Abstract
The nano-Bi2WO6/reduced graphene oxide composite obtained by a simple hydrothermal reaction demonstrates a larger specific capacitance of 922 F/g at a charge and discharge currents of 3 A/g with longer cycle life. The As comparison, pristine Bi2WO6 nanoparticles have poor specific capacitance of 574 F/g at a charge and discharge currents of 2 A/g with weak cycle life. Though analyzing the Cyclic voltammetry curves, it is found that there are two oxidation reaction occurring in the materials: oxidation of Bi (III) to Bi (IV) and Bi (III) to Bi (V). The oxidation of Bi (III) to Bi (IV) is reversible while Bi (III) to Bi (V) will cause nonreversible destroy on structure. In this nano-Bi2WO6/reduced graphene oxide composite, graphene with well conductivity will enhance the electrically conducting as charge transfer channel, so that electrons will be transfer much faster in oxidation and most Bi (III) is oxidized to be Bi (IV) which ensure larger specific capacitance and long cycle life. This nano-Bi2WO6/reduced graphene oxide composite has application prospect in high-performance pseudo-capacitors.
Similar content being viewed by others
Introduction
Energy storage and conversion from semiconductor and alternative energy sources have stimulated a large of research due to the urgency demand for energy. Photovoltaic cells, batteries, fuel cells and supercapacitors are mainly Energy storage and conversion devises1,2,3,4,5. Supercapacitors are considered as a ideal short-term energy storage due to fast charge-discharge, long cycle life, high power performance and low maintenance cost6,7,8,9,10,11. For using as primary power sources as batteries, it is highly desirable to increase the energy density. The energy density of traditional double layer capacitors can hardly increase. Some redox during charge-discharge of pseudo-capacitive materials such as hydroxides12,13,14, oxides15,16,17,18,19,20,21 and polymers22,23,24 can sharply increase the specific capacitance and high energy density. However, the electrical conductivity this pseudo-capacitive materials are too low to support high rate electron transport.
Graphene is a two-dimensional material with an ideal single-atom-thick or several layers of limited structure and it has attracted much attention recently due to its many potential applications in electronic, optical, catalytic and energy fields25,26,27,28. Due to the high surface area, good electrical conductivity, light weight, high flexibility and mechanical strength, graphene is a ideal substrate as charge transfer channel for photoelectrochemistry29,30,31, or energy storage32,33,34,35,36. Pt32, metal oxides33,34,35 and polymers36 were coupled with reduced graphene oxide or highly conducting graphene sheets to improve performance applied in Li-ion battery and supercapacitor. Bi2WO6 is a kind of Aurivillius phase oxide with a layered structure of the perovskite layer (WO4)2− lies between (Bi2O2)2+ layers37. Bi2WO6 has excellent physical and chemical properties such as oxide anion conducting, a nonlinear dielectric susceptibility, ferroelectric piezoelectricity, pyroelectricity, catalytic behavior and luminescent properties38,39,40,41. Bi2WO6-grphene has been prepared and the photocatalytic performance was shown42,43,44. However, Bi2WO6-grphene as pseudocapacitor electrodes has not been reported.
In this work, we reported a simple method to obtain nano-Bi2WO6-graphene composite which demonstrate larger specific capacitance, higher energy density and longer cycle life. As comparison, plain nano-Bi2WO6 has poor specific capacitance and weak cycle life due to the low electric conduction resulted in most Bi (III) will be over oxidized to be Bi (V) which causes nonreversible destroy on structure. In this nano-Bi2WO6-graphene, graphene will render the electrically conducting as charge transfer channel. In this case, Bi (III) is more inclined to be Bi (IV) in charging instead of Bi (V) which ensure the material has long cycle life.
