Abstract
Rituximab is considered to be a promising drug for treating childhood refractory nephrotic syndrome. However, the efficacy and safety of rituximab in treating childhood refractory nephrotic syndrome remain inconclusive. This meta-analysis aimed to investigate the efficacy and safety of rituximab treatment compared with other immunosuppressive agents in children with refractory nephrotic syndrome. Three randomized controlled trials and two comparative control studies were included in our analysis. The included studies were of moderately high quality. Compared with other immunotherapies, rituximab therapy significantly improved relapse-free survival (hazard ratio = 0.49, 95% confidence interval [CI], 0.26–0.92, P = 0.03). Rituximab also achieved a higher rate of complete remission (risk ratio,1.62; 95% CI, 0.92 to 2.84, P = 0.09) and reduced the occurrence of proteinuria (mean difference = −0.25, 95% CI = −0.29 to −0.21, P < 0.00001); however, a more targeted rituximab treatment did not significantly increase serum albumin levels and did not significantly reduce adverse events. Rituximab might be a promising treatment for childhood refractory nephrotic syndrome; however, the long-term effects and cost-effectiveness of rituximab treatment were not fully assessed and there were limited studies that evaluated the clinical benefits of a concurrent infusion of rituximab plus a steroid compared with an infusion of rituximab only. Additional studies are required to address these issues.
Similar content being viewed by others
Introduction
Nephrotic syndrome (NS) is a disorder characterized by large amounts of proteinuria, hypoalbuminemia, edema and hyperlipidemia1. This disorder affects the kidneys by increasing the permeability of the glomerular basement membrane. NS occurs in 16 of every 100,000 children and is a major challenge in pediatric nephrology2. Moreover, NS places a large financial burden on the patient's family. Although most affected children have steroid-sensitive nephrotic syndrome (SSNS), approximately 20% of children do not achieve complete remission and have steroid-resistant nephrotic syndrome (SRNS)3. Moreover, 80%–90% of children with SSNS experience relapses. Among these relapsing children, 50% relapse frequently and develop steroid-dependent nephrotic syndrome (SDNS)4,5,6. The long-term use of corticosteroids can adversely affect children's growth and development7. The treatment of SRNS, SDNS and SSNS remains challenging. Patients with SRNS who do not achieve remission will develop end-stage renal failure. The exact pathogeneses of SRNS, SDNS and SSNS have not been fully elaborated, but immunological factors might play a vital role and the use of immunosuppressants and immunological treatment interventions appear to have achieved promising results7. These immunosuppressants include cyclophosphamide8,9, chlorambucil10, cyclosporin11, levamisole12 and mycophenolate mofetil11. However, some of these immunosuppressants can have serious adverse effects such as nephrotoxicity, hyperglycemia, headaches and dyslipidemia13. Novel drugs are needed to address these problems.
Rituximab is a monoclonal antibody that acts directly against CD20 expressed on B lymphocytes. It is widely used to treat lymphoma14 and rheumatoid arthritis15. Rituximab administration results in rapid and sustained B cell depletion. Several reports have proposed rituximab as a new treatment strategy for children with SDNS or SSNS13,16,17. However, the use of rituximab in the treatment of steroid- and calcineurin inhibitor-dependent SSNS requires further investigation. A single open-labeled, randomized controlled trial (RCT) that enrolled 54 children with SDNS who were dependent on prednisone and calcineurin inhibitors found that rituximab significantly reduced the relapse rate at 3 months (18.5% and 48.1% in the experimental and control arms, respectively) and it also increased the likelihood of a child not requiring prednisone or calcineurin inhibitor treatment18. Many studies have reported that rituximab treatment prolonged remission in patients with refractory NS19.
The aim of this study was to combine the current evidence from all eligible comparative studies to systematically evaluate the use of rituximab versus current immunosuppressive agents in treating children with refractory NS.
Methods
Literature search
This meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement20. Our meta-analysis searches were conducted using the PubMed, Web of Science Knowledge and Cochrane Library databases from their inception dates to August 1, 2014. The search applied the following search terms: “rituximab”, “CD20” and “nephrotic syndrome”.
