Abstract
Noninvasive glucose detections are convenient techniques for the diagnosis of diabetes mellitus, which require high performance glucose sensors. However, conventional electrochemical glucose sensors are not sensitive enough for these applications. Here, highly sensitive glucose sensors are successfully realized based on whole-graphene solution-gated transistors with the graphene gate electrodes modified with an enzyme glucose oxidase. The sensitivity of the devices is dramatically improved by co-modifying the graphene gates with Pt nanoparticles due to the enhanced electrocatalytic activity of the electrodes. The sensing mechanism is attributed to the reaction of H2O2 generated by the oxidation of glucose near the gate. The optimized glucose sensors show the detection limits down to 0.5 μM and good selectivity, which are sensitive enough for non-invasive glucose detections in body fluids. The devices show the transconductances two orders of magnitude higher than that of a conventional silicon field effect transistor, which is the main reason for their high sensitivity. Moreover, the devices can be conveniently fabricated with low cost. Therefore, the whole-graphene solution-gated transistors are a high-performance sensing platform for not only glucose detections but also many other types of biosensors that may find practical applications in the near future.
Similar content being viewed by others
Introduction
Glucose plays a key role in human metabolism processes and abnormal glucose levels in blood or body fluids are directly related to some diseases, such as diabetes mellitus1. Therefore, high-performance glucose sensors have promising applications in medical diagnosis and healthcare products. Various types of glucose sensors including optical and electrochemical sensors have been investigated, in which the electrochemical sensors can be more conveniently used in practical applications. Many electrochemical glucose sensors have been successfully developed by using different functional materials, including carbon-based nanomaterials (e.g. carbon nanotubes, graphene), metal nanoparticles and oxides2,3,4,5. Graphene has been successfully used in glucose sensors for its remarkable physical properties, such as high conductivity, unique electrochemical activity and chemical stability6,7,8. The detection limits of the glucose sensors can be down to several μM, which are low enough for non-invasive glucose detections in body fluids9.
Among graphene-based biosensors7,10, solution-gated graphene transistors (SGGT) have drawn much attention because they are suitable for real-time, highly sensitive and high-throughput detections11. SGGTs could operate in aqueous solutions at low voltages (less than 1 V), which is essential to biological sensing. Furthermore, SGGT-based sensors can exhibit higher sensitivity than conventional electrochemical methods because of the inherent amplification function of the transistors11. They have been widely used in the detection of pH, ion12,13,14, glucose15,16, DNA17,18,19, dopamine20, cells21,22, etc. Regarding the sensing mechanism, the sensors are generally based on the interactions between analytes and their channels or gates11,15,16,17,18,19. So SGGTs with functionalized gate electrodes are good candidates for high-performance biosensors, such as a dopamine sensor with a low detection limit (~1 nM) and good selectivity20. On the other hand, considering that high-quality large-area graphene can be prepared by chemical vapor deposition (CVD) methods, it is convenient to fabricate high-performance SGGT-based chemical and biological sensors that can be used in clinical diagnosis11, environmental monitoring and food safety tests23, etc.
In 2010, Huang et al. firstly reported glucose sensors based on SGGTs by modifying the graphene layers with an enzyme glucose oxidase (GOx)15. The devices can detect the glucose levels down to 0.1 mM. They considered that the products from the oxidative reaction of glucose were responsible for the increase of channel current while the detailed mechanism was not investigated. In 2012, Kwak et al. reported a similar SGGT glucose sensor with GOx immobilized on its graphene channel16. The response of the device was assumed to be related to H2O2 generated in the oxidation of glucose catalyzed by GOx1. However, the actual interaction between H2O2 and graphene is unclear. The device could detect glucose levels in the range of 3.3–10.9 mM, which was not sensitive in comparison with other types of glucose sensors24. It is noteworthy that many transistor-based glucose sensors can detect glucose down to μM levels25. Therefore the mechanisms of the SGGT-based glucose sensors should be further studied and novel device designs need to be explored to achieve better sensing performance.
