Abstract
Lithium Sulfur (Li/S) chemistries are amongst the most promising next-generation battery technologies due to their high theoretical energy density. However, the detrimental effects of their intermediate byproducts, polysulfides (PS), have to be resolved to realize these theoretical performance limits. Confined approaches on using porous carbons to entrap PS have yielded limited success. In this study, we deviate from the prevalent approach by introducing catalysis concept in Li/S battery configuration. Engineered current collectors were found to be catalytically active towards PS, thereby eliminating the need for carbon matrix and their processing obligatory binders, additives and solvents. We reveal substantial enhancement in electrochemical performance and corroborate our findings using a detailed experimental parametric study involving variation of several kinetic parameters such as surface area, temperature, current rate and concentration of PS. The resultant novel battery configuration delivered a discharge capacity of 700 mAh g−1 with the two dimensional (2D) planar Ni current collectors and an enhancement in the capacity up to 900 mAh g−1 has been realized using the engineered three dimensional (3D) current collectors. The battery capacity has been tested for stability over 100 cycles of charge-discharge.
Similar content being viewed by others
Introduction
Past decade has witnessed a renewed interest in development of high energy storage devices, the interest is further bolstered by their potential applications for plug-in hybrid and electric vehicles. Intercalation materials employed in conventional Li-ion batteries impose limitations on the energy density that can be achieved. These shortcomings have stimulated research in alternative chemistries labelled beyond lithium ion batteries1,2,3,4,5. Among several re-visited chemistries, rechargeable lithium/sulfur (Li/S) batteries have gained attraction due to their high theoretical capacity of 1675 mAh g−1 of sulfur cathode, wide range of temperature operation and low cost6,7,8,9,10,11. In spite of several research efforts on this subject, key issues related to “redox shuttle reactions” between sulfur cathode and Li anode have not been fully addressed yet12,13. Poor understanding and lack of control on the series of intermediate lithium polysulfides (PS) are commonly identified problems in all Li/S battery configurations such as solid, liquid and flow cells9,14,15,16,17,18. Though the overall redox reaction is primarily driven by the dissolution of lithium polysuflides into the electrolyte, the insulating nature of the polysulfides and its predisposition to corrode the lithium anode results in low charging efficiency, short cycle life and high self-discharges.
In order to retain power density and cycle life of the device in the face of insulating nature of dissolved polysulfides, it is necessary to substantially increase the contact of active sulfur with the conductive matrix. Though several carbonaceous materials modified at the nanoscale are extensively used as electronic conductors, problems of processing nano/micro porous carbons, binders and achieving high sulfur loading have not yet been thoroughly addressed8,19,20,21,22,23,24,25,26. In spite of some success on effective sulfur loading in variety of porous carbon structures, the intrinsic issues of pore clogging due to deposition of lower order polysulfides (Li2S2 and Li2S) remains to be unaddressed. Deposition of such solid insulating blocks on electrochemically active surfaces increases internal resistances resulting in substantial raise in overpotential and capacity fade upon extended cycling of the cell27,28. Recent research reports have bypassed the sulfur loading step by incorporating intermediate polysulfides (catholyte) in the electrolyte itself7,16,29. Irrespective of the nature of the starting cathode, i.e. either C-S composite or liquid catholyte, it is recognized that Li/S battery configuration eventually morphs itself into a liquid electrochemical cell due to the formation of intermediate polysulfides at the very beginning of the discharge step. Hence, understanding and controlling kinetics of thus formed intermediate polysulfides plays a key role in commercializing Li/S battery technology.
It is well known that the insulating nature of polysulfides causes poor reaction kinetics and hence influences overall redox process. On the other hand, use of electrocatalytic electrodes has found to enhance the reaction kinetics of aqueous polysulfides in photoelectrochemical solar cells30,31,32,33,34 and redox flow cells35,36,37,38,39. However, to the best of our knowledge, there have been no reports on utilizing electrocatalysis concept in non-aqueous polysulfides redox reactions. For the first time, here we have conducted detailed investigations on electrocatalyst effect on Li-polysulfides redox reactions and developed a novel Li/S battery configurations without use of any carbon matrix. Different electrocatalyst such as Pt, Au and Ni have been coated on conventional current collectors such as aluminum and stainless steel (SS) foils and used them to serve the dual role of current collector and electrode for Li/S battery configuration. Further, an engineered porous SS and Ni foils itself were found to act as efficient current collectors and electrodes thereby resulting novel battery configuration called “Metal/PS/Metal” battery (Figure 1). We believe the electrocatalyst concept in emerging Li/S chemistries will open a new avenue for developing efficient energy storage technologies.