Results
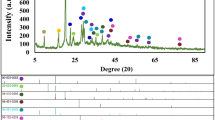
The BWO was obtained by a hydrothermal route while the BWO/RGO was synthesized via a two-step method with two hydrothermal progress. The crystal structure of BWO and BWO/RGO were studied by XRD analysis in Figure 1. In the patterns, all peaks demonstrate sharp and well defined shape corresponding to the Bi2WO6 phase with orthorhombic russellite structure. The lattice parameter values deduced from the XRD peaks are in concordant with the standard JCPDS data file no 73-2020. No other matter is found confirmed the hydrothermal reaction occurred completely. Moreover, except there is little enhancement of crystallinity (about 10%), two XRD patterns of the BWO and BWO/RGO are almost the same which indicates that although under an extra hydrothermal progress, the BWO nanoparticles in the BWO/RGO composites have less change compared with pristine BWO. It seems that when exceeding 24 h, extra time for hydrothermal reaction has little influence on the structure of BWO.
The TEM and HRTEM images of BWO and BWO/RGO are shown in Figure 2. The typical morphology of the BWO is found to be spherical in shape and uniform size distribution of nanoparticles (Figure 2A). The maximum number of BWO particles are found to be around 20 nm. The uniform BWO nanoparticles diffuse well on the graphene (Figure 2B and C) which can lead to a well contact between BWO and graphene. The results reveal that addition of graphene would not change the structure and morphology of the BWO nanoparticles. In the high-resolution image (Figure 2D), the lattice spacing of 0.315 nm can corresponding to the (113) plane of Bi2WO6.
As compared, another composite was obtained by a hydrothermal reaction between reduced graphene oxide (RGO) and BWO nanoparticles. As Figure S1 shown, little BWO nanoparticles composited on the surface of graphene. It seems the composite progress and the contact between BWO and graphene were promoted by the reduction of GO during the hydrothermal reaction. Raman spectra can further confirm the reduction of GO. Figure S2 illustrates Raman spectra of the GO synthesized chemical exfoliation and RGO from hydrothermal treatment. It is known that there are two Raman peaks can be observed from GO and RGO, that is, G peak at 1570 cm−1, D peak at 1360 cm−1. In general, the value of Id/Ig ratio reflects the surface defects density of graphene, which also give an information about the degree of reduction. From Figure S2, we can clearly see that more surface defects exist in the GO (Id/Ig = 1.08) than RGO (Id/Ig = 0.91). The results could confirm that the GO was reduced greatly after hydrothermal treatment.
Cyclic voltammetry (CV) is the most important measurement in electrochemistry which provides the qualitative information regarding the electrochemical processes that takes place in the material. The CV curves of the BWO at various scan rates are shown in Figure 3A. There is a pair of redox peaks observed for Bi2WO6 in the curves which indicates the pseudo capacitive behavior and these redox peaks are due to the oxidation and reduction reactions occurred in the material. The background signal due to the Ni foam was negligible14. The average specific capacitance of the BWO was calculated to be 632 F/g (based on mass of BWO) at a scan rate of 5 mV/s which is similar with other reports (Figure 3B)45 and 302 F/g at a high scan rate of 100 mV/s, about 48% of that at 5 mV/s. Figure 3C shows galvanostatic discharge curves of the BWO at various current densities. The BWO showed a specific capacitance of 574 F/g (based on mass of BWO) at a charge and discharge current density of 2 A/g (Figure 3D). When the charge and discharge current density was raised to be 20 A/g, the specific capacitance was only 332 F/g, about 58% of that at 2 A/g. Moreover, the columbic efficiency fell obviously when the cycle index of charge and discharge exceeded about 500 and was only 50% when the cycle index reach 1000. The results indicate that BWO should be improved for applying in supercapacitor.
Electrochemical characterizations of BWO: (A) CV curves of BWO at various scan rates, (B) average specific capacitance of BWO at various scan rates; (C) galvanostatic discharge curves of BWO at various discharge current densities, (D) average specific capacitance of BWO at various discharge current densities; (E) average specific capacitance versus cycle number of BWO at a galvanostatic charge and discharge current density of 10 A/g.
