Abstract
Cinnamon extract is associated to different health benefits but the active ingredients or pathways are unknown. Cinnamaldehyde (CIN) imparts the characteristic flavor to cinnamon and is known to be the main agonist of transient receptor potential-ankyrin receptor 1 (TRPA1). Here, expression of TRPA1 in epithelial mouse stomach cells is described. After receiving a single-dose of CIN, mice significantly reduce cumulative food intake and gastric emptying rates. Co-localization of TRPA1 and ghrelin in enteroendocrine cells of the duodenum is observed both in vivo and in the MGN3-1 cell line, a ghrelin secreting cell model, where incubation with CIN up-regulates expression of TRPA1 and Insulin receptor genes. Ghrelin secreted in the culture medium was quantified following CIN stimulation and we observe that octanoyl and total ghrelin are significantly lower than in control conditions. Additionally, obese mice fed for five weeks with CIN-containing diet significantly reduce their cumulative body weight gain and improve glucose tolerance without detectable modification of insulin secretion. Finally, in adipose tissue up-regulation of genes related to fatty acid oxidation was observed. Taken together, the results confirm anti-hyperglycemic and anti-obesity effects of CIN opening a new approach to investigate how certain spice derived compounds regulate endogenous ghrelin release for therapeutic intervention.
Similar content being viewed by others
Introduction
Beyond the taste impact of spices, numerous health benefits have been ascribed to naturally derived flavoring molecules. However, the molecular mechanisms by which these molecules mediate their effects remain largely unknown.
Cinnamon is a spice used in cooking since biblical times. It is a brown powder obtained from the inner side of the bark of different species of the genus Cinnamomun. 90% of the essential oil of the cinnamon bark is Cinnamaldehyde (CIN), one of the active components of cinnamon that gives its typical flavor and odor. Cinnamon extract has been linked to many different health benefits. There are numerous reports about the role of cinnamon in obesity and diabetic conditions1,2,3,4. So far, there is neither consensus about the constituents of cinnamon driving these effects nor the molecular pathways responsible for its benefits. CIN has been already described to be responsible for the anti-diabetic5,6 and anti-obesity properties of cinnamon3,4.
CIN is also well-known as the principal agonist of TRPA1 (transient receptor potential-ankyrin receptor 1)7. TRP channels are non-selective cation channels that participate in diverse sensory processes, including cold and warm nociception, hearing, or inflammatory pain8,9,10. Besides CIN, several naturally occurring compounds activate TRPA1 such as mustard, garlic and horseradish, which produce a pungent sensation7,11. TRPA1 is expressed in sensory neurons but recently has been shown to be expressed in the gastrointestinal tract as well as in many different epithelial cells in the intestine and also in the lungs12,13,14. TRPA1 expressed in the gut has been described as a sensor molecule of enterochromaffin cells for the regulation of gastrointestinal functions14,15 through serotonin release.
Obesity is one of the greatest public health challenges of the 21st century. Its prevalence has tripled in many countries of the European Region since the 1980s and the number of people affected continues to rise at an alarming rate. Excess weight drastically increases a person's risk of developing a number of noncommunicable diseases, including cardiovascular disease, cancer and diabetes. Many different spices are known for their use in traditional medicine and positive effects in human health. Here the effect of CIN in glycemia and weight management control is reported.
The physiological effects of cinnamaldehyde ingestion in the gastrointestinal tract, caused by TRPA1 activation are: modifications in gut motility14,16, gastric emptying15, cholecystokinin secretion in the small intestine17 and serotonin release from intestinal enterochromaffin cells14. Very recently also the expression and role of TRPA1 in insulin release from pancreatic β-cells has been described18. In addition, CIN through TRPA1 activation can have an effect on the metabolism as an enhancer of energy expenditure through adrenaline secretion19 and autonomic thermoregulation20 Confirming this pathway, it has been also shown that CIN stimulates interscapular brown adipose tissue, by an increase of thermogenin (UCP1) protein and visceral fat deposition in mice fed with high fat and sucrose diet19,21.