Results
To understand the effect of electrocatalyst on polysulfides redox reactions and hence overall electrochemical properties of this novel concept of using current collectors itself as carbon free electrodes for Li-S battery configuration, we have considered different traditional electrocatalysts such as Pt, Au and Ni and a non-electrocatalyst Al (for control experiments). All these metals were separately coated (~50 nm) on two different substrates such as Al and stainless steel (SS) foil and used them as working electrodes to fabricate standard 2032 type coin cells. Crystal structures of coated thin films were confirmed from X-ray diffraction studies (Supplementary information Figure S1). Coin cell fabrication is performed under inert atmosphere (Ar filled glove box) using Li metal anode and catholyte (10 μl) as an active material and quartz membrane as a separator. Galvanostatic measurements conducted at a constant current rate of 0.1 C (based on sulfur mass in the cell) and obtained results have been monitored for 50 cycles of charge/discharge. From Figure 2a and 2b, well defined plateaus of charge/discharge and reduction in polarization at any depth of discharge (DOD) are clear evidence for electrocatalysis of PS. Though Al electrodes were found to be inactive for polysulfides conversion reaction due to their ultra-low capacity and huge polarization of charge-discharge profiles, other electrodes (Pt, Au and Ni) show enhanced performance with plateaus of charge-discharge around 2.4 and 2.0 V respectively, which is similar to conventional carbon electrode7. Such trend has been observed to be consistent over the long cycles of charge-discharge on both Al and SS substrates (Supplementary Information Figure S2 and Figure S3).
Screening of different metals for Li/S battery configuration.
(a) Comparison of charge-discharge plateaus, (b) cycling behavior for Ni, Au and Pt electrocatalysts with 50 nm thickness on Al substrate (foil) vs. Li/Li+ towards lithium polysulfide conversion reactions in the potential range 3.0 ~ 1.5 V at 0.1 C rate and (c) tabulation of discharge plateau voltage values for electrocatalysts and corresponding capacity values at the end of 50th cycle.
Among different electrocatalysts studied, Ni and Pt electrodes are promising to use as electrocatalyst in lithium-polysulfide battery as it exhibits comparable discharge capacity of 370 and 395 mAh g−1 at the end of 50th cycle respectively (Figure 2c). With the inherent electrochemical activity of Pt electrode exhibits good cycle life over 50 charge-discharge cycles but it shows larger polarization in charge-discharge curves compared to Ni electrode (Figure 2a). On the other hand, despite of its great promise as an electrocatalyst for polysulfide conversion process, thermally evaporated Ni films of 50 nm thickness were found to be partially oxidised due to their high sensitivity towards ambient atmosphere. However, surface oxidation issues of 50 nm Ni films were resolved by increasing its thickness to 200 nm (Supplementary Information, Figures S4, S5 & S6). Further, the thickness of thermally evaporated Ni film (200 nm) has been confirmed experimentally using cross section SEM study (Supplementary Information, Figure S7) and hence is used for further fundamental electrochemical investigations.
Cyclic voltammetry (CV) studies have been carried out on Ni (200 nm) electrocatalyst and compared its performance with conventional carbon electrode. Figure 3a shows that representative CV of Ni electrode vs. Li/Li+ at a scan rate of 0.05 mV s−1 with 10 μl of Li2S8 in TEGDME solvent containing 1 M LiTFSI and 0.1 M LiNO3 as catholyte. Recorded CV consist of two reduction peaks at 2.43 and 1.94 V corresponding to conversion of elemental sulfur to higher plysulfides (Li2S8) and subsequent disproportion in electrolyte and further reduction to lower lithium polysulfides respectively. Electrochemical potential and corresponding current were monitored as a function of cycle number. (Supplementary Information, Figure S8). The constancy of redox peak currents upon increasing number of CV scans indicates the overall stability and activity of electrocatalyst towards long cycling performance.