The BWO/RGO composites were carried out similar electrochemical measurements. In the CV curves (Figure 4A), the average specific capacitance of the BWO/RGO was improved to be 932 F/g (based on mass of BWO/RGO) at a scan rate of 5 mV/s (Figure 4B), even 630 F/g at a high scan rate of 100 mV/s, about 68% of that at 5 mV/s. As graphene has high surface area and good double layer capacitance46,47, the contribution of RGO to the total capacitance can not be ignored. The CV curves of RGO are shown as Figure S3. The double layer capacitance (about 150 F/g) of RGO was almost equal to the above one. Combining the CV curves of RGO/BWO, the contribution of RGO to the total capacitance could be calculated to be about 128 F/g. In the galvanostatic discharge curves of the BWO/RGO at various current densities in Figure 4C, the BWO/RGO showed a specific capacitance of 922 F/g (based on mass of BWO/RGO) at a charge and discharge current density of 3 A/g (Figure 4D). When the charge and discharge current density was raised to be 40 A/g, the specific capacitance was 663 F/g, about 72% of that at 3 A/g. Importantly, the columbic efficiency was nearly 100% for each cycle of charge and discharge (Figure 4E). There was no obvious capacitance decrease observed over 2000 cycles of charge and discharge at a current density of 10 A/g (Figure 4F). The results reveal the high specific capacitance and remarkable rate capability of the nano-Bi2WO6/reduced graphene oxide composite material for high-performance pseudo-capacitors.
Electrochemical characterizations of BWO/RGO: (A) CV curves of BWO/RGO at various scan rates, (B) average specific capacitance of BWO/RGO at various scan rates; (C) galvanostatic discharge curves of BWO/RGO at various discharge current densities, (D) average specific capacitance of BWO/RGO at various discharge current densities; (E) galvanostatic charge and discharge curves of BWO/RGO at a current density of 10 A/g, (F) average specific capacitance versus cycle number of BWO/RGO at a galvanostatic charge and discharge current density of 10 A/g.
The BWO/RGO exhibits excellent electrochemical characteristics and high cycling stability, making that potentially useful for high performance supercapacitor material. In same CV or galvanostatic method in three-electrode systems, the BWO/RGO composites show higher stable specific capacitance at higher charge/discharge rates than pristine BWO nanoparticles. There are several features lead to higher capacity and faster energy storage and releasing of BWO/RGO. First, the BWO nanoparticles are directly grown and anchored on reduced graphene oxide sheets after the hydrothermal reaction. The interactions between BWO nanoparticles and RGO could be both covalent chemical bonding and van der Waals interactions. This intimate binding offers facile electron transport between individual nanoparticles and RGO. Although BWO nanoparticles are electrically insulating, the RGO is well conductor and can transfer the electrons of BWO in redox which is key to both high specific capacitance and rate capability of the BWO/RGO. Most of the BWO nanoparticles in the system are electrochemically active through the graphene network. Rapid charge transport from BWO nanoparticles to the underlying RGO provides fast redox reactions. The better conductivity of BWO/RGO can be confirmed by the Electrochemical impedance spectroscopy (EIS) (Figure 5). In the Nynquist plots of EIS, the BWO/RGO electrode has a smaller frequency semicircle compared with BWO electrode which meanings the resistance of BWO/RGO electrode is lower.
The decline of columbic efficiency for BWO in the cycle of charge and discharge can be attributed to the irreversible oxidation in the redox reactions. A comparison of CV curves for BWO and BWO/RGO is shown in Figure 6. There are two oxidation peaks due to the oxidation of Bi (III) to Bi (IV) (labeled as P1) and Bi (III) to Bi (V) (labeled as P2) respectively, while only one reduction peak due to the reduction of Bi (IV) to Bi (III) (labeled as P1'). So that the oxidation of Bi (III) to Bi (IV) is reversible while oxidation of Bi (III) to Bi (V) is irreversible which results in the decline of columbic efficiency in the cycle of charge and discharge. The intensity of P2 peak of BWO and BWO/RGO are similar while the intensity of P1 peak of BWO/RGO is higher than that of BWO. Compared with BWO, the intensity ratio of P1/P2 of BWO/RGO is much higher. The result illustrates that in BWO/RGO, more Bi (III) is oxidized to Bi(IV) leads to a much high cycling stability. When graphene is absent, in oxidizing reaction, electrons are likely to gather in BWO nanoparticles due to the weak electrical conductivity which causes over oxidation of Bi (III) to Bi (V). As RGO is well conductor, the electrons will be transfer timely by the underlying graphene with less aggregation. To study the chemical state and structure changes after electrochemistry tests, the pristine BWO and cyclic BWO after 1000 cycles were detected by XRD and XPS (Figure 7). After 1000 cycles. the crystalline of cyclic BWO became weak means there is some damage on the crystal structure (Figure 7A). In the XPS spectra of BWO (Figure 7B), the binding energy at 158.8 eV and 164.1 eV belong to the Bi (III) 4f7/2 and 4f5/2 states48. The two peaks of Bi show a shift of about 0.5 eV toward higher banding energy. As higher binding energy meanings higher valence state and combining with the P2 peak in CV curve (Figure 6), it can be confirmed that during electrochemistry progress, more Bi (III) is over-oxidized to Bi(V). Taken together, it is high important to use high electrical conductivity graphene as grown substrate to produce advanced nanocrystal/graphene composite materials for energy application.
