Abstract
Serotonin (5-HT) receptors are valuable molecular targets for antipsychotic drug discovery. Current reported methods for detecting 5-HT receptors, such as cAMP accumulation and calcium influx assay, are often demanding specialized instruments and inconvenient. The luciferase reporter gene assay, based on the responsible-element-regulated expression of luciferase, has been widely applied in the high-throughput functional assay for many targets because of its high sensitivity and reliability. However, 5-HT receptors couple to multiple G-proteins regulate respective downstream signalling pathways and are usually detected using different response elements. Hence, finding a suitable response element to fulfil the detection of different 5-HT receptors and make the results of luciferase reporter gene assays generalizable is very useful for active compounds screening. Here, we conducted three luciferase reporter assays using CRE, NFAT and SRE response elements attached to 5-HT to detect the activation of different 5-HT receptors in CHO-K1 cells. The potencies and efficacies of the reported ligands (agonists and antagonists) were determined and compared. Our results indicate that CRE-luciferase reporter gene is sensitive and reliable to detect the activities of G protein-coupled 5-HT receptors.
Similar content being viewed by others
Introduction
Currently, more than 50% of therapeutic drugs elicit their biological effect via GPCR. These drugs, which are involved in a broad range of biological processes, such as pain, cognition, hypertension, gastric ulcers, rhinitis and asthma, are responsible for a large portion of global pharmaceutical sales1,2,3. In 1988, the first 5-HT receptor was cloned and the others 5-HT receptors were cloned and studied functionally after that. The 5-HT receptor family is the largest G-protein coupled neurotransmitter receptor family except that 5-HT3 receptor is a ligand-gated ion channel receptor. The 5-HT1A, 5-HT2A and 5-HT7A receptors are the three major members of G-protein-coupled 5-HT receptors and are less than 25% homologous3, belonging to Gi, Gq, Gs-protein-coupled receptors respectively and mediate different signalling pathways. The 5-HT receptors regulate many physiological functions, such as sleep, mood, circadian rhythm, cognition and exercise, as well as the cardiovascular, respiratory and intestinal systems. Moreover, they are also closely linked to many mental status disorders, such as depression, anxiety, schizophrenia, obesity and hypertension. Furthermore, the 5-HT receptors are the targets of many drugs, such as Buspirone, Sarpogrelate and Paroxetine4. Therefore, establishing a simple and sensitive screening method for 5-HT receptor is particularly important.
Current methods employed in 5-HT reporter screening programs measure G protein signalling are mainly through determining the changes of second messengers, such as cAMP, inositol trisphosphate (IP3) and intracellular Ca2+, which often demand setting up different assay platforms, require specialized instruments for each pathway and are always costly and inconvenient5,6. While, the reporter gene assay is fast, highly sensitive and easy to set up6 and is based on reporter molecules, such as choline acetyl transferase, green fluorescent protein, firefly luciferase, β-galactosidase and secreted alkaline phosphatase, whose expression are under the control of second-messenger inducible response elements3. Among them, firefly luciferase is more suited to be used as a reporter molecule because of its high sensitivity and the quick development of specified reagents for firefly luciferase assays. Besides the diversification of reporter molecule, there are many available response elements for detecting the 5-HT receptors activation, such as the cAMP response element (CRE), serum response element (SRE) and nuclear factor of activated T cells (NFAT), making it hard to figure out which response element should be used.
As reported before, the binding of agonists to Gs-coupled 5-HT7A receptor causes a rise in intracellular cAMP, which activates protein kinase A (PKA), phosphorylating a CRE-binding protein (CREB), which then binds to a CRE in the promoter of a target gene and increases transcription7. Several previous studies demonstrated that the stimulation of the 5-HT7A reporter can also activate the expression of luciferase reporter mediated by SRE in NIH3T3 cells and a weak intracellular calcium influx in HEK 293 cells8,9. As for Gq-coupled 5-HT2A receptor, it can induce intracellular calcium influx, which dephosphorylates cytoplasmic NFAT, results in the activation of NFAT response element and stimulates gene expression. Meanwhile, Gq-coupled receptors can also activate SRE-mediated reporter-gene transcription via protein kinase C (PKC)-dependent MAP kinase activation7. Different from 5-HT7A receptor, stimulation of Gi-coupled 5-HT1A receptor decreases intracellular cAMP and reduces CRE-mediated reporter-gene transcription. During the above process, the βγ subunits of Gi activate SRE-mediated reporter-gene transcription via Ras-dependent MAP kinase activation7. Moreover, the 5-HT1A reporter can also activate intracellular calcium influx in certain cell types10,11. The signalling pathways of three 5-HT receptors were showed in figure 1.