In this paper, we demonstrate a new type of glucose sensors based on SGGTs, in which both channel and gate are made of CVD grown graphene. Graphene is a semi-metal and can be used as not only semiconductor channels but also gate electrodes of SGGTs. So the devices can be regarded as whole-graphene SGGTs. The graphene gate electrodes are modified with the enzyme GOx and biocompatible polymers including chitosan (CHIT) and Nafion26,27. To improve their electrocatalytic activity, the graphene gates are also modified with Pt nanoparticles (PtNPs) by electrochemical deposition. The optimized SGGT glucose sensors show the detection limits down to 0.5 μM and thus have great potential for non-invasive glucose detections in human body fluids, such as saliva28. The sensing mechanism is attributed to the GOx-catalyzed oxidation of glucose that generates H2O2 near the gate electrodes and the oxidation of H2O2 that modulates the effective gate voltages applied on the transistors. Being potentiometric transducers, SGGT-based sensors have sensitivity dependent on their transconductances29. We find that the whole-graphene SGGTs have much higher transconductances (~several mS) than that of conventional transistors, such as Si-based field effect transistors. Therefore, these type of devices are a highly sensitive platform for not only glucose detections but also many other types of sensing applications.
Results
Device design and sensing mechanism
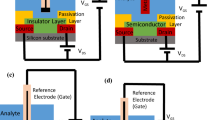
Figure 1 shows the schematic diagram of the device structure and the sensing mechanism of our SGGT-based sensors. The devices were fabricated on glass substrates and characterized in a PBS solution. CVD Graphene was prepared on Cu foils and transferred on the substrates. The graphene film is mainly single-layer, which is confirmed by the Ramen spectrum that shows the 2D peak with the height about two times of that of the G peak (See supporting information, Figure S1)30. As shown in Figure 1b, the gate voltage (VG) is actually applied on the two electric double layers (EDLs) since the electrolyte is ion conductive and VG = VEDL1 + VEDL2, where VEDL1 and VEDL2 are the potential drops across the EDLs on the channel and gate, respectively. So the device performance is sensitive to the changes of potential drops induced by analytes in sensing applications. As shown in Fig. 1c, the graphene gate electrode of our SGGT was modified with GOx on the surface, which can catalyze the oxidation of glucose in the PBS solution and generate H2O2 near the gate electrode in the following reaction24:

(a) Schematic diagram of the glucose sensor based on a whole-graphene SGGT; (b) Potential drops across the two electric double layers (EDLs) on the surfaces of graphene channel and gate. (c) The GOx-catalyzed oxidation of glucose and the oxidation of H2O2 cycles on the GOx-CHIT/Nafion/PtNPs/graphene gate electrode of a SGGT.
It is notable that the enzymatically produced H2O2 will be oxidized on the surface of graphene under a bias voltage24:

In this reaction, the electrons (2e−) will be transferred to the gate electrode, which will change the potential distribution in the device as indicated by the dash line in Figure 1b. For a potentiometric transducer, although the redox current is very low, the reaction of H2O2 will change the potential drop on the gate electrode according to the Nernst equation24:

where k is the Boltzmann constant; T is the temperature; q is electronic charge; [H2O2] is the concentration of H2O2 and C is a constant. Consequently, the voltage applied on the channel surface VEDL1 can be modulated by the reaction of H2O2 and thus

It is notable that the channel current IDS is dependent on the voltage VEDL1 applied on the surface of the channel due to the field effect. Therefore the channel current is sensitive to the concentration of H2O2. Consequently, the channel current can also indicate the glucose level in glucose sensing because the concentration of H2O2 increases with the increase of the glucose level.