Study selection criteria and study types
RCTs or comparative cohort studies that evaluated the efficacy and safety of rituximab in treating pediatric patients with refractory NS were included.
Type of participants
The patients were diagnosed with NS at 18 years of age and younger.
Type of interventions
Rituximab and current immunotherapy were compared. Different schedules and modalities of rituximab were included.
Analyzed outcomes
The primary outcome was relapse-free survival. The secondary outcomes were (1) complete remission events, (2) biological indicators, including proteinuria, serum albumin, serum cholesterol and serum creatinine and (3) adverse events.
Study selection and data extraction
The references obtained from the electronic search were evaluated by two independent reviewers (Zhao Z and Liao G) using a study selection form. The initial assessment was based on screening the titles and abstracts; studies that did not meet the inclusion criteria were excluded. The studies that were not excluded after an initial evaluation were retrieved for full text screening and, according to the inclusion criteria, it was determined whether the study should be included in our analysis. In cases of disagreement, the final decision for inclusion was made by consensus among the authors. Review articles, case reports, comments, meeting abstracts and editorials were excluded.
The data were extracted by two independent reviewers (Zhao Z and Liao G). The extraction data included the (1) study characteristics (authors, publication year), (2) study design features, (3) study participants (e.g., eligibility criteria and baseline characteristics), (4) study interventions and (5) study outcomes (efficacy and safety outcomes).
Bias and quality assessments of the included studies
The risk of bias in each included RCT was evaluated using the Cochrane Collaboration's ‘Risk of bias’ tool21. The quality assessment of the comparative cohort studies was performed using the Newcastle-Ottawa scale (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp), which included three main categories: (1) selection of cohort, (2) comparability of cohort and (3) determination of outcomes.
Statistical analysis
Our meta-analysis was performed using the RevMan software (version 5.20, The Nordic Cochrane Centre, Copenhagen, Denmark) and Stata software (version 11.0, Stata corporation, College Station, TX, USA). For relapse-free survival, a hazard ratio (HR) and its 95% confidence interval (CI) were applied for analysis. For dichotomous outcomes (adverse events and response rate), risk ratios (RRs) were calculated and these RRs were then pooled. Continuous variables were analyzed using mean differences (MDs) and 95% CIs. The heterogeneity of the included studies was analyzed using the Cochrane Q test and the I2 statistic and P < 0.1 or I2 > 50% represented significant heterogeneity. If there was significant heterogeneity, we used a random effects model for the data analysis. Otherwise, we used a fixed effect model.
Results
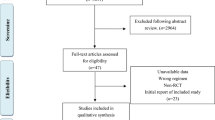
Our literature search identified 600 articles, of which 243 were from PubMed, 349 from Web of Science and 8 from the Cochrane Library. Using Endnote software, 190 repeated studies were removed. After screening the titles and abstracts, 400 studies were excluded and the remaining 10 articles underwent full-text screening. Five studies were excluded for the following reasons: two studies compared treatment effects before and after using rituximab in the same series of patients19,22; another study was a comparison of two groups that both used rituximab, with or without mycophenolate mofetil23; the other two studies compared different doses of rituximab infusion24,25. Finally, five studies were included for our analysis, three of which were RCTs18,26,27; one study was a retrospective comparative control study28 and one was a prospective comparative control study29. The study selection process is shown in Figure 1. The basic characteristics of the included studies are listed in Table 1.
Assessing the quality of the studies
The risk of bias for each of the included RCTs is shown in Table 2. The Newcastle-Ottawa scale scores awarded 7 stars for a study reported by Sinha A28 and 5 stars for a study reported by Delbe-Bertin L29.