Electrochemical characterization of Ni thin film electrodes.
(a) Representative cyclic voltammogram of newly designed Li-polysulfide battery contains Ni film (200 nm) electrocatalyst as an electrode to lithium polysulfide conversions vs. Li/Li+ at scan rate of 0.05 mV s−1 between 1.5 to 3.0 V, (b) CV derived electrochemical parameters to understand the electro-catalytic activity of Ni film towards polysulfide conversions, (c) cycling behavior and coulombic efficiency of Ni film (200 nm) as an electrode in Li-polysulfide battery for 100 cycles and (d) rate capability behavior of newly invented electrode/electrocatalyst (Ni, 200 nm) towards lithium polysulfide conversions.
Further, CVs of Ni electrode were recorded at different scan rates ranging from 0.2 to 1.0 mV s−1 with an increment of 0.2 mV s−1 in order to understand the kinetics of Ni electrocatalyst towards polysulfides conversions (Supplementary Information, Figure S9). Shift in the anodic and cathodic peaks of CV with increase in scan rate is a general trend in any carbon based Li-S battery configurations, which is considered as an evidence for quasi reversible reaction of polysulfides40. As expected, conventional carbon electrode shows shift in the anodic/cathodic peak positions towards positive/negative potentials respectively indicating quasi reversible process of polysulfides. Interestingly, Ni electrode showed almost stable peak position with increase of scan rate, thereby indicating its suitablility towads higher current rates of charge/discharge process. Further, we have observed the rate of increase in peak currents of both anodic and cathodic peaks as a function of applied scan rate. The cathodic and anodic peak potentials of conventional carbon electrode and Ni electrode are summarized along with exchange current density values in Figure 3b. The lower oxidation potential and higher reduction potentials are always indication for efficient electrochemical system as it reflects in low polarization in the cell configuration. Herein, the Ni electrode shows the lower oxidation potentials of 2.51 and 2.54 V (vs 2.79 V of carbon) and higher reduction potentials of 2.43 and 1.94 V (vs 2.40 and 1.84 V of carbon). In addition, exchange current density values calculated from Tafel plot reveals that Ni electrode has better kinetics than that of carbon electrode (Supplementary Information, Figure S10). Therefore, newly designed lithium polysulfide battery system containing electrocatalyst (Ni film) as electrode is expected to result in superior charge/discharge characteristics due to its better reaction kinetics towards PS conversion process.
To evaluate specific capacity, cycleability, coulombic efficiency and rate capabilities of Ni electrode based Li-S battery, galvanostatic charge/discharge measurements were performed. The cycleability vs. specific capacity and coulombic efficiency plots of 200 nm thick Ni electrode at 0.1 C rate are described in Figure 3c. Fascinatingly, Ni film acting as both current collector and electrode delivered a steady state capacity up to 700 mAh g−1 over 100 cycles of charge/discharge with an excellent coulombic efficiency of <98%. Further, to investigate its suitability for high power applications, rate capability tests were also performed on Ni electrocatalyst (Figure 3d). Ni electrode based Li-S cell was first subjected to low C-rate of 0.1 C to obtain stable nominal capacity. Subsequent cycling was conducted at higher current rates of 0.2 and 0.5 C for each 10 cycles. At the end of the high C-rate (0.5 C) test, the cell was operated at 0.1 C rate in order to understand the capacity retention. Observed specific capacity values of 780, 605 and 510 mAh g−1 at 0.1, 0.2 and 0.5 C rates respectively, clearly supports its suitability towards high power applications. Hence, good cycle life with the negligible capacity fade over 100 cycles, excellent coulombic efficiency and rate capability were found to be appealing feaures of electrocatalysis driven Li-S battery.
It is well known that the energy density per unit area of Li-S cell can be increased by increasing the amount of sulfur content in catholyte. However, such increase in sulfur loading drastically reduces electrochemical properties of the cell. In order to understand the effect of polysulfide concentration on electrochemical performance of electrocatalyst, different catholytes having 100, 200 and 600 mM concentration of Li2S8 were prepared and tested against Ni (200 nm film) electrode. Specific capacity vs. cyclic number shown in Figure 4a was a result of galvanostatic charge/discharge measurements conducted constant current rate of 0.1C. The decrease in overall specific capacity of the Li-S cell with increase in the concentration of polysulfides was due to increase in viscosity of the electrolyte. However, it is interesting to note that at any given concentration of the polysulfides, all cells showed very good cyclic stability over 50 cycles. Especially, cells with 100 mM and 200 mM concentrations of Li2S8 show the specific capacity of 650 and 500 mAh g−1 respectively are evidence of extending this electrocatalysis concept to higher concentration of polysulfides.