Discussion
In this work, we reported a simple method to obtain nano-Bi2WO6/reduced graphene oxide composite which demonstrate larger specific capacitance, higher energy density and longer cycle life. As comparison, pristine Bi2WO6 nanoparticles have poor specific capacitance and weak cycle life due to the low electric conduction and over oxidation of Bi (III) to Bi (V) which causes nonreversible destroy on structure. In this nano-Bi2WO6/reduced graphene oxide composite, graphene with well conductivity will enhance the electrically conducting as charge transfer channel. In this composite, electrons will be transfer timely in oxidation and most Bi (III) is oxidized to be Bi (IV) which ensure long cycle life.
Methods
Synthesis of Bi2WO6 nanoparticles
The Bi2WO6 (BWO) nanoparticles were prepared via a solvothermal method. A typical process is as following: 0.97 g Bi(NO3)3·5H2O and 0.33 g Na2WO4·2H2O were dissolved in 40 ml ethylene alcohol, after being stirred for 6 h, the transparent solution was added into a 50 ml Teflon-lined autoclave up to 80% of the total volume. Then the autoclave was sealed in a stainless steel tank and heated at 180°C for 24 h, in which the heating rate is 1°C/min. Subsequently, the reactor was cooled to room temperature naturally, the products were washed by de-ionized water and alcohol in turn and then the BWO nanoparticles were obtained after being dried at 80°C in air.
Synthesis of nano-Bi2WO6/reduced graphene oxide composites
Graphene oxide (GO) was prepared from natural graphite (99.95, 32 um) by a modified Hummers method14. 5 mg of GO, 95 mg of BWO nanoparticles and 30 ml of distilled water were mixed in a 50 ml Teflon-lined autoclave and then followed by hydrothermal treatment of the mixture at 180°C for 24 hours. After the reaction, the products were harvested by centrifugation and washed with distilled water and ethanol for three times and then dried in an oven at 80°C for 3 hours. The obtained Bi2WO6/reduced graphene oxide composite is labeled as BWO/RGO. As compared, reduced graphene oxide (RGO) was obtained under a similar hydrothermal progress without adding of BWO.
Characterization
The crystal structures of the samples were characterized by using an X-ray diffractometer (XRD) (AXS D8 Advanced XRD, Germany) with Cu-Ka radiation. The microstructures were observed by using a high-resolution transmission electron microscope (HRTEM) (JEOL JEM 2010, Japan). X-ray photoelectron spectroscopy (XPS) measurements were made on a Kratos AXIS Ultra DLD (Kratos, Japan) spectrometer with a charge neutralizer to gain information on the chemical binding energy of the samples. The C 1s peak at 284.6 eV of the adventitious carbon was referenced to rectify the binding energies. Raman measurement was carried out using a Raman spectroscopy (HORIBA Jobin Yvon LabRAM HR, France). The power of the laser was 10 mW and the laser excitation was 488 nm. Scans were taken on an extended range (100–3000 cm−1) and the exposure time was 2 s.