Efficacy of rituximab in children with nephrotic syndrome
Relapse-free survival
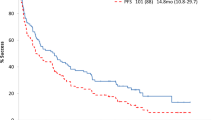
One study27 reported that the median relapse-free survival rate favored the rituximab group over the placebo group, with a statistically significant difference (HR = 0.27; 95% CI, 0.14 to 0.53; P < 0.0001). In another study28, the relapse-free survival rate was similar in the two groups (rituximab versus tacrolimus) (P = 0.86). The third study reported that three patients in each group achieved remission26. Ravani P18 reported that the drug-free rates at three months were 62.9% and 3.7% in the rituximab group and the other immunotherapy group, respectively; we considered that these results could potentially represent the relapse-free survival rate. The pooled HRs of the four included studies revealed a significant difference between the rituximab treatment group and the control therapy group (HR = 0.49, 95% CI, 0.26–0.92, P = 0.03); a random-effect model was used because of significant heterogeneity (I2 = 55%, P = 0.08) and the results are outlined in Figure 2.
Complete remission rate
A complete remission rate was reported in three studies18,26,28. Pooled data from the three studies indicated that rituximab treatment seemed to achieve a better complete remission rate than other immunotherapy drugs (39.62% versus 25.45%) (RR: 1.62, 95% CI: 0.92–2.84, P = 0.09), as shown in Figure 3.
Biochemical indicators
Serum albumin
Two studies evaluated the serum albumin index after treatment26,28. Pooled analysis of the data revealed that there was no significant difference between the two groups (MD = 0.18 g/dl, 95% CI = −0.24 to 0.60 g/dl), with no heterogeneity among these studies (I2 = 0, P = 0.53) (Figure 4A).
Serum creatinine
Only two studies reported serum creatinine at the end of treatment26,28. Pooled data from the two included studies suggested that there were no significant difference between the two treatments (MD = 0.01 mg/dl, 95% CI, −0.11 to 0.13, P = 0.89). Moreover, there was no heterogeneity and the analysis was performed using a fixed effect model (I2 = 0, P = 0.58) (Figure 4B).
Proteinuria
Two studies reported proteinuria at the end of treatment18,26. Compared with other immunotherapies, rituximab treatment reduced proteinuria by 0.25 g/d (MD = −0.25, 95% CI = −0.29 to −0.21, P < 0.00001). The results are showed in Figure 4C.
Other parameters
Sinha A reported that there was no between-group difference (2 groups) in the estimated glomerular filtration rate at the 1-year follow-up28. Furthermore, another included study reported that rituximab did not decrease the IgG levels in patients with SDNS compared with the control treatment, but it prolonged preexisting low IgG levels29.
Safety
Adverse events
It was reported that the adverse effects included bronchospasm, hypotension and skin rash in patients receiving rituximab treatment. Because of their minor severity, these adverse events rapidly and completely resolved by reducing the drug infusion rate or providing minor supportive treatment. We only included grade 3–4 adverse events in the analysis. No significant differences were observed in events of bronchospasm (RR = 5.84, 95% CI, 0.73 to 46.34, P = 0.10), hypotension (RR = 2.94, 95% CI, 0.48 to 18.07, P = 0.24), acute renal failure (RR = 0.97, 95% CI = 0.14 to 6.54, P = 0.98), or skin rash (RR = 2.91, 95% CI = 0.32 to 26.79, P = 0.35) between the two groups.
Publication bias
Relapse-free survival was assessed for publication bias. No evidence of publication bias was disclosed among the included studies by statistical testing, using Stata software, version 11.0 (Egg's test, P = 0.238; Begg's test, P = 0.734).
Discussion
Our meta-analysis included three RCTs and two comparative control studies involving 184 patients. There were 89 patients in the rituximab arm and 95 patients in the control arm. Our results indicated that rituximab treatment could significantly improve the relapse-free survival rate in patients with NS compared with control therapy. Moreover, rituximab treatment seemed to achieve a higher complete remission rate (39.62% for rituximab versus 25.45% for the control). Furthermore, rituximab treatment significantly reduced the incidence of proteinuria. There were no significant differences in the serum albumin and serum creatinine levels or the estimated glomerular filtration rate between the two groups. The adverse effects were similar between the two arms and no significance differences were observed.