H2O2 detections by SGGTs
Since our glucose sensors are based on the detections of H2O2, the first step is to fabricate highly sensitive H2O2 sensors based on SGGTs1,23. We noticed that the electrocatalytic activity of graphene to H2O2 can be enhanced by modifying PtNPs on its surface31. So PtNPs were deposited on CVD graphene films by an electrochemical method and the deposition time was optimized in the experiments. Figure 2a and 2b shows the AFM images of the CVD graphene films with and without PtNPs on the surfaces. The average size of the PtNPs is about 30 nm. Figure 2c and 2d show the cyclic voltammetry (CV) curves of a graphene electrode before and after the modification of PtNPs on its surface. It is notable that the electrocatalytic activity of the graphene electrode to H2O2 is dramatically improved by the PtNPs since the redox current is increased for several hundred times after the modification.
Figure 3a shows the channel current (IDS) of a SGGT without PtNPs on the graphene gate measured at VG = 0.7 V and VDS = 0.05 V. The channel current increases with the additions of H2O2 and shows the detection limit of about 10 μM. The inset of Figure 3a shows the transfer curve of the SGGT characterized in the PBS solution, which exhibits a reasonable ambipolar behavior with the Dirac point at about 0.3 V. The transfer curve of the device characterized in the PBS solution with the addition of 1 mM H2O2 shifts horizontally to lower gate voltages, being consistent with the channel current increase with the additions of H2O2 measured at fixed voltages.
(a) The channel current (IDS) responses of a SGGT without any modification on the graphene gate to additions of H2O2 with different concentrations measured at the fixed voltages of VG = 0.7 V and VDS = 0.05 V. Inset: the transfer curves of the SGGT measured in pure PBS and 1 mM H2O2 PBS solutions. (b) The channel current responses of a SGGT with PtNP-modified graphene gate to additions of H2O2 with different concentrations measured at the fixed voltages of VG = 0.7 V and VDS = 0.05 V. Inset: the transfer curves of the device measured in PBS and 1 mM H2O2 PBS solutions. (c) Effective gate voltage change (ΔVGeff) versus H2O2 concentration ([H2O2]) for the SGGTs with or without PtNPs on graphene gate electrodes.
To improve the sensitivity of the device to H2O2, the graphene gate electrode was modified with PtNPs by electrochemical deposition. We observed that the deposition time can influence the density and the shape of PtNPs and thus the detection limits of the devices (see supporting information, Figure S2). Figure 3b shows the channel current response of a device (PtNPs deposition time: 120 s) to additions of H2O2 measured at VG = 0.7 V and VDS = 0.05 V. The detection limit (Signal/Noise ≥3) of the device to H2O2 is about 30 nM, which is much lower than that of the device without the PtNPs. Similarly, as shown in the inset of Figure 3b, the transfer curve of the device shifts horizontally to lower gate voltages when H2O2 is added in the PBS solution, which is consistent with the increase of channel current measured at the fixed gate voltage of 0.7 V. In comparison, the devices with PtNPs deposited on the gates for 90 s and 150 s show the detection limits of 300 nM and 100 nM, respectively (See supporting information, Figure S3 and S4). So the optimum deposition time is ~120 s, which corresponds to the PtNPs with the average size of ~30 nm and a high density. In addition, we find that other metal nanoparticles like Au nanoparticles also can improve the sensitivity of the device to H2O2, which however results in lower sensitivity compared with PtNPs. As shown in Figure S5 in the supporting information, the device modified with Au nanoparticles by electrochemical deposition shows the detection limit of about 300 nM. Therefore, the graphene gates of the SGGTs were modified with PtNPs for the optimum period of 120 s in the following experiments for glucose sensing applications.
The shift of the transfer curve can be regarded as the change of the effective gate voltage induced by the reaction of H2O2 at the gate electrode. The effective gate voltage change (ΔVGeff) can be calculated according to the change of the channel current measured at a fixed gate voltage. The detailed calculation process is as follows. For different channel current value, the corresponding gate voltage can be read out from the transfer curve. The difference between the gate voltages before and after the addition of H2O2 is ΔVGeff for this concentration of H2O2. Therefore, the effective gate voltage changes (ΔVGeff) as a function of [H2O2] can be obtained, as shown in Figure 3c. It is notable that the SGGT with a PtNP-modified gate electrode shows much bigger response than the device without the Pt modification.
