Abstract
Two-dimensional (2D) molybdenum disulphide (MoS2) atomic layers have a strong potential to be used as 2D electronic sensor components. However, intrinsic synthesis challenges have made this task difficult. In addition, the detection mechanisms for gas molecules are not fully understood. Here, we report a high-performance gas sensor constructed using atomic-layered MoS2 synthesised by chemical vapour deposition (CVD). A highly sensitive and selective gas sensor based on the CVD-synthesised MoS2 was developed. In situ photoluminescence characterisation revealed the charge transfer mechanism between the gas molecules and MoS2, which was validated by theoretical calculations. First-principles density functional theory calculations indicated that NO2 and NH3 molecules have negative adsorption energies (i.e., the adsorption processes are exothermic). Thus, NO2 and NH3 molecules are likely to adsorb onto the surface of the MoS2. The in situ PL characterisation of the changes in the peaks corresponding to charged trions and neutral excitons via gas adsorption processes was used to elucidate the mechanisms of charge transfer between the MoS2 and the gas molecules.
Similar content being viewed by others
Introduction
Over the past few decades, metal oxide semiconductors have been applied as conventional chemical sensing materials because of their high sensitivity and relatively low cost1,2,3. However, they still have some critical drawbacks. First, metal oxide semiconductors exhibit poor sensitivity and selectivity at room temperature. This obstacle has led to the development of alternative materials such as carbon nanotubes4, graphene5 and transition metal dichalcogenides (TMDs)6,7,8,9,10,11. Recently, 2D TMDs have attracted much attention for use in next-generation nanoelectronic devices12,13,14, with a single-layer MoS2 transistor having been reported to exhibit outstanding performance15. The intrinsic merits of TMDs, including their high surface-to-volume ratio and semiconducting properties, have accelerated the development of a diverse range of applications of these materials as chemical sensors. A recent flurry of research involving MoS2-based gas detection has mitigated the wide chasm between metal oxide materials and alternatives6,7,8,9,10,11. However, the fundamental mechanism of chemical sensing using MoS2 remains unclear, limiting its practical applications. Here, we demonstrate highly sensitive and selective gas detection of NO2 and NH3 using uniform wafer-scale MoS2 nanofilms synthesised by thermal chemical vapour deposition (CVD). We elucidate the charge transfer mechanism of MoS2 gas adsorption using in situ photoluminescence (PL) and computational calculations involving first-principles density functional theory. The peak intensities from the positively charged trions (A+) and neutral excitons (A0) in the PL spectrum show trade-off phenomena by adsorption of each different gas molecule (NO2 or NH3) onto the MoS2. The electron depletion of MoS2 by NO2 adsorption leads to an increase in the intensity of the A+ peak and a suppression of the intensity of the A0 peak, whereas electron accumulation by NH3 adsorption suppresses the intensity of the A+ peak and increases the intensity of the A0 peak. These in situ PL characterisation results clarify the mechanisms of charge transfer between the MoS2 and the gas molecules. These findings will help to implement future gas sensing technologies using diverse two dimensional TMDs nanomaterials.
Results
Wafer-scale synthesis of atomic-layered MoS2
Most approaches use direct/indirect sulphurisation of Mo-containing thin films to synthesise atomic-layered MoS2 thin films. The precursor is a key factor in the synthesis of MoS2. In previous studies, most authors adopted one of three precursors: molybdenum thin films16; molybdenum trioxide17; or ammonium thiomolybdate18. However, previous methods have involved complex precursor preparations, yielding films with inconsistent quality. In our search for strategies for synthesising uniform wafer-scale MoS2 (see schematic in Fig. 1a), we have focused on the development of a thermal CVD system and process. Atomic-layered MoS2 was grown using molybdenum trioxide (MoO3) deposited onto a sapphire substrate and a sulphur powder source. The sublimated sulphur served as a precursor to sulphurise the MoO3 film. To achieve our overall goal of preparing MoS2 films of consistent quality on the desired substrates, we turned our attention to pressure control during the CVD reaction. A recent report indicated that an increase in the amount of either Mo or S atoms results in increased formation of energetically favourable defects on the MoS2 surface during film growth19. Thus, we systematically controlled the reaction pressure to provide sufficient sublimated sulphur using a custom-made automatic pressure control system (Supplementary Fig. S1).