Our study indicated that rituximab treatment demonstrated benefits in terms of relapse-free survival, which was consistent with previous studies. Gulati et al. reported that rituximab treatment in patients with SRNS or SDNS that was refractory to standard therapy could sustain long-term relapse-free survival30. A study from China reported that rituximab treatment demonstrated a 91.67% effective rate22. A recent review revealed that rituximab treatment reduced the number of relapses per year, with minimal change in disease and little focal segmental glomerulosclerosis31. Tellier et al. reported that 4 (22%) of 18 patients with idiopathic NS who were treated with rituximab experienced remission without relapse and the remaining patients had increased durations of remission32. In patients who received one dose of rituximab (375 mg/m2), 25%–40% were in sustained remission at 12–17 months33,34. Sinha A indicated that therapy with rituximab could reduce relapse rates in children with refractory NS35.
NS is characterized by a large amount of proteinuria. Reducing proteinuria is part of the treatment for NS. Kong et al. reported that approximately 90.1% of patients receiving rituximab treatment achieved complete or partial remission of proteinuria36. Another study reported that rituximab treatment could significantly reduce 24-hour proteinuria in patients with NS25. Indeed, the combined data indicated that rituximab treatment could reduce proteinuria. However, rituximab did not significantly increase serum albumin. This disparity might be attributed to the following causes. First, the patients who were included for analysis had different pathological patterns and different treatment responses to rituximab, which might have influenced the results. For example, the pathogenetic differences between children who are resistant ab initio and those with delayed resistance would have resulted in different treatment effects. The study reported by Magnasco A26 might have included patients with different genetic backgrounds or sensitivities to rituximab and, thus, might have indicated that rituximab might not be a good choice in children who were unresponsive to steroids and calcineurin inhibitors, particularly for those unresponsive ab initio. By contrast, some case series have reported that rituximab might have a good effect on NS who are unresponsive to prednisone and calcineurin inhibitors30,37. Second, the administration of rituximab differs. Magnasco A26 adopted a treatment that consisted of two infusions of rituximab and two to three infusions of rituximab were used in the Sinha A28 study. A higher dose of rituximab could effectively reduce proteinuria and increase serum albumin in refractory NS. Third, only two studies were included for the analysis of serum albumin and there was a small number of cases (26 in the rituximab group and 28 in the other immunotherapy group). Indeed, rituximab therapy reduced proteinuria with the amelioration of serum albumin, as shown in Figure 4 and the mean serum albumin level in the rituximab group was higher than that in the other immunotherapy group (2.63 g/dl versus 2.53 g/dl in the Magnesco A study and 3.8 g/dl versus 3.4 g/dl in the Sinha A study). However, the statistical power did not reach the level of statistical significance. If more cases were analyzed (6 times more), rituximab would have had a positive effect. A systematic review reported that rituximab treatment significantly reduced proteinuria and increased serum albumin in idiopathic NS31. As mentioned above, larger, well-designed, prospective, controlled studies should be performed to assess these issues.
Rituximab therapy was well tolerated in most patients. One review reported that the most frequent adverse event was infusion-related reactions to rituximab therapy, which accounted for 22.4% of all reported adverse events. The second most common adverse event was acute reaction (22.2%). Other related adverse effects included anaphylaxis, rash, bronchospasm, abdominal pain, vomiting, chills and so on13. The study demonstrated that using a more targeted rituximab treatment did not significantly reduce the incidence of adverse events. The following factors may explain the occurrence of these adverse events. Most of the adverse effects (e.g., hypotension, bradycardia, chest tightness and body ache) were infusion reactions due to non-humanized anti-CD20 antibodies (rituximab) and they usually occurred at the initial infusion. These adverse effects could be well managed with premedication (with steroid and antihistamines) or by reducing the infusion rate or discontinuing the drug. Currently, humanized anti-CD20 antibodies (ofatumumab and obinutuzumab) are under clinical investigation; whether these new agents could reduce the incidence of adverse events must be further evaluated. However, rituximab did demonstrate some advantages over other immunotherapies. Rituximab therapy significantly reduced the use of steroids and immunosuppressive agents. Ruggenenti et al. reported that the median per-patient steroid maintenance dose decreased from 0.27 mg/kg to 0 mg/kg and the median cumulative dose to achieve remission from relapse decreased from 19.5 mg/kg to 0.5 mg/kg after rituximab treatment in patients with steroid-dependent or frequently relapsing idiopathic NS19. Some studies have revealed that rituximab treatment significantly reduced steroid doses compared with other immunotherapies19,27. Therefore, reducing the steroid dose might prevent steroid-related side effects. Rituximab therapy resulted in the discontinuation of steroids for more than 200 days without relapses in more than half of the patients and it seemed to improve the peak Z score27. Sato M reported that rituximab treatment could improve the growth and obesity indices of some children with SDNS who were suffering from the severe side effects of steroids38.