The fabrication and electrochemical test of the materials
The working electrode was prepared by coating the active material onto nickel foam (1 cm × 1 cm) followed by pressing with a pressure of 0.5 tons. While the active material was the mixture of BWO or BWO/RGO, carbon black and PDFE binder (ratio 7:2:1). Before the coating, the mixture was ground in a mortar using a pestle and the Grinding time was one hour. Then, the samples were uniformly distributed upon the Ni foam and allowed to dry in a vacuum oven for 2 hours. The end loading of active material for each electrode was 3 ~ 5 mg cm−2. At last, all of these electrodes were studied by Cyclic voltammetry (CV), Galvanostatic discharge and Electrochemical impedance spectroscopy (EIS) measurements on a commercial electrochemical workstation with three electrode system. Electrodes made from carbon materials worked as the working electrode, Ag/AgCl electrode as the reference electrode and platinum network electrode as the counter electrode, the electrolyte is the 6 M KOH solution.
References
Winter, M. & Brodd, R. J. What are batteries, fuel cells and supercapacitors? Chem. Rev. 104, 4245–4270 (2004).
Aricò, A. S., Bruce, P., Scrosati, B., Tarascon, J. M. & Van Schalkwijk, W. Nanostructured materials for advanced energy conversion and storage devices. Nat. Mater. 4, 366–377 (2005).
Peng, Z., Liu, Y., Chen, K., Yang, G. & Chen, W. Fabrication of the protonated pentatitanate nanobelts sensitized with CuInS2 quantum dots for photovoltaic applications. Chemi. Eng. J. 244, 335–342 (2014).
Peng, Z. et al. Efficiency enhancement of TiO2 nanodendrite array electrodes in CuInS2 quantum dot sensitized solar cells. Electrochim. Acta 111, 755–761 (2013).
Han, W. et al. Self-assembled three-dimensional graphene-based aerogel with embedded multifarious functional nanoparticles and its excellent photoelectrochemical activities. ACS Sustain. Chem. Eng. 2, 741–748 (2014).
Simon, P. & Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 7, 845–854 (2008).
De Souza, V. H. R., Oliveira, M. M. & Zarbin, A. J. G. Thin and flexible all-solid supercapacitor prepared from novel single wall carbon nanotubes/polyaniline thin films obtained in liquid-liquid interfaces. J. Power Sources 260, 34–42 (2014).
Li, Z. et al. Colossal pseudocapacitance in a high functionality-high surface area carbon anode doubles the energy of an asymmetric supercapacitor. Energy Environ. Sci. 7, 1708–1718 (2014).
Chen, X., Wang, H., Yi, H., Wang, X. & Guo, Z. Anthraquinone on Porous Carbon Nanotubes with Improved Supercapacitor Performance. J. Phys. Chem. C 118, 8262–8270 (2014).
Liu, X. et al. One-step electrochemical deposition of nickel sulfide/graphene and its use for supercapacitors. Ceram. Int. 40, 8189–8193 (2014).
Lu, F. et al. Electrochemical properties of high-power supercapacitors using ordered NiO coated Si nanowire array electrodes. Appl. Phys. A-Mater. Sci. Process. 104, 545–550 (2011).
Kong, L. B., Lang, J. W., Liu, M., Luo, Y. C. & Kang, L. Facile approach to prepare loose-packed cobalt hydroxide nano-flakes materials for electrochemical capacitors. J. Power Sources 194, 1194–1201 (2009).
Patil, U., Gurav, K., Fulari, V., Lokhande, C. & Joo, O. S. Characterization of honeycomb-like “β-Ni(OH)2” thin films synthesized by chemical bath deposition method and their supercapacitor application. J. Power Sources 188, 338–342 (2009).
Wang, H., Casalongue, H. S., Liang, Y. & Dai, H. Ni(OH)2 nanoplates grown on graphene as advanced electrochemical pseudocapacitor materials. J. Am. Chem. Soc. 132, 7472–7477 (2010).
Yuan, C., Zhang, X., Su, L., Gao, B. & Shen, L. Facile synthesis and self-assembly of hierarchical porous NiO nano/micro spherical superstructures for high performance supercapacitors. J. Mater. Chem. 19, 5772–5777 (2009).
Chen, S. et al. Shape-controlled synthesis of one-dimensional MnO2 via a facile quick-precipitation procedure and its electrochemical properties. Cryst. Growth & Des. 9, 4356–4361 (2009).