Large-scale synthesis of MoS2.
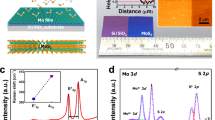
(a) Schematic of the atomic-layered MoS2. The quasi-2D MoS2 was occupied by one Mo (a trigonal prismatic structure) and two S atoms (hexagonal planes). (b) Image of the as-synthesised MoS2 film on the 2-inch sapphire substrate. The as-synthesised MoS2 film was semi-transparent. (c) Cross-sectional TEM images of the as-grown MoS2 films. The image clearly demonstrates that the synthesised MoS2 films consisted of three layers of MoS2. (d) Raman spectrum of the triple-layered MoS2. The spectrum reveals a strong in-plane vibrational mode for the Mo and S atoms (E2g) and an out-of-plane vibrational mode for the S atoms (A1g). The peak position difference (Δ) between the E2g and A1g bands is approximately 22.9, indicating triple-layered MoS2. (e, f) Raman maps of E2g (blue) and A1g (red), respectively. The Raman mapping area was 50 × 50 μm2 with 0.3 μm steps. The Raman images show the spatial distribution on the surface of the substrates.
The new CVD system design was very effective for the uniform synthesis of MoS2 films on 2-inch sapphire substrates, as illustrated in Fig. 1b. Cross-sectional transmission electron microscopy (TEM) was used to examine the number of layers formed by CVD (Fig. 1c). The MoS2 films contained double, triple, and, in some cases, more than three layers (additional TEM images, TEM energy-dispersive X-ray spectroscopy (EDS) maps, TEM EDS point spectra, atomic force microscopy images, X-ray photoelectron spectra and absorption spectra are provided in Supplementary Figs. S2–7). The Raman spectrum in Fig. 1d shows the in-plane vibrational mode of the Mo and S atoms (E2g) and the out-of-plane vibrational mode of S atoms (A1g) in the as-synthesised MoS2 films20. The difference in peak position (Δ) between the E2g and A1g bands, which is a strong indicator of the number of layers, was approximately 22.9. This result indicates that the as-synthesised MoS2 was mainly composed of three layers18,21,22. To confirm the wafer-scale synthesis of MoS2, we conducted a large-scale structural analysis using Raman mapping and an imaging technique. The Raman mapping area was 50 × 50 μm2 with 0.3 μm steps (the original Raman mapping spectra are shown in Supplementary Fig. S8). The corresponding Raman images revealed the spatial distribution of MoS2 over a 250-μm2 area of the substrate (Figs. 1e and f). The blue and red models show the spatial distributions of the E2g and A1g bands, respectively. The as-synthesised MoS2 was highly uniform over a large area of the surface (Figs. 1e and f). Thus, systematic pressure control during the CVD process resulted in highly uniform MoS2 films on the wafer scale.