Schematic diagram showing the major 5-HT receptors signalling pathways.
Upon stimulation, Gs coupled 5-HT7A receptors activate cAMP; Gi-coupled 5-HT1A receptors inhibit cAMP production and the βγ subunits activate the MAPK pathway; Gq-coupled 5-HT2A receptors activate phospholipase C (PLC) to produce inositol trisphosphate (IP3) and diacylglycerol (DAG), which increases intracellular Ca2+ concentration.
Although the detection of 5-HT receptors activation by the luciferase reporter gene assay have been reported by other groups8, the three available response elements mentioned above have not been systemically compared. To simplify the experiments, we tried to determine if the activation of different 5-HT receptors could be detected using a single response element. We compared three different response elements and found that CRE was reliable to be used to detect the activation of three different 5-HT receptors conveniently. Furthermore, the stability of CRE-based luciferase reporter gene assay had been proven with the reported ligands and a screening of a small compounds library was performed to demonstrate the availability of the assay. In conclusion, our experiments indicate that CRE-based luciferase reporter gene for detecting the activation of G protein-coupled 5-HT receptors is conveniently and effectively.
Results
Activity of 5-HT on different response elements based luciferase assay
For the preliminary experiments, different cell lines were used to transfect the reporter plasmid CRE-luc and the internal control plasmid pRL-SV40. Finally, CHO-K1 and 293T were selected for further analysis because they were widely used in cell transfection. Both cell lines were observed a cAMP-inducible luciferase expression after transfection of the reporter plasmid CRE-luc. Stimulated by 5-HT for 12–24 h, no nonspecific and endogenous activation was observed on CHO-K1 cells, while a significantly expression of luciferase was detected on 293T cells, which indicated that 293T cells may had endogenous receptors (Fig. 2A). Therefore, we used the CHO-K1 cell line for all further experiments.
Activity of 5-HT on different response elements based luciferase assay.
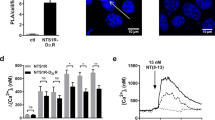
(A) Transfection of either 293T or CHO-K1 cells with CRE-luc and pRL-SV40 plasmids followed by stimulation with 10 μM 5-HT. (B) The maximum S/B resulting from 10 μM 5-HT treatment when using CRE, SRE and NFAT response elements to detect different receptors. (C) Transfection of CHO-K1 cells with the CRE-luc, 5-HT1A receptor and pRL-SV40 plasmids followed by stimulation with 5-HT in the presence of FSK (0.5 μM or 1 μM), luciferase activity was measured. The inhibition rate is expressed as a percentage of the indicated FSK-induced value. (D-F) Detection the activation of 5-HT1A, 5-HT2A and 5-HT7A receptors to different concentrations 5-HT in CRE-luc, NFAT-luc, SRE-luc assay. Luciferase activity was measured. Three independent experiments were performed in triplicate.
5-HT receptors could be detected by the expression of luciferase linked to three response elements: SRE, CRE and NFAT (Fig. 1). However, which one is more suitable to be used to establish general screening assay for 5-HT receptors is unclear. We compared the EC50 values and maximum S/B (Signal/Background, equate to Relative Luciferase Activity) of 5-HT in individual experiments with the three different response elements (Table 1, Fig. 2B). When we co-trancfected the 5-HT7A receptor plasmid with different response element-linked luciferase reporter plasmids in CHO-K1 cells, receptor activation by 5-HT could induce dose-dependent expression of luciferase with distinguishable potencies (Fig. 2D–F) and indicated in Table 1. The maximum S/B of 5-HT was highest in the CRE-luc reporter assay although the EC50 value of 5-HT obtained through the CRE-luc reporter assay was slightly larger than those obtained through SRE-luc reporter assays (Fig. 2B). After activation of 5-HT2A receptor by 5-HT, similar EC50 value was observed in the NFAT-luc, SRE-luc and CRE-luc reporter assays (Table 1), but the maximum S/B was the lowest in the NFAT-luc reporter assay (Fig. 2B). For Gi-coupled 5-HT1A receptor, CRE-luc based assays were usually performed in the presence of forskolin (FSK), a stimulator of adenylylcyclase, to raise the cAMP level so that the inhibition was easier to detect7. Compared to 1 μM FSK, 5-HT could achieve a better inhibitory effect when stimulated with 0.5 μM FSK (Fig. 2C). NFAT-luc based reporter assay was more efficient than SRE-luc reporter assay because of higher maximum S/B and lower EC50 values of 5-HT (Table 1, Fig. 2B). But when we used the NFAT-luc reporter assay to detect the activation of 5-HT1A receptor, Gα16 was need to co-transfected to couple the receptor to calcium mobilization, inducing dephosphorylates cytoplasmic NFAT and results in gene expression.