Wang, Y. & Zhitomirsky, I. Electrophoretic deposition of manganese dioxide− multiwalled carbon nanotube composites for electrochemical supercapacitors. Langmuir 25, 9684–9689 (2009).
Ko, J. M. & Kim, K. M. Electrochemical properties of MnO2/activated carbon nanotube composite as an electrode material for supercapacitor. Mater. Chem. Phys. 114, 837–841 (2009).
An, G. et al. Low-temperature synthesis of Mn3O4 nanoparticles loaded on multi-walled carbon nanotubes and their application in electrochemical capacitors. Nanotechnology 19, 275709 (2008).
Bi, R. R. et al. Highly dispersed RuO2 nanoparticles on carbon nanotubes: facile synthesis and enhanced supercapacitance performance. J. Phys. Chem. C 114, 2448–2451 (2010).
Wang, J. G., Yang, Y., Huang, Z. H. & Kang, F. Effect of temperature on the pseudo-capacitive behavior of freestanding MnO2@ carbon nanofibers composites electrodes in mild electrolyte. J. Power Sources 224, 86–92 (2013).
Fang, Y. et al. Self-supported supercapacitor membranes: polypyrrole-coated carbon nanotube networks enabled by pulsed electrodeposition. J. Power Sources 195, 674–679 (2010).
Roberts, M. E., Wheeler, D. R., McKenzie, B. B. & Bunker, B. C. High specific capacitance conducting polymer supercapacitor electrodes based on poly (tris (thiophenylphenyl) amine). J. Mater. Chem. 19, 6977–6979 (2009).
Zhang, K., Zhang, L. L., Zhao, X. & Wu, J. Graphene/polyaniline nanofiber composites as supercapacitor electrodes. Chem. Mater. 22, 1392–1401 (2010).
Bao, Q. et al. Broadband graphene polarizer. Nat. Photonics 5, 411–415 (2011).
Zhang, H. et al. Z-scan measurement of the nonlinear refractive index of graphene. Optics letters 37, 1856–1858 (2012).
Zheng, Z. et al. Microwave and optical saturable absorption in graphene. Opt. Express 20, 23201–23214 (2012).
Niu, Z. et al. A Universal Strategy to Prepare Functional Porous Graphene Hybrid Architectures. Adv. Mater. 26, 3681–3687 (2014).
Qi, X. et al. Ultraviolet, visible and near infrared photoresponse properties of solution processed graphene oxide. Appl. Surf. Sci. 266, 332–336 (2013).
Ren, L. et al. Upconversion-P25-graphene composite as an advanced sunlight driven photocatalytic hybrid material. J. Mater. Chem. 22, 11765–11771 (2012).
Han, W. et al. Synthesis of CdS/ZnO/graphene composite with high-efficiency photoelectrochemical activities under solar radiation. Appl. Surf. Sci. 299, 12–18 (2014).
Yoo, E. et al. Enhanced electrocatalytic activity of Pt subnanoclusters on graphene nanosheet surface. Nano Lett. 9, 2255–2259 (2009).
Wang, D. et al. Self-assembled TiO2–graphene hybrid nanostructures for enhanced Li-ion insertion. ACS Nano 3, 907–914 (2009).
Wang, H., Robinson, J. T., Diankov, G. & Dai, H. Nanocrystal growth on graphene with various degrees of oxidation. J. Am. Chem. Soc. 132, 3270–3271 (2010).
Wu, C. et al. Preparation of Novel Three-Dimensional NiO/Ultrathin Derived Graphene Hybrid for Supercapacitor Applications. ACS Appl. Mater. Interfaces 6, 1106–1112 (2014).
Kulkarni, S. B. et al. High-performance supercapacitor electrode based on a polyaniline nanofibers/3D graphene framework as an efficient charge transporter. J. Mater. Chem. A 2, 4989–4998 (2014).
Cao, X. F., Zhang, L., Chen, X. T. & Xue, Z. L. Microwave-assisted solution-phase preparation of flower-like Bi2WO6 and its visible-light-driven photocatalytic properties. CrystEngComm 13, 306–311 (2011).
McDowell, N. A., Knight, K. S. & Lightfoot, P. Unusual High-Temperature Structural Behaviour in Ferroelectric Bi2WO6 . Chem.-Eur. J. 12, 1493–1499 (2006).