Gas detection characteristics of the MoS2 gas sensor
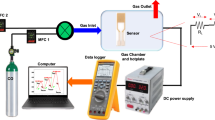
The uniform atomic-layered MoS2 films were used for gas molecule detection (Fig. 2a). Transient resistance responses were investigated using two analyte gases (NO2 or NH3 at concentrations from 1.2 to 50 ppm). The gas sensitivity was calculated using ΔR/Ra = (Rg - Ra)/Ra, where Ra and Rg represent the resistances of the device to air and the analyte gas, respectively. In the NO2 gas mode, the resistance increased (positive sensitivity) (Fig. 2b). The NO2 sensitivity values were comparable to those in a previous report8. NO2 acts as an electron acceptor, resulting in p-doping (Supplementary Fig. S12). The NO2 molecules on the surface of MoS2 bring the Fermi level closer to the valence-band edge. During the desorption process, thermal energy (heating to 100°C) enhances the rate of desorption of the NO2 molecules from the MoS2 film (Fig. 2b, red line). We next compared the gas sensing characteristics for NH3 to those for NO2 (Fig. 2c). In contrast to the resistance recorded for NO2 molecules, the resistance of the MoS2 sensing device decreased with the adsorption of NH3 gas molecules, i.e., negative sensitivity was observed. NH3 acts as an electron donor (i.e., n-doping) such that it shifts the Fermi level of the MoS2 to the conduction-band edge. However, theoretical calculations indicated that the Fermi-level shift induced by the NH3 molecules is negligible (Supplementary Fig. S12). The measured overall NH3 sensitivities were lower than those of NO2 (Figs. 2b and c) because of the smaller charge transfer of NH3 compared to that of NO223. The dependence of the gas response on the gas concentration at different operating temperatures is plotted in Fig. 2d. The surface chemical reaction between the MoS2 channel and the NO2 molecules saturated at approximately 20 ppm, irrespective of the operating temperature (Fig. 2d, red and blue lines). By contrast, in the case of NH3, the sensitivity at RT gradually increased from 5 to 50 ppm and the sensitivity under 5 ppm was undetectable. However, the sensing signal at 100°C was imperceptible at all concentrations (Supplementary Fig. S9). Thus, the recovery rate of NO2 at 100°C is clearly superior to that of NH3, which is closely related to the faster desorption process of NO2 molecules as a result of the thermal energy24,25. At 20 ppm and 100°C, we obtained the best selectivity for NO2 relative to NH3 (~400% increase compared to that at RT).
Gas-sensing using the MoS2 device.
(a) 3D schematic of the MoS2 gas-sensing device for NO2 and NH3. (b) Transient NO2 gas response at 1.5 to 50 ppm gas at operating temperatures of RT and 100°C. In the NO2 gas mode, the resistance increases (positive sensitivity). The recovery rate of NO2 is higher at 100°C than at RT. (c) The transient NH3 gas response at 1.5 to 50 ppm gas at operating temperatures of RT and 100°C. The resistance decreases with the adsorption of NH3 gas molecules (negative sensitivity). The NH3 sensing signal is negligible at 100°C. (d) Comparison of the NO2 and NH3 sensitivities at different gas concentrations and operating temperatures. The highest selectivity of NO2 to NH3 was obtained when the concentration reached 20 ppm at 100°C.
In situ photoluminescence of the MoS2-based gas sensor
To explore the gas adsorption characteristics of the MoS2, we adopted theoretical and experimental approaches. First-principles density functional theory (DFT) calculations were conducted using the screened hybrid functional of Heyd-Scuseria-Ernzerhof with the D2 correction for van der Waals interactions26,27 (see the detailed methods in the Supplementary Information). To simulate NO2 and NH3 adsorption onto the MoS2 monolayer, supercells containing 16 Mo and 32 S atoms with NO2 and NH3 were employed using a 2 × 2 × 1 k-point grid. The most stable configurations of NO2 and NH3 reported in a recent study that compared the total energy between different adsorption configurations28 were considered. The NO2 and NH3 molecules were preferentially adsorbed onto the top of the hexagon of the MoS228 (Figs. 3a and b). The adsorption energies of the NO2 and NH3 gas molecules were evaluated using  , where
, where  is the total energy of a supercell containing both an MoS2 monolayer and a gas molecule (NO2 or NH3),
is the total energy of a supercell containing both an MoS2 monolayer and a gas molecule (NO2 or NH3),  is the total energy of the host MoS2 supercell and E(molecule) is the total energy of a supercell containing a gas molecule. The calculated adsorption energies of NO2 and NH3 were −0.14 eV and −0.16 eV, respectively. These values were ~0.1 eV smaller than the values obtained using the local density approximation (LDA) because the LDA functional overestimates the adsorption energy28. The negative adsorption energies indicate that the adsorption process is exothermic. Thus, NO2 and NH3 molecules are likely to be adsorbed onto the surface of MoS2.