Further verification of the reliability of the CRE-based system
As indicated by the results above, the CRE-luc reporter assay looks more efficiently than SRE-luc or NFAT-luc reporter assays for detecting the 5-HT receptors activation. To further optimize this assay, we tried to adjust the amounts and proportions of the plasmids to achieve maximum S/B. We used 5-HT2A receptor as an example and selected five conditions for the experiment and found that the expression levels of luciferase were maximum when the cells were transfected with 5-HT2A expression vectors, CRE-luc and PRL-SV40 at 30, 40 and 90 ng, respectively (Fig. 3A). The similar results were also observed in the 5-HT1A and 5-HT7A receptors (data not shown).
Further verification of the reliability of the CRE-based system.
(A) CHO-K1 cells were co-transfected with CRE-luc, 5-HT2A receptor and pRL-SV40 plasmids in the different proportions. After the cells were incubated with 5-HT, luciferase activity was measured. (B) Cells transfected with 5-HT1A receptor, were incubated with agonist with or without antagonist, in the presence of 0.5 μM FSK. Luciferase activity was measured. The inhibition rate is expressed as a percentage of the indicated FSK-induced value. (C, E) Cells transfected with 5-HT2A receptor, were incubated with agonist with or without antagonist, or antagonist with 1 μM agonist. (D, F) Cells transfected with 5-HT7A receptor, were incubated with agonist with or without antagonist, or antagonist with 1 μM agonist. Three independent experiments were performed in triplicate.
Then, we used several reported agonists and antagonists of 5-HT receptor to find out if CRE-luc assay was suitable for the evaluation of 5-HT receptor ligands. 8-OH-DPAT is a potent agonist of 5-HT1A receptor and a partial agonist of 5-HT7A receptor. It had a weaker effect than 5-HT in the cells transfected with the 5-HT7A receptor, but it had a similar effect to 5-HT in the cells transfected with the 5-HT1A receptor and displayed an inhibition on the expression of luciferase caused by FSK (Fig. 3B, D). DOI is a potent agonist of 5-HT2A receptor with an estimated EC50 value of 33.2 nM, which was less than that for 5-HT (0.18 μM) in 5-HT2A transfected cells (Fig. 3C). All the EC50 values were shown in Table 1 and were similar to those reported by previous studies13,14,15,16,17.
Methiothepin, a universal antagonist of three 5-HT receptors, could dose-dependently inhibit the expression of the luciferase reporter gene induced by agonists in cells transfected with the 5-HT2A or 5-HT7A receptor (Fig. 3E, F) and caused a rightward parallel shift of the 5-HT dose-response curve when co-incubated with 5-HT in 5-HT1A receptor transfected cell, as expected for a competitive antagonist (Fig. 3B). 0.1 μM methiothepin could significantly suppress the expression of the luciferase reporter gene in 5-HT2A or 5-HT7A receptor transfected cells if the addition of 5-HT was lower than 1 μM (Fig. 3C, D). The selective 5-HT2A antagonist Katanserin and the selective 5-HT7A antagonist SB269970 also exhibited inhibition effect and were similar to the previous reports18 (Table 1, Fig. 3E, F). WAY100635, like methiothepin, a selective antagonist of 5-HT1A receptor also caused a rightward parallel shift of the 5-HT dose-response curve in 5-HT1A receptor transfected cells (Fig. 3B). All the EC50 and IC50 values were shown in Table 1 and were similar to those reported studies. To further identify the reliability of the CRE-based assay, we also performed a cAMP assay, which was usually applied in detecting the activation of 5-HT receptors and the results showed that EC50 of 5-HT and the IC50 of methiothepin were similar to those generated by CRE-luc assay in 5-HT1A receptor or 5-HT7A receptor transfected cells (Table 1).