Assuming the capacitances of EDL1 on the channel and EDL2 on the gate are CEDL1 and CEDL2, respectively, the effective gate voltage is given by20,32:

where γ and C2 are constants. Therefore, it is reasonable to find that the effective gate voltage changes for 91 mV/decade in the linear region (3 μM to 300 μM) shown in Figure 3c and we can obtain γ ≈ 0.5 from equation (5) at room temperature (T = 300 K).
Glucose sensing by enzyme-functionalized SGGTs
Enzyme-functionalized glucose sensors were then prepared on the basis of the PtNP-modified SGGTs because H2O2 is a major product of glucose oxidation catalyzed by GOx. The gate electrode was coated with a thin layer of Nafion and then the mixture of CHIT and GOx. GOx plays an important role in glucose sensing because a control SGGT device without the modification of GOx did not show any response to glucose additions with the concentrations up to 3 mM (See supporting information, Figure S6). We found that the Nafion layer can be uniformly coated on graphene by a solution process20. Nafion has negative charges in PBS (pH = 7.2) solution and can dramatically improve the selectivity of the device to main interferences, including uric acid (UA) and ascobic acid (AA)33,34, because UA and AA are negatively charged and are thus difficult to diffuse through the negatively charged Nafion film due to the electrostatic interaction. Secondly, we noticed that it is difficult to directly coat GOx and CHIT aqueous solution on graphene for the hydrophobic surface of graphene. So the Nafion film is also used as an interlayer for coating the GOx-CHIT film on the surface. We also observed that the device with GOx modified on the graphene gate without PtNPs could not show obvious response to glucose due to the poor electrocatalytic activity of the gate (See supporting information, Figure S7). So only the devices with GOx-CHIT/Nafion/PtNPs/graphene gate electrodes show high sensitivity to glucose.
Figure 4 shows the current response of a GOx-modified SGGT to additions of glucose. The detection limit (Signal/Noise ≥3) is about 0.5 μM. The current change is attributed to the change of the effective gate voltage (ΔVGeff) induced by the reaction of glucose on the gate electrode. Therefore, ΔVGeff is calculated according to the current responses and is found to change for about 173 mV/decade in the linear region, as shown in Figure 4c. So the effective voltage change can be fitted with the equation:

where [Glu] is the concentration of glucose and C3 is a constant. According to equation (6), γ is calculated to be γ ≈ 1.9. The reason for having higher γ in the glucose sensor is presumably due to the decreased capacitance of the graphene gate for the modification of the insulating polymers (Nafion and CHIT) on the surface. In addition, the selectivity of the device was characterized. The main interference AA could not induce an obvious current response when its concentration was less than 3 μM (See supporting information, Figure S8). Considering that the AA concentration in the human body is more than 10 times lower than that of glucose35, the selectivity of the device is good enough for practical applications.
(a) The transfer characteristics of a SGGT with a GOx-CHIT/Nafion/PtNPs/graphene gate electrode measured in PBS and 1 mM glucose PBS solutions. Dash line: The transconductance (gm) of the SGGT characterized in PBS solution. (b) The channel current responses of the SGGT to additions of glucose with different concentrations. (c) The corresponding effective gate voltage change (ΔVGeff) at different glucose concentrations ([Glu]).
Discussion
The detectable range of the SGGT glucose sensor is from 0.5 μM to 1 mM, which is a suitable range for the detections of glucose in body fluids, such as sweat, tar and human saliva28. For blood glucose tests, conventional electrochemical methods are sensitive enough and have been successfully used in commercial products. However, blood glucose tests are painful and not very convenient in clinical diagnosis. Non-invasive detections of glucose levels in body fluids have been proposed to be an alternative way since the glucose levels in body fluids can reflect the glucose level in plasma. However, the glucose levels in body fluids are much lower than that in plasma36,37. Therefore, highly sensitive glucose sensors such as the SGGT-based sensors are potentially useful for non-invasive diagnosis of glucose levels in the human body.