is the total energy of the host MoS2 supercell and E(molecule) is the total energy of a supercell containing a gas molecule. The calculated adsorption energies of NO2 and NH3 were −0.14 eV and −0.16 eV, respectively. These values were ~0.1 eV smaller than the values obtained using the local density approximation (LDA) because the LDA functional overestimates the adsorption energy28. The negative adsorption energies indicate that the adsorption process is exothermic. Thus, NO2 and NH3 molecules are likely to be adsorbed onto the surface of MoS2.
Adsorption configurations and in situ PL.
(a, b) Top views of the most favourable configurations for NO2 (a) and NH3 (b) on the MoS2. The calculated adsorption energies were −0.14 eV for NO2 and −0.16 eV for NH3. The negative adsorption energies indicate that the adsorption process is exothermic, indicating that NO2 and NH3 molecules are likely to be adsorbed onto the surface of the MoS2. (c, d) In situ PL spectra recorded from the MoS2 with NO2 (c) and NH3 (d) molecules. The overall intensity of the PL spectra changes in the presence of NO2 and NH3 molecules. The PL intensities of the A+ trions and A0 excitons are either suppressed or increased by changes in the concentrations of the charge carriers. (e, f) Schematics of the charge density differences for MoS2 in the presence of NO2 (e) and NH3 (f) gas molecules. NO2 molecules on the surface of MoS2 act as electron acceptors, whereas NH3 molecules act as electron donors.
Next, we turned our attention to the in situ characterisation of PL to study the sensing mechanism in depth. The high temperature during film growth can induce unintentional defects on the substrate/MoS2 interface. To prevent defects, we transferred the as-grown MoS2 from the sapphire substrate to a SiO2/p + Si substrate. Interestingly, the atomic-layered MoS2 transferred onto the SiO2/p + Si substrate exhibited opposite gas sensitivity (i.e., p-type behaviour) compared to the n-type behaviour of MoS2 on the sapphire substrate (see Supplementary Fig. S13 for details). Dangling bonds at the semiconductor/substrate interface can redefine the effective Fermi levels within gap states, modulating the conductive properties of the MoS229. The dangling oxygen bonds on the SiO2 surface can result in a p-type MoS2 semiconductor29. In the in situ PL characterisation with the two analyte gases (see Supplementary Fig. S10 for details), we observed various intensity changes in the PL spectra for NO2 and NH3 molecules (Figs. 3c and d). The atomic-layered MoS2 had two main PL peaks associated with the A and B excitons30 (Supplementary Figs. S11). Spin-orbit coupling-induced valence-band splitting can give rise to A and B excitons31. After the gas molecules are adsorbed, the PL intensities of the A and B excitons can either be suppressed or increased by changing the concentrations of the charge carriers32,33. We here focused on the signal peak of the A exciton. The relatively low-energy A exciton signal expands to two features: a trion of A−/+ (two electrons to a hole, resulting in a negatively charged exciton, or an electron to two holes, resulting in a positively charged exciton) and a neutral exciton of A0 32,33. With the emergence of the trion, we assumed that the exciton is coupled to either another electron or to a hole at the Fermi level. In the case of MoS2, the A+ and A0 peaks correspond to the trions (1.8413 eV) and to the neutral excitons (1.8424 eV), respectively. The bare MoS2 notwithstanding, the positive trion (in this case, an electron to two holes, resulting in positively charged excitons, A+) emission dominates the PL spectra because the bare MoS2 on the SiO2 substrate exhibits p-type characteristics, as previously mentioned32. As schematically shown in Fig. 3e, the NO2 molecules on the surface of the MoS2 act as electron acceptors (p-type dopants), whereas the NH3 molecules act as electron donors (n-type dopants)28. By adsorption of NO2 gas molecules, a neutral exciton (A0) can be converted into a quasi-particle (A+) because of excessive holes generated by electron extraction from the MoS234. As a result, the A+ peak in the PL spectrum increases in intensity and the A0 peak is suppressed (Fig. 3c). By contrast, when additional electrons are introduced from the NH3 molecules, the intensity of the A+ peak in the PL spectrum is suppressed because of dissociation of the positive trions from the neutral excitons, resulting in increasing neutral excitons (A0)35, as shown in Fig. 3d. The in situ PL characterisation clarifies the mechanisms of charge transfer between the MoS2 and the gas molecules.