In the studies included in this meta-analysis, the schedules and modalities of rituximab administration were not uniform. A single dose of 375 m/m2 rituximab accompanied by a B cell-driven infusion protocol administered over 4 weeks constitutes the most commonly applied regimen for NS in clinics. Other studies have used one, two or three infusions. In clinical practice, a dose of intravenous corticosteroid is often administered with an infusion of rituximab. The infusion of rituximab in combination with infused/oral corticosteroid drugs was reported in all included studies. In three studies27,28,29 (Lijima K, Sinha A and Delbe-Bertin L), the patients were treated 30 minutes before the infusion of rituximab with corticosteroid therapy. This additional dose of intravenous steroid could reduce the adverse infusion effects resulting from rituximab and might yield additional benefits. A study from the Rituximab in Nephrotic Syndrome of Steroid-Dependent or Frequently Relapsing Minimal Change Disease Or Focal Segmental Glomerulosclerosis (NEMO) Study Group reported that in a series of 30 patients with NS, 29 patients received an infusion of rituximab combined with corticosteroid; 28 patients experienced complete remission and 2 patients achieved partial remission19. Additional studies are required to assess the clinical benefits of a concurrent infusion of rituximab plus a steroid compared with an infusion of rituximab only.
A number of reports have reported using anti-CD20 antibodies (rituximab) in children with NS. From the literature, we might speculate that the effects of rituximab are better in SDNS and frequent-relapse NS than in SRNS6,17,22,27,30,35,39. In a systematic review that summarized 155 patients undergoing rituximab treatment for SRDS, 52 patients (33.6%) achieved remission13.
Currently, the most widely used agents for SRNS are calcineurin inhibitors; nearly 60%–70% of patients with SRNS have promising results with calcineurin inhibitor therapy; to date, no other alternative agents have shown superior efficacy13.
The use of rituximab in SRNS occurs under the following circumstances. First, rituximab could play a role in SRNS by maintaining remission and reducing the dose of steroids and other immunosuppressants and rituximab is an alternative treatment when patients experience serious adverse events with other immunosuppressants. Second, in some SRNS patients who are resistant to immunosuppressants, treatment with rituximab might yield encouraging results13.
However, it is challenging to treat patients with SRNS who resistant to rituximab. New anti-CD20 monoclonal antibodies have been utilized in clinics. Ofatumumab and obinutuzumab were both approved to treat chronic lymphocytic leukemia(CLL)40. Unlike chimeric rituximab, both of these monoclonal antibodies are humanized. The two drugs seem to offer more advantages over rituximab41,42. A study reported that ofatumumab showed promising results in managing refractory SRNS patients who are resistant to rituximab43. A recent study found that obinutuzumab was superior to rituximab when combined with chlorambucil in patients with CLL44. A phase II, multi-center clinical trial suggested that ofatumumab plus bendamustine was feasible and effective in relapsed/refractory CLL patients45. Another study also indicated that ofatumumab was safe, with modest activity in heavily pretreated, rituximab-refractory patients with follicular lymphoma46. Radford J and coworkers reported that obinutuzumab combined with chemotherapy demonstrated an encouraging response rate (93%–96%) in patients with relapsed/refractory follicular lymphoma47. However, few studies have explored the use of humanized anti-CD20 monoclonal antibodies in NS. Future studies could evaluate the effect of new human anti-CD20 monoclonal antibodies in treating NS.