It is noteworthy that the whole-graphene transistors have many advantages. Firstly, the devices have a very simple structure and can be prepared with one layer of graphene. So transistor arrays can be easily prepared by patterning high-quality large-area graphene. Besides glucose sensors, many other types of enzyme sensors can be realized based on the detection of H2O238. Therefore, multifunctional SGGT arrays for different types of highly sensitive biosensors can be realized by modifying the gate electrodes with specific biomaterials. Secondly, the devices show high transconductance gm ( )11, which is important for amplifying a potential signal into a current signal. As shown in Figure 4a, gm has the maximum value of about 2 mS even at the drain voltage of only 0.05 V. It is notable that a typical Si transistor used as a biosensor has a transconductance of only around 20 μS for VDS = 0.5 V39. So the transconductance of a SGGT is two orders of magnitude higher than that of a Si transistor, which is the main reason for the high sensitivity of the SGGT-based biosensor.
)11, which is important for amplifying a potential signal into a current signal. As shown in Figure 4a, gm has the maximum value of about 2 mS even at the drain voltage of only 0.05 V. It is notable that a typical Si transistor used as a biosensor has a transconductance of only around 20 μS for VDS = 0.5 V39. So the transconductance of a SGGT is two orders of magnitude higher than that of a Si transistor, which is the main reason for the high sensitivity of the SGGT-based biosensor.
In conclusion, whole-graphene SGGTs with PtNP-functionalized graphene gate electrodes can be used for highly sensitive detections of H2O2 with the detection limits down to 30 nM. The devices are further demonstrated as highly sensitive glucose sensors after the immobilization of GOx, CHIT and Nafion on the PtNPs/graphene gate electrodes and can detect glucose concentrations down to 0.5 μM. The high sensitivity of the SGGT-based sensors is attributed to the inherent amplification function of the transistors and the improved electrocatalytic activity of the graphene gate electrodes after the modification of PtNPs. Since most of enzyme sensors are based on the detection of H2O2, SGGTs are expected to be developed as many other types of enzyme sensors in the future.
Methods
Materials
Chloroplatinic acid (H2PtCl6·6H2O AR) was purchased from Sinopharm Chemical Reagent Co., Ltd. Glucose oxidase (GOx) (100 kU·g−1) was purchased from Sigma-Aldrich Reagent Database Inc. and stored at −20°C for future use. CHIT and hydrogen peroxide (H2O2, 30%, AR) were purchased from Advanced Technology & Industrial Co., Ltd and used as received. Phosphate buffered saline (PBS) solution (pH 7.2) was purchased from Invitrogen Co., Ltd and used as received. Glucose was also purchased from Sigma- Aldrich Co. Deionized water was used throughout in the whole process.
CHIT solution was prepared by dissolving CHIT (0.5 g) in acetic acid solution (100 mL, 50 mM, pH 5–6). The solution was electromagnetic stirred overnight and stored in 4°C refrigerator. GOx stock solution was prepared by dissolving in PBS and stored in 4°C refrigerator before use. 5%wt Nafion solution was diluted for 10 times by isopropanol before use.
Device Fabrication
The schematic structure of a whole-graphene SGGT is shown in Figure 1a. Au/Cr source and drain electrodes were deposited on glass substrates by magnetron sputtering through a shadow mask. Cr was used as an adhesion layer for Au. Single-layer graphene was grown on Cu foils by CVD method and transferred on glass substrates with Au electrodes30. Then the graphene films are patterned by the standard lithography method to have the graphene channel and gate14. The area of the graphene gate electrode is defined to be 3 mm × 3 mm and the channel width and length are 0.2 mm and 3 mm, respectively. A Poly(dimethylsiloxane) (PDMS) (Sylgard 184, Dow Corning, USA) wall was attached to the substrate to enable the test of the device in liquid (PBS solution).