Discussion
The gas sensing characteristics of wafer-scale layered MoS2 fabricated by CVD were determined. The gas sensor based on the CVD-fabricated MoS2 exhibited excellent sensitivity and high selectivity. The in situ PL characterisation and theoretical studies elucidated the charge-transfer mechanism between the gas molecules and the MoS2. In-depth PL studies verified that the electron depletion of the MoS2 by NO2 adsorption increased the intensity of the A+ peak and suppressed that of the A0 peak, whereas electron accumulation by NH3 adsorption suppressed the intensity of the A+ peak and increased that of the A0 peak. Intensive PL characterisation clarified the charge transfer phenomena between the MoS2 and the gas molecules. The results of this study will enable more extensive applications of gas sensing using two dimensional transition metal dichalcogenides nanomaterials.
Methods
CVD synthesis of the MoS2 nanofilms
MoS2 nanofilms were synthesised using chemical vapour deposition (CVD) (Teraleader Co., Ltd., South Korea) (Supplementary Fig. S1). First, C-plane sapphire substrates were prepared using a typical cleaning process (sonication in acetone, isopropyl alcohol and deionised water for 10 min each). MoO3 films (5 nm) were deposited onto the clean substrates using a thermal evaporator. The pre-deposited MoO3 samples were placed at the centre of the furnace and ~1 g of sulphur powder, which was used as a sulphur precursor, was subsequently loaded into a quartz boat in an independently temperature-controllable flange heater located near the inlet of the furnace. The furnace and the flange heater were heated to ~850°C and ~180°C, respectively, for 1 h. The process was maintained for an additional 1 h under flowing Ar/H2 gas (volume ratio: Ar:H2 = 85:15%) at a chamber pressure of 760 torr. The MoO3 film was converted into a MoS2 nanofilm via a two-step reaction (the reduction of MoO3 by hydrogen gas, followed by sulphurisation of the reduced MoO3 with sublimated sulphur gases). Finally, the furnace was rapidly cooled to room temperature by opening the chamber box after the furnace was turned off.
Fabrication of the MoS2 sensing device
The SiO2 (300 nm)/C-plane sapphire was cleaned using a typical cleaning process (sequential sonication in acetone, isopropyl alcohol and deionised water for 10 min each). A MoO3 film (~5 nm thick) was patterned with an active shadow mask using a thermal evaporator. The patterned MoO3 film was converted into a MoS2 nanofilm by CVD. Using a thermal evaporator and a shadow mask with an interdigitated electrode array structure consisting of two opposing comb-shaped electrodes with a width of 400 μm and a gap of 100 μm, we deposited an Ag film (100 nm; used for the electrodes) onto the MoS2 nanofilm. Detailed fabrication schemes are provided in Supplementary Fig. S14.
Characterisation of CVD-synthesised MoS2, gas sensing, DFT calculations, in situ PL tests
See the detailed methods in the Supplementary Information.
References
Fine, G. F., Cavanagh, L. M., Afonja, A. & Binions, R. Metal oxide semi-conductor gas sensors in environmental monitoring. Sensors (Basel). 10, 5469–5502 (2010).
Pearton, S. J. et al. Recent advances in wide bandgap semiconductor biological and gas sensors. Prog. Mater. Sci. 55, 1–59 (2010).
Huang, J. & Wan, Q. Gas sensors based on semiconducting metal oxide one-dimensional nanostructures. Sensors (Basel). 9, 9903–9924 (2009).