This study has some limitations that should be mentioned. First, our study included three RCTs and two cohort observation studies. The clinical evidence was not sufficiently strong for an observation study. Second, our study included patients at different locations and with different basic characteristics, different pathological types and different follow-up times; all of these factors could explain some of the heterogeneity in some of our results. However, there was no significant publication bias, according to the statistical analysis. Third, only Sinha et al. evaluated renal function after 1 year of follow-up28; longer follow-up evaluations assessing renal function outcomes were not available among the selected studies. Fourth, due to limited information, the relapse rates were not evaluated in our study. Fifth, the cost of rituximab treatment is high24; therefore, not all the included studies evaluated the cost-effectiveness of rituximab in treating NS. It is essential to evaluate the cost-effectiveness of rituximab in treating NS. Sixth, the number of included cases was small. Future studies should address these issues.
In conclusion, rituximab can be considered an effective and safe treatment option for SNNS and SDNS because it prolongs relapse-free survival, increases the complete remission rate and reduces proteinuria. However, the long-term effects and cost-effectiveness of rituximab treatment were not fully assessed. Additional studies with well-designed and longer follow-up periods are needed to address these issues.
References
Certikova-Chabova, V. & Tesar, V. Recent insights into the pathogenesis of nephrotic syndrome. Minerva Med 104, 333–347 (2013).
Eddy, A. A. & Symons, J. M. Nephrotic syndrome in childhood. Lancet 362, 629–639 (2003).
McKinney, P. A., Feltbower, R. G., Brocklebank, J. T. & Fitzpatrick, M. M. Time trends and ethnic patterns of childhood nephrotic syndrome in Yorkshire, UK. Pediatr Nephrol 16, 1040–1044 (2001).
Koskimies, O., Vilska, J., Rapola, J. & Hallman, N. Long-term outcome of primary nephrotic syndrome. Arch Dis Child 57, 544–548 (1982).
Tarshish, P., Tobin, J. N., Bernstein, J. & Edelmann, C. J. Prognostic significance of the early course of minimal change nephrotic syndrome: report of the International Study of Kidney Disease in Children. J Am Soc Nephrol 8, 769–776 (1997).
Lombel, R. M., Gipson, D. S. & Hodson, E. M. Treatment of steroid-sensitive nephrotic syndrome: new guidelines from KDIGO. Pediatr Nephrol 28, 415–426 (2013).
Pravitsitthikul, N., Willis, N. S., Hodson, E. M. & Craig, J. C. Non-corticosteroid immunosuppressive medications for steroid-sensitive nephrotic syndrome in children. Cochrane Database Syst Rev 10, CD002290 (2013).
Abeyagunawardena, A. S. et al. Predictors of long-term outcome of children with idiopathic focal segmental glomerulosclerosis. Pediatr Nephrol 22, 215–221 (2007).
Prasad, N., Gulati, S., Sharma, R. K., Singh, U. & Ahmed, M. Pulse cyclophosphamide therapy in steroid-dependent nephrotic syndrome. Pediatr Nephrol 19, 494–498 (2004).
Ueda, N. et al. Beneficial effect of chlorambucil in steroid-dependent and cyclophosphamide-resistant minimal change nephrotic syndrome. J Nephrol 22, 610–615 (2009).
Gellermann, J., Ehrich, J. H. & Querfeld, U. Sequential maintenance therapy with cyclosporin A and mycophenolate mofetil for sustained remission of childhood steroid-resistant nephrotic syndrome. Nephrol Dial Transplant 27, 1970–1978 (2012).
Al-Saran, K., Mirza, K., Al-Ghanam, G. & Abdelkarim, M. Experience with levamisole in frequently relapsing, steroid-dependent nephrotic syndrome. Pediatr Nephrol 21, 201–205 (2006).