Graphene gate electrodes were modified with Pt nanoparticles (PtNPs) to enhance the electrocatalytic activity. PtNPs were eletrochemically deposited on the graphene electrodes in 5 mM H2PtCl6/0.1 M HCl aqueous solution23. A constant voltage of −0.2 V relative to an Ag/AgCl reference electrode was applied on the needed graphene electrode and the deposition time was optimized (~120 s). Then the PtNPs/graphene electrodes were rinsed with DI water and used as the gate electrodes of the SGGTs.
For the preparation of GOx-CHIT/Nafion/PtNPs/graphene electrode, 50 uL GOx stock solution was mixed with 0.5%wt CHIT solution and the mixture is sonicated for 15 min before use. The graphene gate electrode was firstly modified with PtNPs as described above, then 10 μL 0.5%wt Nafion was dropped on the surface of the gate and dried at room temperature. After that, 10 uL GOx-CHIT mixture was drop coated on the Nafion/PtNPs/graphene gate electrode. The as-prepared SGGT was placed in a 4°C refrigerator overnight to dry the GOx-CHIT film. The devices were finally rinsed with DI water to remove unexpected residue and stored in the refrigerator for future use.
Device characterization
The SGGTs were characterized by a semiconductor parameter analyzer (4156C, Agilent Technology) in PBS solution at room temperature as shown in Figure 1a. The transfer characteristic (channel current IDS versus gate voltage VG) was tested under a fixed drain voltage VDS = 0.05 V and variable VG. In sensing applications, the channel current as a function of time was characterized under the constant voltages of VDS = 0.05 V and VG = 0.7 V with the additions of analytes (H2O2 or glucose). The transconductance gm of the device at a gate voltage is calculated with the equation:

The electrochemical properties of the graphene electrodes were characterized by EG&G PAR2273 electrochemical workstation (Princeton Co. Ltd). During the measurements, Ag/AgCl (sat. KCl) was used as a reference electrode and Pt wire was used as a counter electrode. The graphene and PtNPs/graphene electrodes were studied by cyclic voltammetry (CV) measurements with a scan rate of 50 mV/s. The device can be used for several times without obvious degradation when they are kept in refrigerator after each experiment.
The surface morphology of graphene films was characterized by Atom force microscopy (AFM, Digital instruments).
References
Heller, A. & Feldman, B. Electrochemical glucose sensors and their applications in diabetes management. Chem. Rev. 108, 2482–2505 (2008).
Lin, Y. H., Lu, F., Tu, Y. & Ren, Z. F. Glucose biosensors based on carbon nanotube nanoelectrode ensembles. Nano. Lett. 4, 191–195 (2004).
Song, Y. J., Qu, K. G., Zhao, C., Ren, J. S. & Qu, X. G. Graphene Oxide: Intrinsic Peroxidase Catalytic Activity and Its Application to Glucose Detection. Adv. Mater. 22, 2206–2210 (2010).
Ikariyama, Y., Yamauchi, S., Yukiashi, T. & Ushioda, H. One step fabrication of microbiosensor prepared by the codeposition of enzyme and platinum particles. Anal. Lett. 20, 1791–1801 (1987).
Tang, H., Yan, F., Tai, Q. D. & Chan, H. L. W. The improvement of glucose bioelectrocatalytic properties of platinum electrodes modified with electrospun TiO2 nanofibers, Biosens. Bioelectron. 25, 1646–1651 (2010).
Novoselov, K. S. Electric Field Effect in Atomically Thin Carbon Films. Science 306, 666–669 (2004).
Liu, Y. X., Dong, X. C. & Chen, P. Biological and chemical sensors based on graphene materials. Chem. Soc. Rev. 41, 2283–2307 (2012).
Huang, X. et al. Graphene-Based Materials: Synthesis, Characterization, Properties and Applications. Small 7, 1876–1902 (2011).