Kong, J. Nanotube Molecular Wires as Chemical Sensors. Sience 287, 622–625 (2000).
Schedin, F. et al. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 6, 652–655 (2007).
Late, D. J. et al. Sensing Behavior of Atomically Thin-Layered MoS2 Transistors. ACS Nano 7, 4879–4891 (2013).
Li, H. et al. Fabrication of single- and multilayer MoS2 film-based field-effect transistors for sensing NO at room temperature. Small 8, 63–67 (2012).
He, Q. et al. Fabrication of flexible MoS2 thin-film transistor arrays for practical gas-sensing applications. Small 8, 2994–2999 (2012).
Lee, K., Gatensby, R., McEvoy, N., Hallam, T. & Duesberg, G. S. High Performance Sensors Based on Molybdenum Disulfide Thin Films. Adv. Mater. 25, 6699–6702 (2013).
Yao, Y. et al. High-concentration aqueous dispersions of MoS2 . Adv. Funct. Mater. 23, 3577–3583 (2013).
Perkins, F. K. et al. Chemical vapor sensing with monolayer MoS2 . Nano Lett. 13, 668–673 (2013).
Wang, Q. H., Kalantar-Zadeh, K., Kis, A., Coleman, J. N. & Strano, M. S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 7, 699–712 (2012).
Ganatra, R. & Zhang, Q. Few-Layer MoS2: A Promising Layered Semiconductor. ACS Nano 8, 4074–4099 (2014).
Jariwala, D., Sangwan, V. K., Lauhon, L. J., Marks, T. J. & Hersam, M. C. Emerging Device Applications for Semiconducting Two-Dimensional Transition Metal Dichalcogenides. ACS Nano 8, 1102–1120 (2014).
Radisavljevic, B., Radenovic, A., Brivio, J., Giacometti, V. & Kis, A. Single-layer MoS2 transistors. Nat. Nanotechnol. 6, 147–150 (2011).
Lee, Y. et al. Synthesis of wafer-scale uniform molybdenum disulfide films with control over the layer number using a gas phase sulfur precursor. Nanoscale 6, 2821–2826 (2014).
Lin, Y. -C. et al. Wafer-scale MoS2 thin layers prepared by MoO3 sulfurization. Nanoscale 4, 6637–6641 (2012).
Liu, K. -K. et al. Growth of large-area and highly crystalline MoS2 thin layers on insulating substrates. Nano Lett. 12, 1538–1544 (2012).
Najmaei, S. et al. Vapour phase growth and grain boundary structure of molybdenum disulphide atomic layers. Nat. Mater. 12, 754–759 (2013).
Bertrand, P. A. Surface-phonon dispersion of MoS2 . Phys. Rev. B 44, 5745–5749 (1991).
Li, S. et al. Quantitative raman spectrum and reliable thickness identification for atomic layers on insulating substrates. ACS Nano 6, 7381–7388 (2012).
Liu, Y. et al. Layer-by-layer thinning of MoS2 by plasma. ACS Nano 7, 4202–4209 (2013).
Yue, Q., Shao, Z., Chang, S. & Li, J. Adsorption of gas molecules on monolayer MoS2 and effect of applied electric field. Nanoscale Res. Lett. 8, 425 (2013).
Yavari, F. et al. High sensitivity gas detection using a macroscopic three-dimensional graphene foam network. Sci. Rep. 1, 166 (2011).
Choi, H. et al. Flexible and transparent gas molecule sensor integrated with sensing and heating graphene layers. Small 10, 3812 (2014).
Heyd, J., Scuseria, G. E. & Ernzerhof, M. Hybrid functionals based on a screened Coulomb potential. J. Chem. Phys. 118, 8207–8215 (2003).
Krukau, A. V., Vydrov, O. a., Izmaylov, A. F. & Scuseria, G. E. Influence of the exchange screening parameter on the performance of screened hybrid functionals. J. Chem. Phys. 125, 224106 (2006).