Sinha, A. & Bagga, A. Rituximab therapy in nephrotic syndrome: implications for patients' management. Nat Rev Nephrol 9, 154–169 (2013).
Senff, N. J. et al. European Organization for Research and Treatment of Cancer and International Society for Cutaneous Lymphoma consensus recommendations for the management of cutaneous B-cell lymphomas. Blood 112, 1600–1609 (2008).
Murray, E. & Perry, M. Off-label use of rituximab in systemic lupus erythematosus: a systematic review. Clin Rheumatol 29, 707–716 (2010).
Kamei, K. et al. Rituximab treatment combined with methylprednisolone pulse therapy and immunosuppressants for childhood steroid-resistant nephrotic syndrome. Pediatr Nephrol 29, 1181–7 (2014).
Ito, S. et al. Survey of rituximab treatment for childhood-onset refractory nephrotic syndrome. Pediatr Nephrol 28, 257–264 (2013).
Ravani, P. et al. Short-term effects of rituximab in children with steroid- and calcineurin-dependent nephrotic syndrome: a randomized controlled trial. Clin J Am Soc Nephrol 6, 1308–1315 (2011).
Ruggenenti, P. et al. Rituximab in steroid-dependent or frequently relapsing idiopathic nephrotic syndrome. J Am Soc Nephrol 25, 850–863 (2014).
Moher, D., Liberati, A., Tetzlaff, J. & Altman, D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6, e1000097 (2009).
Higgins, J. P. et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 343, d5928 (2011).
Sun, L. et al. Efficacy of rituximab therapy in children with refractory nephrotic syndrome: a prospective observational study in Shanghai. World J Pediatr 10, 59–63 (2014).
Ito, S. et al. Maintenance therapy with mycophenolate mofetil after rituximab in pediatric patients with steroid-dependent nephrotic syndrome. Pediatr Nephrol 26, 1823–1828 (2011).
Cravedi, P., Ruggenenti, P., Sghirlanzoni, M. C. & Remuzzi, G. Titrating rituximab to circulating B cells to optimize lymphocytolytic therapy in idiopathic membranous nephropathy. Clin J Am Soc Nephrol 2, 932–937 (2007).
Cravedi, P. et al. Efficacy and safety of rituximab second-line therapy for membranous nephropathy: a prospective, matched-cohort study. Am J Nephrol 33, 461–468 (2011).
Magnasco, A. et al. Rituximab in children with resistant idiopathic nephrotic syndrome. J Am Soc Nephrol 23, 1117–1124 (2012).
Iijima, K. et al. Rituximab for childhood-onset, complicated, frequently relapsing nephrotic syndrome or steroid-dependent nephrotic syndrome: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet 384, 1273–1281 (2014).
Sinha, A., Bagga, A., Gulati, A. & Hari, P. Short-term efficacy of rituximab versus tacrolimus in steroid-dependent nephrotic syndrome. Pediatr Nephrol 27, 235–241 (2012).
Delbe-Bertin, L., Aoun, B., Tudorache, E., Lapillone, H. & Ulinski, T. Does rituximab induce hypogammaglobulinemia in patients with pediatric idiopathic nephrotic syndrome? Pediatr Nephrol 28, 447–451 (2013).
Gulati, A. et al. Efficacy and safety of treatment with rituximab for difficult steroid-resistant and -dependent nephrotic syndrome: multicentric report. Clin J Am Soc Nephrol 5, 2207–2212 (2010).
Kronbichler, A. et al. Rituximab treatment for relapsing minimal change disease and focal segmental glomerulosclerosis: a systematic review. Am J Nephrol 39, 322–330 (2014).
Tellier, S. et al. Long-term outcome of children treated with rituximab for idiopathic nephrotic syndrome. Pediatr Nephrol 28, 911–918 (2013).
Kamei, K. et al. Single dose of rituximab for refractory steroid-dependent nephrotic syndrome in children. Pediatr Nephrol 24, 1321–1328 (2009).