Evaluation of concentration and temperature effects on electrocatalysis of PS.
(a) Feasibility of electrocatalyst (Ni film −200 nm) towards lithium polysulfide conversion reaction for increased molar concentration of lithium polysulfide solution (60 mM to 600 mM) per cell at 0.1 C rate in the potential range of 1.5 to 3.0 V and (b) temperature effect on the charge-discharge polarization of the cell contains electrocatalyst (Ni film −200 nm) as an electrode in Li-polysulfide battery.
Another interesting property of an efficient electrocatalyst is its positive response towards temperatures as kinetics of catalytic process is expected to enhance with increase in temperature. In the present scenario, it is also expected that Ni electrocatalyst should show enhanced battery performance with increase in temperature due to increase in kinetic energy and molecular motion of polysulfides. Figure 4b reveals the effect of temperature on electrochemical performance of lithium-polysulfide battery whose capacity values were found to be enhanced by 10% when the cell is heated to 40°C from room temperature (Supplementary Information, Figure S11). More importantly, the polarization between the charge and discharge plateaus was found to be reduced drastically with increase of cell temperature, which is most important and beneficial point in view of battery applications. The plateau region at 2.4 V associated to the conversion of elemental sulfur to Li2S8 was also found to be enhanced at 40°C. Such enhancement in plateau region may be related to efficient conversion efficiency of insoluble end products to elemental sulfur upon charging since the plateau depends on available elemental sulfur18.
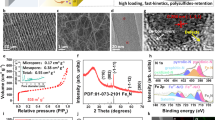
Further, it is also well known that electrocatalytic activity of any electrocatalyst depends on its accessible surface area41. Hence, by increase in the surface area of Ni electrocatalyst (electrode) should result in enhanced polysufides conversion properties. To prove this hypothesis in Li-S configurations, we have chosen two different Ni structures with high surface area namely microporous 3D Ni (prepared by electrodeposition at −10 mA cm−2 42) and commercially available 3D Ni foam and compared their electrocatalytic properties with that of planar 2D Ni electrode and as well as with conventional carbon electrode. Figure 5a–c are the SEM images of 2D planar Ni film, microporous 3D Ni and macroporous 3D Ni foam respectively and Figure 5d depicts their electrochemical properties. As expected, specific capacity values of Ni electrode were found to increase with increase in surface area. Microporous 3D Ni exhibits the discharge capacity of 800 mAh g−1 for 50 cycles, whereas macroporous 3D Ni foam shows further improvement, reaching stable discharge capacity value of 900 mAh g−1 (Supplementary Information, Figure S12). Conversely, the conventional cell with carbon as working electrode showed significant reduction in capacity values upon cycling due to its intrinsic issues of pore clogging by lower polysulfides (Li2S) and poor adsorption of electrophilic PS on carbon electrode43. It is noted that, in general, electrocatalysis surface accessibility is maximum with meso or macroporous structure than that of microporous structures41,44. Hence, the observed superior performance of macroporous 3D Ni over microporous 3D Ni structures is in good agreement with traditional electrocatalysis process. Further, stability and morphological integrity of substrates after long cycling were confirmed by conducting post SEM analysis (Supplementary Information, Figures S13, S14 and S15).
Surface area effects on electrochemical properties.
(a) SEM images of 2D planar Ni thin film (b) SEM image of 3D Ni deposited on SS substrate by galvanostatically at 10 mA cm−2 (c) SEM image of commercially available Ni foam, (d) comparison of discharge capacity values exhibited by different Ni electrode surfaces along with conventional carbon paper as an electrode towards lithium polysulfide conversions in Li-polysulfide battery at 0.1C rate in the potential window of 1.5 to 3.0 V and (e) demonstration of Metal/PS/Metal battery.