Screening of a small compound library
In order to verify the ability of the CRE-luc assay, we performed a screening using a small compound library consisting of 133 compounds at 50 μM. Active compounds were defined as those inhibiting at least 50% of the luciferase expression and relative luciferase activity increases to at least 2.0 times. Applying this high threshold, we identified 14 active compounds as agonists and 3 active compounds as antagonist for 5-HT7A receptor; 4 active compounds as agonists and 7 active compounds as antagonist for 5-HT2A receptor. These results demonstrated that the screening assay is available for library screening (Fig. 4).
Screening of a small compound library.
Graphical presentation for the screening results of 133 compounds at 50 μM in the CRE-based luciferase assay. Each dot represents one compound. (A, B) Compounds above the red line showing more than 2.0 fold increasing of relative luciferase activity were defined as active agonists. (C, D) Compounds above the red line showing more than 50% inhibition were defined as active antagonist.
Discussion
Over the past several years, although screening methods targeting GPCRs, such as the radio-ligand binding assay, cAMP accumulation assay and calcium influx assay, developed rapidly, but they require specialized instruments and expensive reagents. Furthermore, the radio-ligand binding assay is harmful to humans and the environment. In contrast, the reporter gene assay is nonradioactive, highly sensitive and widely used. As mentioned above, choosing the best response element is important for reporter gene assays. To choose a single response element that was suitable for establishing a general 5-HT targeted screening assay, we compared three response elements in the reporter gene assay systemically.
To detect negative regulation of adenylyl cyclase by Gi-coupled 5-HT1A receptor, the system must first be active. To achieve this, FSK, the direct chemical modulator of adenylyl cyclase, was used. The EC50 value for FSK may vary depending on cell type and assay type12. Thus, it is important to determine the optimal concentration of FSK in our reporter gene assay system and the EC50 value of FSK was determined as 0.78 μM in our reporter gene assay system (data not shown). Thus, we used 0.5 μM and 1.0 μM FSK to optimize assay performance and the data showed that as 5-HT concentration increased, luciferase expression in CRE-luc cells decreased, with EC50 values of 6.17 nM and 8.56 nM in FSK concentrations of 0.5 μM and 1.0 μM, respectively. Therefore, a concentration of 0.5 μM FSK was used for stimulating the cells in the CRE-based reporter gene assay system. Since the expression of NFAT-luc required the co-transfection of Gα16 and the 5-HT showed less activity in SRE-luc transfected cell (Table 1), CRE-based reporter gene assay seems more reliable in test of 5-HT1A receptor. For the Gq protein-coupled receptor 5-HT2A, similar EC50 values were generated when 5-HT was added to the cells transfected with the three different response elements. The CRE-luc and SRE-luc assays showed higher maximum S/B than the NFAT-luc assay, but SRE-mediated gene transcription is known to be rapidly induced by serum; thus, serum must be removed when transfecting cells with SRE-luc, which may cause cell death. For the Gs protein-coupled 5-HT7A receptor, in the cells transfected with the CRE-based luciferase plasmid, 5-HT generated better EC50 values and maximum S/B than those transfected by other response elements.
The results suggest that the CRE-luciferase reporter gene assay is more easy to perform and can detect the activation of three types of G-protein coupled 5-HT receptors with a good effect. The reported ligands were used to confirm the reliability of this reporter gene assay and the EC50/IC50 values were similar to those reported in the literature and our cAMP quantification assay. To further confirm the reliability of the CRE-luc assay, we performed a screening of a compound library for 5-HT2A and 5-HT7A receptor. The screening campaign had identified several active compounds and could be developed for novel 5-HT agonist or antagonist in future. In summary, the CRE-based luciferase assay was suitable to monitor the activation of three different 5-HT receptors and can be easily adapted for compound screening. We suggest that the CRE-based luciferase assay may be also useful for detecting other 5-HT receptors and is beneficial for discovering novel 5-HT receptor ligands.