Amaral, C. E. F. & Wolf, B. Current development in non-invasive glucose monitoring, Med. Eng. Phys. 30, 541–549 (2008).
He, Q. Y., Wu, S. X., Yin, Z. Y. & Zhang, H. Graphene-based electronic sensors. Chem. Sci. 3, 1764–1772 (2012).
Yan, F., Zhang, M. & Li, J. Solution-Gated Graphene Transistors for Chemical and Biological Sensors. Adv. Healthcare Mater. 3, 313–331 (2014).
Ohno, Y., Maehashi, K., Yamashiro, Y. & Matsumoto, K. Electrolyte-Gated Graphene Field-Effect Transistors for Detecting pH Protein Adsorption. Nano. Lett. 9, 3318–3322 (2009).
Sudibya, H. G., He, Q., Zhang, H. & Chen, P. Electrical Detection of Metal Ions Using Field-Effect Transistors Based on Micropatterned Reduced Graphene Oxide Films. ACS Nano 5, 1990–1994 (2011).
He, R. X. et al. Solution-Gated Graphene Field Effect Transistors Integrated in Microfluidic Systems and Used for Flow Velocity Detection. Nano. Lett. 12, 1404–1409 (2012).
Huang, Y. X. et al. Nanoelectronic biosensors based on CVD grown graphene. Nanoscale 2, 1485–1488 (2010).
Kwak, Y. H. et al. Flexible glucose sensor using CVD-grown graphene-based field effect transistor. Biosens. Bioelectron. 37, 82–87 (2012).
Mohanty, N. & Berry, V. Graphene-Based Single-Bacterium Resolution Biodevice and DNA Transistor: Interfacing Graphene Derivatives with Nanoscale and Microscale Biocomponents. Nano. Lett. 8, 4469–4476 (2008).
Dong, X. C., Shi, Y. M., Huang, W., Chen, P. & Li, L. J. Electrical Detection of DNA Hybridization with Single-Base Specificity Using Transistors Based on CVD-Grown Graphene Sheets. Adv. Mater. 22, 1649–1653 (2010).
Chen, T. Y. et al. Label-free detection of DNA hybridization using transistors based on CVD grown graphene. Biosens. Bioelectron. 41, 103–109 (2013).
Zhang, M. et al. High-Performance Dopamine Sensors Based on Whole-Graphene Solution-Gated Transistors. Adv. Funct. Mater. 24, 978–985 (2014).
Cohen-Karni, T., Qing, Q., Li, Q., Fang, Y. & Lieber, C. M. Graphene and Nanowire Transistors for Cellular Interfaces and Electrical Recording. Nano. Lett. 10, 1098–1102 (2010).
Ang, P. K. et al. Flow Sensing of Single Cell by Graphene Transistor in a Microfluidic Channel. Nano. Lett. 11, 5240–5246 (2011).
Wen, Y. et al. The Electrical Detection of Lead Ions Using Gold-Nanoparticle- and DNAzyme-Functionalized Graphene Device. Adv. Healthcare Mater. 2, 271–274 (2012).
Tang, H., Yan, F., Lin, P., Xu, J. & Chan, H. L. W. Highly Sensitive Glucose Biosensors Based on Organic Electrochemical Transistors Using Platinum Gate Electrodes Modified with Enzyme and Nanomaterials. Adv. Funct. Mater. 21, 2264–2272 (2011).
Lin, P. & Yan, F. Organic Thin-Film Transistors for Chemical and Biological Sensing. Adv. Mater. 24, 34–51 (2012).
Yang, M. H., Yang, Y. H., Liu, B., Shen, G. L. & Yu, R. Q. Amperometric glucose biosensor based on chitosan with improved selectivity and stability. Sensor. Actuat. B-Chem. 101, 269–276 (2004).
Lim, S. H., Wei, J., Lin, J. Y., Li, Q. T. & KuaYou, J. A glucose biosensor based on electrodeposition of palladium nanoparticles and glucose oxidase onto Nafion-solubilized carbon nanotube electrode. Biosens. Bioelectron. 20, 2341–2346 (2005).