Discussion
The concept of using metallic current collectors as electrodes is novel. Hydrophilic, catalytic and porous nature of current collector has three fold advantage over conventional carbon electrodes, it can (i) adsorb intermediate PS on current collector due to hydrophilic nature, (ii) efficiently convert higher PS to lower PS and vice versa due to catalytic nature and (iii) host the insulating solid precipitates at the end of each discharge cycle due to porous nature7,18,43. Reduction in polarization and increase in discharge capacity of Au, Pt and Ni coated current collectors over conventional Al substrate is an evidence of electrocatalysis process (from Figure 2). Stability over 100 cycles of charge-discharge and capability to withstand high current rates (C-rate test) further supports the argument (from Figure 3). Tests on evaluating some of catalytically sensitive properties such as concentration, temperature and surface area showing (i) good stability towards converting high concentration of PS (from Figure 4a), (ii) fast electron transfer and hence low polarization with increase of temperature (from Figure 4b) and (iii) increase in specific capacity with increase in active surface area of the electrode (from Figure 5), clearly proves the electrocatalysis in this novel Li-S battery configuration.
In summary, a carbon free Li-S battery configuration has been demonstrated using concept of electrocatalysis. Lithium polysulfide conversions reactions have been found to take place on electrocatalytic surfaces such as Pt, Au and Ni. Use of Ni in Li-S battery configuration has found to be two folded, acting as current collector and also as electrode, thereby eliminating the traditional tedious process of synthesis and fabrication of highly porous micro/nano carbon structures. Detail electrochemical studies involving specific capacity, cyclic stability, rate capability and coulombic efficiency as a function of polysulfides concentration, temperature and surface area of electrode/current collector revealed that Ni based electrodes were capable of delivering stable capacities up to 900 mAh g−1. Thus, we believe this novel concept of electrocatalysis of lithium polysulfides, a carbon free cathode, will open up a new avenue for developing most awaiting Li-S battery technology for both stationary and portable applications.
Methods
Preparation of different polysulfides
Polysulfide solutions used in this study were prepared by heating the stoichiometric amounts of Li2S and S to attain Li2S8 in tetraethylene glycol dimethyle ether (TEGDME) at 90°C with effective stirring for 12 h. Such prepared polysulphides used directly as active material along with an electrolyte consists of 1 M lithium bis (trifluoromethanesulfonyl) imide (LiTFSI) and 0.1 M lithium nitrate (LiNO3) in TEGDME. The polysulfide concentrations used here as 60, 100, 200 and 600 mM and these are calculated based on the sulfur content in the polysulfide solutions
Preparation of different electrocatalytic electrodes
Ni, Pt, Au and Al metal thin films were deposited using e-beam evaporator on Al foil and SS foil substrates individually with the film thickness of 50 nm (50 & 200 nm for Ni films) to use them as electrode materials towards lithium polysulfide conversions. In e-beam evaporation process, a high intensity electron beam was used to vaporize the desired metal sources, which are placed on sample holder. Upon e-beam focusing, the metal atoms evaporated and condense on the surface of the Al and SS substrate positioned in face of the precursor source material. Thickness monitor placed in front of the substrate would permit us to control and monitor desired thickness of the evaporated thin films. Temescal FC/BJD2000 deposition system was used to depositing all thin-films with different thickness at 250°C under vacuum system with base pressure of 5 × 10−6 Torr. Two types of 3D Ni electrodes were used, one of them is commercially available 3D Ni foam (MTI Corporation) and other is prepared by galvanostatic electrodeposition method. Firstly, the Ni−Cu alloy films were deposited on surface roughened stainless steel (SS) foils and then remove Cu component from alloy. The electrodeposition of Ni-Cu alloy was carried out using three-electrode cell consisting of 4 ml aqueous solution of NiSO4 (1 M), CuSO4 (0.05 M) and citric acid as an electrolyte, stainless steel foil (Type 304, 0.1 mm thick, Alfa Aesar) as working electrode, Ag/AgCl reference electrode (CH Instruments) and the stainless steel strip as counter electrode. Electrochemical deposition was typically conducted under galvanostatic conditions of −10 mA cm−2 at room temperature for 2 h using GAMRY potentiostat/galvanostat.
