Höland, W. et al. Future perspectives of biomaterials for dental restoration. J. Eur. Ceram. Soc. 29, 1291–1297 (2009).
Huang, S., Cao, P., Wang, C., Huang, Z. & Gao, W. Fabrication of a high-strength lithium disilicate glass-ceramic in a complex glass system. J. Asian Ceram. Soc. 1, 46–52 (2013).
Bischoff, C., Eckert, H., Apel, E., Rheinberger, V. M. & Holand, W. Phase evolution in lithium disilicate glass-ceramics based on non-stoichiometric compositions of a multi-component system: structural studies by 29Si single and double resonance solid state NMR. Phys. Chem. Chem. Phys. 13, 4540–4551 (2011).
Soares Jr, P. C., Zanotto, E. D., Fokin, V. M. & Jain, H. TEM and XRD study of early crystallization of lithium disilicate glasses. J. Non-Cryst. Solids 331, 217–227 (2003).
Lutterotti, L., Matthies, S. & Wenk, H.-R. MAUD: a friendly Java program for material analysis using diffraction. IUCr: Newsletter of the CPD 21, 14–15 (1999).
Acknowledgements
This research was undertaken on the Powder Diffraction beamline at the Australian Synchrotron Centre, Victoria, Australia (project ID: AS121/PDFI/5330). We thank the beamline scientists Dr. Qinfen Gu and Dr. Justin Kimpton for their technical assistance. S.H. would like to acknowledge the China Scholarship Council (CSC) for providing a doctoral scholarship for his Ph.D. study at the University of Auckland.
Author information
Authors and Affiliations
Contributions
S.H., P.C. and W.G. conceived and designed the experiments. S.H. carried out the experiments. S.H. and P.C. analyzed the data. P.C., W.G. and Z.H. supervised the experiments and discussed the results. S.H. wrote the paper with the input from all co-authors.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Huang, S., Huang, Z., Gao, W. et al. Trace phase formation, crystallization kinetics and crystallographic evolution of a lithium disilicate glass probed by synchrotron XRD technique. Sci Rep 5, 9159 (2015). https://doi.org/10.1038/srep09159
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep09159
This article is cited by
-
Improved performances of lithium disilicate glass-ceramics by seed induced crystallization
Journal of Advanced Ceramics (2021)
-
Control of crystallization behaviour of supercooled liquid composed of lithium disilicate on platinum substrate
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.