Yamaguchi, M., Mitsumori, M. & Kano, Y. Noninvasively measuring blood glucose using saliva. IEEE Eng. Med. Biol. 17, 59–63 (1998).
Khodagholy, D. et al. High transconductance organic electrochemical transistors. Nature Commun. 4, 2133 (2013).
Li, X. et al. Large-Area Synthesis of High-Quality and Uniform Graphene Films on Copper Foils., Science 324, 1312–1314 (2009).
Rong, L.-Q., Yang, C., Qian, Q.-Y. & Xia, X.-H. Study of the nonenzymatic glucose sensor based on highly dispersed Pt nanoparticles supported on carbon nanotubes. Talanta 72, 819–824 (2007).
Cicoira, F. et al. Influence of Device Geometry on Sensor Characteristics of Planar Organic Electrochemical Transistors. Adv. Mater. 22, 1012–1016 (2010).
Wang, H. S., Li, T. H., Jia, W. L. & Xu, H. Y. Highly selective and sensitive determination of dopamine using a Nafion/carbon nanotubes coated poly(3-methylthiophene) modified electrode. Biosens. Bioelectron. 22, 664–669 (2006).
Liao, C. Z., Zhang, M., Niu, L. Y., Zheng, Z. J. & Yan, F. Organic electrochemical transistors with graphene-modified gate electrodes for highly sensitive and selective dopamine sensors. J. Mater. Chem. B 2, 191–200 (2014).
Yang, Q., Atanasov, P. & Wilkins, E. Development of needle-type glucose sensor with high selectivity. Sensor. Actuat. B-Chem 46, 249–256 (1998).
Amer, S., Yousuf, M., Siddqiui, P. Q. & Alam, J. Salivary glucose concentrations in patients with diabetes mellitus–a minimally invasive technique for monitoring blood glucose levels. Pak. J. Pharm. Sci. 14, 33–37 (2001).
Liao, C. Z., Mak, C. H., Zhang, M., Chan, H. L. W. & Yan, F. Flexible organic electrochemical transistors for highly selective enzyme biosensors and used for saliva testing. Adv. Mater. in press, 10.1002/adma.201404378 (2014).
Erden, P. E. & Kilic, E. A review of enzymatic uric acid biosensors based on amperometric detection. Talanta 107, 312–323 (2013).
Fromherz, P., Offenhausser, A., Vetter, T. & Weis, J. A Neuron-Silicon Junction - a Retzius Cell of the Leech on an Insulated-Gate Field-Effect Transistor. Science 252, 1290–1293 (1991).
Acknowledgements
This work is financially supported by the Research Grants Council (RGC) of Hong Kong, China (project number: N_PolyU506/13) and the Hong Kong Polytechnic University (project number: G-SB07, A-PL49, G-YM45).
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript. F.Y. conceived the experiments. M.Z. fabricated and characterized the devices. C.L., C.H.M., P.Y. and C.L.M. assisted the experiments. F.Y. and M.Z. wrote the paper together.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supporting information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Zhang, M., Liao, C., Mak, C. et al. Highly sensitive glucose sensors based on enzyme-modified whole-graphene solution-gated transistors. Sci Rep 5, 8311 (2015). https://doi.org/10.1038/srep08311
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep08311
This article is cited by
-
A direct electrochemical biosensor for rapid glucose detection based on nitrogen-doped carbon nanocages
Rare Metals (2024)
-
Hexagonal-shaped graphene quantum plasmonic nano-antenna sensor
Scientific Reports (2023)
-
Plasmonic organic electrochemical transistors for enhanced sensing
Nano Research (2023)
-
Coaxial fiber organic electrochemical transistor with high transconductance
Nano Research (2023)
-
Cu and Ni Co-sputtered heteroatomic thin film for enhanced nonenzymatic glucose detection
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.