Cell fabrication and characterizations
Coin cells of standard 2032 were constructed to evaluate the electrochemical performance of the different electrocatalysts towards polysulfide conversions or as a cathode for Li-polysulfide batteries. The coin cell fabrication was carried out in an argon-filled glove box using 10 μl Li2S8 polysulfide place on elecrocatalyst, metallic lithium anode and an electrolyte along with quartz separator. In the present study, we have fixed the volume of catholyte (per cell) and varied the concentration of catholyte from 0.06 M to 0.6 M, which translates to sulfur loading from 0.152 to 1.52 mg/cm−2. Coin cells were tested for cyclic voltammograms (CV) in the potential range 1.5 ~ 3.0 V with different scan rates from 0.2 to 1.0 mV s−1 and impedance (EIS) studies from 100 KHz to 200 mHz using Bio-logic electrochemical work station. Charge- discharge studies for different electrocatalysts at 0.1 C rate and rate capability test at different current rates (0.2 and 0.5 C rate) were carried out in the potential range of 1.5 ~ 3.0 V using ARBIN charge-discharge cycle life tester. The capacity values were calculated using mass of sulfur in polysulfide solution and corresponding current rates are considered based on 1674 mAh g−1 (1C) equivalent to full discharge or charge in 1 h. The morphology of the samples were characterized by a JSM 401F (JEOL Ltd., Tokyo, Japan) SEM operated at 3.0 kV and a JEM 2010 (JEOL Ltd, Tokyo, Japan). X-ray diffraction (XRD) patterns were recorded on a Bruker D8 Advance diffractometer at 40.0 kV and 120 mA with Cu-Kα radiation.
References
Bruce, P. G., Freunberger, S. A., Hardwick, L. J. & Tarascon, J. M. Li-O2 and Li-S batteries with high energy storage. Nat. Mater. 11, 19–29 (2012).
Ogasawara, T., Débart, A., Holzapfel, M., Novák, P. & Bruce, P. G. Rechargeable Li2O2 Electrode for Lithium Batteries. J. Am. Chem. Soc. 128, 1390–1393 (2006).
Cabana, J., Monconduit, L., Larcher, D. & Palacin, M. R. Beyond Intercalation-Based Li-Ion Batteries: The State of the Art and Challenges of Electrode Materials Reacting Through Conversion Reactions. Adv. Mater. 22, E170–E192 (2010).
Zhang, H., Yu, X. & Braun, P. V. Three-dimensional bicontinuous ultrafast-charge and -discharge bulk battery electrodes. Nat. Nanotechnol. 6, 277–281 (2011).
Xiao, A., Yang, L., Lucht, B. L., Kang, S.-H. & Abraham, D. P. Examining the Solid Electrolyte Interphase on Binder-Free Graphite Electrodes. J. Electrochem. Soc. 156, A318–A327 (2009).
Ji, X. L. & Nazar, L. F. Advances in Li-S batteries. J Mater. Chem. 20, 9821–9826 (2010).
Demir-Cakan, R. et al. Li-S batteries: simple approaches for superior performance. Energy Environ. Sci. 6, 176–182 (2013).
Ji, X., Lee, K. T. & Nazar, L. F. A highly ordered nanostructured carbon-sulphur cathode for lithium-sulphur batteries. Nat. Mater. 8, 500–6 (2009).
Rauh, R. D., Abraham, K. M., Pearson, G. F., Surprenant, J. K. & Brummer, S. B. A Lithium/Dissolved Sulfur Battery with an Organic Electrolyte. J. Electrochem. Soc. 126, 523–527 (1979).
Yamin, H., Gorenshtein, A., Penciner, J., Sternberg, Y. & Peled, E. Lithium Sulfur Battery: Oxidation/Reduction Mechanisms of Polysulfides in THF Solutions. J. Electrochem. Soc. 135, 1045–1048 (1988).
Evers, S. & Nazar, L. F. New Approaches for High Energy Density Lithium–Sulfur Battery Cathodes. Acc. Chem. Res. 46, 1135–1143 (2012).
Manthiram, A., Fu, Y. & Su, Y.-S. Challenges and Prospects of Lithium–Sulfur Batteries. Acc. Chem. Res. 46, 1125–1134 (2012).
Mikhaylik, Y. V. & Akridge, J. R. Polysulfide Shuttle Study in the Li/S Battery System. J. Electrochem. Soc. 151, A1969–A1976 (2004).