Yue, Q., Shao, Z., Chang, S. & Li, J. Adsorption of gas molecules on monolayer MoS2 and effect of applied electric field. Nanoscale Res. Lett. 8, 425 (2013).
Dolui, K., Rungger, I. & Sanvito, S. Origin of the n-type and p-type conductivity of MoS2 monolayers on a SiO2 substrate. Phys. Rev. B 87, 165402 (2013).
Splendiani, A. et al. Emerging photoluminescence in monolayer MoS2 . Nano Lett. 10, 1271–1275 (2010).
Coehoorn, R., Haas, C. & de Groot, R. A. Electronic structure of MoSe2, MoS2 and WSe2. II. The nature of the optical band gaps. Phys. Rev. B 35, 6203–6206 (1987).
Mak, K. F. et al. Tightly bound trions in monolayer MoS2 . Nat. Mater. 12, 207–211 (2013).
Mouri, S., Miyauchi, Y. & Matsuda, K. Tunable photoluminescence of monolayer MoS2 via chemical doping. Nano Lett. 13, 5944–5948 (2013).
Ross, J. S. et al. Electrical control of neutral and charged excitons in a monolayer semiconductor. Nat. Commun. 4, 1474 (2013).
Mao, N., Chen, Y., Liu, D., Zhang, J. & Xie, L. Solvatochromic effect on the photoluminescence of MoS2, monolayers. Small 9, 1312–1315 (2013).
Acknowledgements
This study was supported financially by the Fundamental Research Program (PNK3770 and PNK4060) of the Korean Institute of Materials Science (KIMS) and by the “Gyeongsangnam, Changwon Science Research Park Project” of the Grant of the Korean Ministry of Science, ICT and Future Planning. M. G. H. and B. C. are grateful for support from the Basic Science Research Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2014R1A1A1006214 and NRF-2014R1A1A1036139). MC was supported by Global Frontier Program through the Global Frontier Hybrid Interface Materials (GFHIM) of the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2013M3A6B1078872).
Author information
Authors and Affiliations
Contributions
B.C., M.G.H. and D.-H.K. designed and supervised the experiments. B.C., M.G.H. and A.R.K. synthesised the MoS2. B.C., M.G.H., J.Y., A.R.K. and Y.-J.L. characterised the MoS2. B.C. and A.R.K. fabricated the MoS2-based devices. B.C., M.G.H., S.L., T.J.Y. and C.G.K. measured the MoS2 sensing devices. M.G.H. and M.C. performed the first-principles DFT calculations for the MoS2 with gas molecules. B.C., M.G.H., M.C., S.-G.P., J.-D.K., C.S.K., M.S., Y.J., K.-S.N., B.H.L., H.C.K., P.M.A. and D.-H.K. analysed the data. B.C., M.G.H. and D.-H.K. co-wrote the paper. All authors discussed the results and commented on the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Charge-transfer-based Gas Sensing Using Atomic-layer MoS2
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Cho, B., Hahm, M., Choi, M. et al. Charge-transfer-based Gas Sensing Using Atomic-layer MoS2. Sci Rep 5, 8052 (2015). https://doi.org/10.1038/srep08052
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep08052
This article is cited by
-
Detection of H2S, HF and H2 pollutant gases on the surface of penta-PdAs2 monolayer using DFT approach
Scientific Reports (2023)
-
Hot wire-CVD grown molybdenum disulfide (MoS2) thin films for photodetector and humidity sensing applications
Journal of Materials Science: Materials in Electronics (2023)
-
Influence of MoS2 film thickness for nitric oxide gas sensing applications
Journal of Materials Science: Materials in Electronics (2023)
-
Adsorption of industry affiliated gases on buckled aluminene for gas sensing applications
Journal of Molecular Modeling (2023)
-
Room-temperature nitrogen dioxide gas sensor based on graphene oxide nanoribbons decorated with MoS2 nanospheres
Applied Physics A (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.