Methods
Materials
(+/−)-1-(2,5-Dimethoxy-4-iodophenyl)-2-aminopropane hydrochloride (DOI), 8-methoxy-2-tetralone (8-OH-DPAT), methiothepin, katanserin and Dulbecco's modified Eagle's/F12 medium (50:50, v/v) without phenol red were purchased from Sigma-Aldrich (St. Louis, MO). 5-HT was obtained from Santa Cruz Biotechnology (Santa Cruz, Inc., CA). SB269970 and WAY100635 were purchased from Selleck (Houston, USA). 5-HT1A and Gα16 plasmids were obtained from Bioworld (Minnesota, USA). pUNIV-5-HT2A was provided by Professor Jeanne Mialet-Perez (The cDNA Resource Center of Missouri-Rolla University, France). pCDNA3-5-HT7A was a gift from professor Kathleen Van Craenenbroeck (Ghent University, Belgium). The pCRE-Luc vector was purchased from Clontech (BD Biosciences Clontech, CA). The pGL3-NFAT-luc vector was kindly provided by Professor Karine Lefort (University of Lausanne, Switzerland) and the SRE-luc vector was kindly provided by professor Jae Young Seong (University College of Medicine, Korea). Dual Luciferase Assay kits were purchased from Promega (Madison, WI). Carbon absorption foetal bovine serum and Lipofectamine 2000 were purchased from Invitrogen (Carlsbad, CA). The CHO-K1 cells and 293T cells were obtained from the Institute of Biochemistry and Cell Biochemistry and Cell Biology (Shanghai, China). White opaque 96-well microplates were obtained from Corning (Acton, MA, USA).
Cell culture
CHO-K1 cells were grown in Dulbecco's modified Eagle's/F12 medium supplemented with 1.2 g/L sodium bicarbonate and 10% carbon absorption foetal bovine serum (FBS). 293T cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% carbon absorption foetal bovine serum (FBS). All cells were grown at 37°C and in an atmosphere of 5% CO2.
Cell transfection
Cells were seeded in 48-well plates and a cell monolayer would be 95% confluent at the time of transfection. A suspension of plasmid and Lipofectamine 2000 was preincubated in culture medium without FBS for 20 min before being added to each well of the 48-well plates. After 4–6 h, fresh medium with varied concentrations compounds was added to the wells.
Dual Luciferase Reporter Assay
Approximately 24 h post transfection, the cells were washed twice with PBS. Cell lysates were prepared by adding 100 µL of PLB to each well to break the cells and the plates were placed on ice for 30 min. The lysates from each well were transferred to wells of white opaque 96-well microplates. Dual-Glo® Reagent was added into each well and the firefly luminescence was measured in a microplate reader after 10 min. Then, an equal volume of Dual-Glo® Stop&Glo Reagent was added to the wells and the Renilla luminescence was measured after 10 min. Transfection experiments were performed in quadruplicate and were repeated at least three times.
Quantitative cAMP Measurements
CHO-K1 cells were transfected with 5-HT1A or 5-HT7A receptor and plated on 12-well plates for 16 h. For 5-HT1Areceptor, cells were pre-incubated with varied concentration of 5-HT and then stimulated for 30 min with 0.5 μM FSK. For 5-HT7A receptor, cells were stimulated with varied concentration of 5-HT or pre-incubated with varied concentration of Methiothepin then stimulated for 30 min with 1 μM 5-HT. Cellular cAMP levels were measured using a cAMP ELISA kit (catalog no. ADI-900-067, Enzo) according to the manufacturer's instructions.
Data Analysis
Luciferase activity of each sample was normalized to Renilla luciferase activity to correct for the differences in transfection efficiency. Relative Luciferase activity is expressed as the fold increases over control (Eq. 1) and the percentage inhibition of luciferase were calculated using the formula below (Eq. 2) and the EC50 and IC50 values were generated using origin7.5 software.


For Relative Luciferase activity, compound is agonist and the negative control is DMSO control. For percentage inhibition, In all 5-HT2A and 5-HT7A receptors transfected cell assay, positive control is 1 μM agonist and compound is antagonist in the presence of 1 μM agonist, negative control is DMSO, but in 5-HT1A receptor transfected CRE-based assay, positive control is 0.5 μM FSK, compound is agonist in the presence of 0.5 μM FSK.
References
Thomsen, W., Frazer, J. & Unett, D. Functional assays for screening GPCR targets. Curr. Opin. Biotechnol. 16, 655–665 (2005).
Wise, A., Jupe, S. C. & Rees, S. The identification of ligands at orphang-protein coupled-receptors. Annu. Rev. Pharmacol. Toxicol. 44, 43–66 (2004).
Roeder, T., Görich, D., Heyden, D. & Geweckeb, M. A green-fluorescent -protein-based assay for the characterization of G-protein-coupled receptors. Anal. Biochem. 332, 38–45 (2004).
Filip, M. & Bader, M. Overview on 5-HT receptors and their role in physiology and pathology of the central nervous system. Pharmacol. Rep. 76, 761–777 (2009).