Zhang, L., Ding, X. & Zhao, K. Enhanced Photocatalytic Removal of Sodium Pentachlorophenate with Self doped Bi2WO6 under Visible Light by Generating More Superoxide Ions. Environ. Sci. Technol. 48, 5823–5831 (2014).
Wang, D. et al. AgBr Quantum Dots Decorated Mesoporous Bi2WO6 Architectures with Enhanced Photocatalytic Activities for Methylene Blue. J. Mater. Chem. A 2, 11716–11727 (2014).
Zhang, Y., Fei, L., Jiang, X., Pan, C. & Wang, Y. Engineering nanostructured Bi2WO6–TiO2 toward effective utilization of natural light in photocatalysis. J. Am. Ceram. Soc. 94, 4157–4161 (2011).
Gao, E. P., Wang, W. Z., Shang, M. & Xu, J. H. Synthesis and enhanced photocatalytic performance of graphene-Bi2WO6 composite. Phys. Chem. Chem. Phys. 13, 2887–2893 (2011).
Ma, H. W. et al. Significant enhanced performance for Rhodamine B, phenol and Cr(VI) removal by Bi2WO6 nancomposites via reduced graphene oxide modification. Appl. Catal. B-Environ. 121, 198–205 (2012).
Sun, Z. H. et al. A high-performance Bi2WO6-graphene photocatalyst for visible light-induced H-2 and O-2 generation. Nanoscale 6, 2186–2193 (2014).
Nithya, V. D., Selvan, R. K., Kalpana, D., Vasylechko, L. & Sanjeeviraja, C. Synthesis of Bi2WO6 nanoparticles and its electrochemical properties in different electrolytes for pseudocapacitor electrodes. Electrochim. Acta 109, 720–731 (2013).
Zhu, Y. et al. Carbon-based supercapacitors produced by activation of graphene. Science 332, 1537–1541 (2011).
Chen, Z. et al. Three-dimensional flexible and conductive interconnected graphene networks grown by chemical vapour deposition. Nat. Mat. 10, 424–428 (2011).
Li, C. et al. Ultrathin nanoflakes constructed erythrocyte-like Bi2WO6 hierarchical architecture via anionic self-regulation strategy for improving photocatalytic activity and gas-sensing property. Appl. Catal. B-Environ. 163, 415–423 (2015).
Acknowledgements
This work was supported by the Chinese National Natural Science Foundation (No. 61072015), Zhejiang Provincial Natural Science Foundation of China (No. ZZ4110503, LQ12E02002 and LY13F04006). Educational Commission of Zhejiang Province (No. Y201121341).
Author information
Authors and Affiliations
Contributions
J.Z. proposed and designed the experiments. P.L., G.X., Z.L., X.W.,Y.W., L.Y. and X.T. carried out the experiments (synthesis and photocatalytic tests) and characterizations. J.Z. wrote the paper. Y.Z., H.W., E.Z. and Z.J. provide scientific advice. J.X. conducted the SEM and HRTEM measurements, respectively. All authors read and approved the final manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supporting Information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Zhang, J., Liu, P., Zhang, Y. et al. Enhanced Performance of nano-Bi2WO6-Graphene as Pseudocapacitor Electrodes by Charge Transfer Channel. Sci Rep 5, 8624 (2015). https://doi.org/10.1038/srep08624
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep08624
This article is cited by
-
Light-Dependent A.C. Transport Properties of Zinc Oxide (ZnO)/Reduced Graphene Oxide (rGO) Heterostructure Device: A Signature of Electrical Memory
Journal of Electronic Materials (2023)
-
Novel aqueous rechargeable nickel//bismuth battery based on highly porous Bi2WO6 and Co0.5Ni0.5MoO4 microspheres
Rare Metals (2023)
-
Cu doped ZnO hierarchical nanostructures: morphological evolution and photocatalytic property
Journal of Materials Science: Materials in Electronics (2019)
-
Improving Photocatalytic Performance from Bi2WO6@MoS2/graphene Hybrids via Gradual Charge Transferred Pathway
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.