Zhang, S. S. Improved Cyclability of Liquid Electrolyte Lithium/Sulfur Batteries by Optimizing Electrolyte/Sulfur Ratio. Energies 5, 5190–5197 (2012).
Zhang, S. S. Liquid electrolyte lithium/sulfur battery: Fundamental chemistry, problems and solutions. J. Power Sources 231, 153–162 (2013).
Zhang, S. S. & Read, J. A. A new direction for the performance improvement of rechargeable lithium/sulfur batteries. J. Power Sources 200, 77–82 (2012).
Zhang, S. S. & Tran, D. T. A proof-of-concept lithium/sulfur liquid battery with exceptionally high capacity density. J. Power Sources 211, 169–172 (2012).
Fan, F. Y. et al. Polysulfide Flow Batteries Enabled by Percolating Nanoscale Conductor Networks. Nano Lett. 14, 2210–2218 (2014).
Zhang, B., Qin, X., Li, G. R. & Gao, X. P. Enhancement of long stability of sulfur cathode by encapsulating sulfur into micropores of carbon spheres. Energy Environ. Sci. 3, 1531–1537 (2010).
Schuster, J. et al. Spherical Ordered Mesoporous Carbon Nanoparticles with High Porosity for Lithium–Sulfur Batteries. Angew. Chem. Int. Ed. 51, 3591–3595 (2012).
Jayaprakash, N., Shen, J., Moganty, S. S., Corona, A. & Archer, L. A. Porous Hollow Carbon@Sulfur Composites for High-Power Lithium–Sulfur Batteries. Angew. Chem. Int. Ed. 50, 5904–5908 (2011).
Guo, J., Xu, Y. & Wang, C. Sulfur-Impregnated Disordered Carbon Nanotubes Cathode for Lithium–Sulfur Batteries. Nano Lett. 11, 4288–4294 (2011).
Zheng, G., Yang, Y., Cha, J. J., Hong, S. S. & Cui, Y. Hollow Carbon Nanofiber-Encapsulated Sulfur Cathodes for High Specific Capacity Rechargeable Lithium Batteries. Nano Lett. 11, 4462–4467 (2011).
Li, N. et al. High-rate lithium-sulfur batteries promoted by reduced graphene oxide coating. Chem. Commun. 48, 4106–4108 (2012).
Ji, L. et al. Graphene Oxide as a Sulfur Immobilizer in High Performance Lithium/Sulfur Cells. J. Am. Chem. Soc. 133, 18522–18525 (2011).
Elazari, R., Salitra, G., Garsuch, A., Panchenko, A. & Aurbach, D. Sulfur-Impregnated Activated Carbon Fiber Cloth as a Binder-Free Cathode for Rechargeable Li-S Batteries. Adv. Mater. 23, 5641–5644 (2011).
Evers, S., Yim, T. & Nazar, L. F. Understanding the Nature of Absorption/Adsorption in Nanoporous Polysulfide Sorbents for the Li–S Battery. J. Phys. Chem. C 116, 19653–19658 (2012).
Hofmann, A. F., Fronczek, D. N. & Bessler, W. G. Mechanistic modeling of polysulfide shuttle and capacity loss in lithium–sulfur batteries. J. Power Sources 259, 300–310 (2014).
Yang, Y., Zheng, G. & Cui, Y. A membrane-free lithium/polysulfide semi-liquid battery for large-scale energy storage. Energy Environ. Sci. 6, 1552–1558 (2013).
Hodes, G., Manassen, J. & Cahen, D. Electrocatalytic Electrodes for the Polysulfide Redox System. J. Electrochem. Soc. 127, 544–549 (1980).
Hao, F. et al. High Electrocatalytic Activity of Vertically Aligned Single-Walled Carbon Nanotubes towards Sulfide Redox Shuttles. Sci. Rep. 2, 368; 10.1038/srep00368 (2012).
Hodes, G., Manassen, J. & Cahen, D. Photo-electrochemical energy conversion: electrocatalytic sulphur electrodes. J. Appl. Electrochem. 7, 181–182 (1977).
Lessner, P., McLarnon, F. R., Winnick, J. & Cairns, E. J. Kinetics of Aqueous Polysulfide Solutions: Part III. Investigation of Homogeneous and Electrode Kinetics by the Rotating Disk Method. J. Electrochem. Soc. 134, 2669–2677 (1987).