Hans, B. O. & Brann, M. R. Pharmacology of muscarinic acetylcholine receptor subtypes (ml-m5)" high throughput assays in mammalian cells. Eur. J. Pharmacol. 295, 93–102 (1996)
Cheng, Z. et al. Luciferase Reporter Assay System for Deciphering GPCR Pathways. Current Chemical Genomics. 4, 84–91 (2010).
Hill, S. J., Baker, J. G. & Rees, S. Reporter-gene systems for the study of G-protein-coupled receptors. Curr. Opin. Pharmacol. 1, 526–532 (2001).
Kvachnina, E. et al. 5-HT7 receptor is coupled to Gα subunits of heterotrimeric G12-protein to regulate gene transcription and neuronal morphology. Ponimaskin. J. Neurosci. 25, 7821–7830 (2005).
Baker, L. P. et al. Stimulation of type 1 and type 8 Ca2+/Calmodulin-sensitive adenylyl cyclases by Gs-coupled 5-hydroxytryptamine subtype 5-HT7A receptor. J. Biol. Chem. 273, 17469–17476 (1998).
Raymond, J. R., Mukhin, Y. V., Gettys, T. W. & Garnovskaya, M. N. The recombinant 5-HT1A receptor: G protein coupling and signalling pathways. Br. J. Pharmacol. 127, 1751–1764 (1999).
Pauwels, P. J. & Colpaert, F. C. Ca2+ responses in Chinese Hamster Ovary-K1 cells demonstrate an atypical pattern of ligand-induced 5-HT1A receptor activation. J Pharmacol Exp Ther. 307, 608–614 (2003).
Williams, C. cAMP detection methods in HTS: selecting the best from the rest. Nat. Rev. Drug Discovery. 3, 125–135 (2004).
Raymond, J. R., Albers, F. J. & Middleton, J. P. Functional expression of human 5-HTIA receptors and differential coupling to second messengers in CHO cells. Naunyn Schmiedebergs Arch Pharmacol. 346, 127–137 (1992).
Philippe, S. & Daniel, H. Centrally acting hypotensive agents with affinity for 5-HTlA binding sites inhibit forskolin-stimulated adenylate cyclase activity in calf hippocampus. Br. J. Pharmacol. 95, 975–985 (1988).
Wurch, T., Colpaert, F. C. & Pauwels, P. J. Mutation in a protein kinase C phosphorylation site of the 5-HT1A receptor preferentially attenuates Ca2+ responses to partial as opposed to higher-efficacy 5-HT1A agonists. Neuropharmacology. 44, 873–881 (2003).
Porter, R. H. P. et al. Functional characterization of agonists at recombinant human 5-HT2A, 5-HT2B and 5-HT2C receptors in CHO-K1 cells. Br. J. Pharmacol. 128, 13–20 (1999).
Lestienne, L. R. et al. Differential profile of typical, atypical and third generation antipsychotics at human 5-HT7a receptors coupled to adenylyl cyclase: detection of agonist and inverse agonist properties. Naunyn Schmiedebergs Arch Pharmacol. 376, 93–105 (2007).
Knight, J. A. et al. Pharmacological analysis of the novel, rapid and potent inactivation of the human 5-Hydroxytryptamine7 receptor by risperidone, 9-OH-risperidone and other Inactivating Antagonists. Mol Pharmacol. 75, 374–380 (2009).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grants 81402482, 91313303, 81102420 and 51003044), the Shanghai Committee of Science and Technology (14ZR1411100), China Postdoctoral Science Foundation grant (2014M551361), the Jiangxi Natural Science Foundation (20132BAB206035) and the Floor plan of scientific and technological projects in Jiangxi (KJLD13023).
Author information
Authors and Affiliations
Contributions
Y.C., W.L., J.H. and C.S. conceived and designed the experiments. Y.C., Z.X., D.W., performed the experiments. Y.C. W.L., J.L. and J.H. analysed the data and wrote the manuscript. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Chen, Y., Xu, Z., Wu, D. et al. Luciferase Reporter Gene Assay on Human 5-HT Receptor: Which Response Element Should Be Chosen?. Sci Rep 5, 8060 (2015). https://doi.org/10.1038/srep08060
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep08060
This article is cited by
-
ACBD3 modulates KDEL receptor interaction with PKA for its trafficking via tubulovesicular carrier
BMC Biology (2021)
-
Ferulic acid alleviates abnormal behaviors in isolation-reared mice via 5-HT1A receptor partial agonist activity
Psychopharmacology (2021)
-
Complementary roles of murine NaV1.7, NaV1.8 and NaV1.9 in acute itch signalling
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.