Fujinaga, S. et al. Single infusion of rituximab for persistent steroid-dependent minimal-change nephrotic syndrome after long-term cyclosporine. Pediatr Nephrol 25, 539–544 (2010).
Sinha, A. et al. Efficacy and safety of rituximab in children with difficult-to-treat nephrotic syndrome. Nephrol Dial Transplant. 29, gfu267; 10.1093/ndt/gfu267 (2014).
Kong, W. Y., Swaminathan, R. & Irish, A. Our experience with rituximab therapy for adult-onset primary glomerulonephritis and review of literature. Int Urol Nephrol 45, 795–802 (2013).
Guigonis, V. et al. Rituximab treatment for severe steroid- or cyclosporine-dependent nephrotic syndrome: a multicentric series of 22 cases. Pediatr Nephrol 23, 1269–1279 (2008).
Sato, M., Ito, S., Ogura, M. & Kamei, K. Impact of rituximab on height and weight in children with refractory steroid-dependent nephrotic syndrome. Pediatr Nephrol 29, 1373–1379 (2014).
Prytula, A. et al. Rituximab in refractory nephrotic syndrome. Pediatr Nephrol 25, 461–8 (2010).
Lim, S. H. & Levy, R. Translational medicine in action: anti-CD20 therapy in lymphoma. J Immunol 193, 1519–1524 (2014).
Rioufol, C. & Salles, G. Obinutuzumab for chronic lymphocytic leukemia. Expert Rev Hematol 7, 533–543 (2014).
Gagez, A. L. & Cartron, G. Obinutuzumab: a new class of anti-CD20 monoclonal antibody. Curr Opin Oncol 26, 484–491 (2014).
Basu, B. Ofatumumab for rituximab-resistant nephrotic syndrome. N Engl J Med 370, 1268–70 (2014).
Goede, V. et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med 370, 1101–1110 (2014).
Cortelezzi, A. et al. Bendamustine in combination with ofatumumab in relapsed or refractory chronic lymphocytic leukemia: a GIMEMA Multicenter Phase II Trial. Leukemia 28, 642–648 (2014).
Czuczman, M. S. et al. Ofatumumab monotherapy in rituximab-refractory follicular lymphoma: results from a multicenter study. Blood 119, 3698–3704 (2012).
Radford, J. et al. Obinutuzumab (GA101) plus CHOP or FC in relapsed/refractory follicular lymphoma: results of the GAUDI study (BO21000). Blood 122, 1137–1143 (2013).
Acknowledgements
This study was supported by the following Science Foundation: 1. EU FP7 Program, UroSense, 2011; The National Natural Science Foundation of China (81270840).
Author information
Authors and Affiliations
Contributions
Z.Z., G.L. and H.Z. conducted the literature search, determined studies for exclusion and inclusion, extracted data from retrieved studies, performed the meta-analysis and drafted the manuscript. Y.L. and S.Z. provided comments on the experiment design and the manuscript, read and approved the final manuscript. All authors reviewed the paper and approved the final manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Zhao, Z., Liao, G., Li, Y. et al. The efficacy and safety of rituximab in treating childhood refractory nephrotic syndrome: A meta-analysis. Sci Rep 5, 8219 (2015). https://doi.org/10.1038/srep08219
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep08219
This article is cited by
-
Efficacy of rituximab therapy in children with nephrotic syndrome: a 10-year experience from an Iranian pediatric hospital
BMC Pediatrics (2022)
-
The efficacy of rituximab in the treatment of refractory nephrotic syndrome: a meta-analysis
International Urology and Nephrology (2020)
-
Low-dose rituximab is no less effective for nephrotic syndrome measured by 12-month outcome
Pediatric Nephrology (2019)
-
Single, very low rituximab doses in healthy volunteers - a pilot and a randomized trial: implications for dosing and biosimilarity testing
Scientific Reports (2018)
-
Cost analysis on the use of rituximab and calcineurin inhibitors in children and adolescents with steroid-dependent nephrotic syndrome
Pediatric Nephrology (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.