Esposito, D. V., Dobson, K. D., McCandless, B. E., Birkmire, R. W. & Chen, J. G. Comparative Study of Tungsten Monocarbide and Platinum as Counter Electrodes in Polysulfide-Based Photoelectrochemical Solar Cells. J. Electrochem. Soc. 156, B962–B969 (2009).
Stephens, I. E. L., Ducati, C. & Fray, D. J. Correlating Microstructure and Activity for Polysulfide Reduction and Oxidation at WS2 Electrocatalysts. J. Electrochem. Soc. 160, A757–A768 (2013).
Ge, S. H., Yi, B. L. & Zhang, H. M. Study of a high power density sodium polysulfide/bromine energy storage cell. J. Appl. Electrochem. 34, 181–185 (2004).
Zhao, P., Zhang, H., Zhou, H. & Yi, B. Nickel foam and carbon felt applications for sodium polysulfide/bromine redox flow battery electrodes. Electrochim. Acta 51, 1091–1098 (2005).
Lessner, P. M., McLarnon, F. R., Winnick, J. & Cairns, E. J. Aqueous polysulphide flow-through electrodes: Effects of electrocatalyst and electrolyte composition on performance. J. Appl. Electrochem. 22, 927–934 (1992).
Licht, S. Massachusetts Institute of Technology. Polysulfide battery. US 4828942. 1998 May 9.
Munaiah, Y., Suresh, S., Dheenadayalan, S., Pillai, V. K. & Ragupathy, P. Comparative Electrocatalytic Performance of Single-Walled and Multiwalled Carbon Nanotubes for Zinc Bromine Redox Flow Batteries. J. Phys. Chem. C 118, 14795–14804 (2014).
Reddy, A. L. M., Shaijumon, M., Rajalakshmi, N. & Ramaprabhu, S. Performance of Proton Exchange Membrane Fuel Cells Using Pt/MWNT-Pt/C Composites as Electrocatalysts for Oxygen Reduction Reaction in Proton Exchange Membrane Fuel Cells. J. Fuel Cell Sci. Technol. 7, 021001, 10.1115/1.3176215 (2009).
Gowda, S. R. et al. Three-Dimensionally Engineered Porous Silicon Electrodes for Li Ion Batteries. Nano Lett. 12, 6060–6065 (2012).
Pang, Q., Kundu, D., Cuisinier, M. & Nazar, L. F. Surface-enhanced redox chemistry of polysulphides on a metallic and polar host for lithium-sulphur batteries. Nat. Commun. 5, 4759, 10.1038/ncomms5759 (2014).
Rolison, D. R. Catalytic nanoarchitectures--the importance of nothing and the unimportance of periodicity. Science 299, 1698–701 (2003).
Acknowledgements
L.M.R.A. acknowledges the support from Wayne State University startup funds. We thank Dr. C.V. Rao for his useful discussions.
Author information
Authors and Affiliations
Contributions
L.M.R.A. and G.B. designed the research. G.B. and K.A. conducted the experiments. K.Y.S.N. contributed to the discussions of results. G.B. and L.M.R.A. wrote the first draft of the manuscript and all authors participated in manuscript revision.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary Information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Babu, G., Ababtain, K., Ng, K. et al. Electrocatalysis of Lithium Polysulfides: Current Collectors as Electrodes in Li/S Battery Configuration. Sci Rep 5, 8763 (2015). https://doi.org/10.1038/srep08763
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep08763
This article is cited by
-
Post lithium-sulfur battery era: challenges and opportunities towards practical application
Science China Chemistry (2024)
-
Li-S Batteries: Challenges, Achievements and Opportunities
Electrochemical Energy Reviews (2023)
-
Space-confined synthesis of CoSe2-NC nanoclusters anchored on honeycomb-like carbon framework towards high-performance lithium sulfur battery
Ionics (2023)
-
Optimization on transport of charge carriers in cathode of sulfide electrolyte-based solid-state lithium-sulfur batteries
Nano Research (2023)
-
A novel modified sulfur cathode to facilitate the adsorption and conversion of polysulfides in lithium–sulfur batteries
Journal of Solid State Electrochemistry (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.