This study aimed to investigate whether TRPA1-agonists affect food intake and gastric emptying via ghrelin release. Ghrelin is an orexigenic hormone produced predominantly in the stomach and proximal small bowel. Ghrelin also acts as endogenous ligand for the growth hormone secretagogue receptor and as a potent stimulator of feeding22. Recently, ghrelin has been implicated in the regulation of glucose homeostasis and insulin levels23,24. It has been suggested that ghrelin antagonists could provide a potential therapeutic solution to stop the progression of type 2 diabetes. The expression pattern and gene expression of TRPA1 in stomach and small intestine were determined. In vivo (acute and long-term treatment) and in vitro evaluation of the CIN effect in the gut physiology and ghrelin secretion respectively was performed. Moreover, the expression of TRPA1 in epithelial cells of the stomach and the enrichment of TRPA1 mRNA in the pyloric part of the stomach of mice have been shown. The co-expression of TRPA1 and ghrelin in enteroendocrine cells (EC) of the duodenum and the potential role and physiological relevance of this receptor in ghrelin cells have been described. Finally, after repeated ingestion of CIN, Diet induced Obese (DIO) mice present a reduction of body weight gain as well as an improvement of insulin sensitivity. The observed up-regulation of ACSL4 in brown adipose tissue (BAT) and Cpt1a in white adipose tissue (WAT) could indicate an increase of fatty acid oxidation and therefore a decrease of fat mass. These results suggest that CIN could be used as a potential anti-hyperglicemic and anti-obesity compound.
Results
TRPA1 and ghrelin expression in the gut
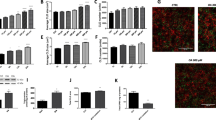
Using qRT-PCR (Fig. 1) and in-situ hybridization (Fig. 2) techniques, the expression of TRPA1 through the gastrointestinal tract has been confirmed and described. The level of expression in the esophagus was undetectable. In the stomach, we observed higher TRPA1 mRNA expression in the inferior half of the stomach body (named “Pyloric”, insert Fig. 1; light grey portion), as opposed to the upper region (named “Cardia”, insert Fig. 1. dark grey portion). TRPA1 mRNA has been found along the small and large intestine (Fig. 1). Furthermore, we observed that the expression pattern of TRPA1 is similar and proportional to the ghrelin expression pattern with higher expression in the pyloric part of the stomach, lower in cardia and both of them decreased toward the lower gastrointestinal tract (Fig. 1).
Tissue and cellular distribution of TRPA1 in gastrointestinal tract and other tissues.
Fluorescent in situ hybridization and immune-localization of TRPA 1 (green) and enteroendocrine cell marker Chromogranin A (CgA) (red), respectively (A1–A3). Note high co-localization of TRPA1 and Cg A in the cells located in the crypts of the duodenal mucosa; under the discontinuous line (A3). Many of the CgA positive cells localized in the villi do not co-localize with TRPA1 (above the discontinuous line, arrows) (A3). Co-localization of ghrelin and TRPA1 in two cells located in two different crypts of the duodenum (arrows) (B1–B3). In situ hybridization with DAB cross section of stomach in the pyloric region under light microscope (C1-C3). TRPA1 positive cells are mostly located in the glandular base of the stomach (C1). Higher magnification of TRPA1 expressing cells. Small and round shape (C2–C3). In situ hybridization with DAB reveals localization of TRPA1 in the crypts of the duodenum (arrows) (D1) and the detailed shape of the positive cells (D2). TRPA1 positive cells in trigeminal root ganglia, as a positive control (E1). Scale bars = 100 μm in (A1–A3); 20 μm in (B1–B3); 200 μm (C1); 5 μm (C2); 5 μm (C3); 50 μm (D1); 20 μm (D2); 50 μm (E1).
To determine which type of cells express TRPA1, a combination of immunohistochemistry and in situ hybridization was performed (Fig. 2). In the duodenum, by double staining with anti-chromogranin A antibody (Chromogranin A; CgA) and a fluorescence labeled anti-TRPA1 probe, the percentage of co-localization between TRPA1 and CgA was identified. The last one marks all enteroendocrine cell types except CCK- and motilin-expressing cells. 60% of the CgA-positive cells express TRPA1 in the villi, however this percentage is higher (96%) in the crypts of the duodenum (Fig. 2A). Furthermore, also by double staining with immunohistochemistry (ghrelin) and in situ hybridization (TRPA1), we observed that ghrelin positive cells of the duodenum, scattered in the epithelia of crypts and villi, displayed up to 65% co-localization with TRPA1 (Fig. 2B). Fluorescence from the ghrelin vesicles was observed mainly in the basal side of cells, while TRPA1 mRNA seemed to be distributed across the whole cytoplasm of the cell or mainly in the apical part of the cells.
Using in-situ hybridization, the precise localization of TRPA1 mRNA expressing cells in the stomach was shown (Fig. 2C). Most of this mRNA appeared in the glandular base decreasing in amount as the glands extend toward the lumen with a few cells observed in the glandular neck (Fig. 2 C1). The morphology of the positive cells was small and round shaped (Fig. 2 C2 and C3). In situ hybridization with DAB confirmed the localization of TRPA1 mainly in the crypts of the duodenum (arrows) (Fig. 2D1 and D2). Finally, as a positive control, we stained TRPA1 positive cells in trigeminal root ganglia, (Fig. 2D3) with DAB.
Ghrelin secretion of the MGN3-1 cell line is reduced by cinnamaldehyde
The MGN3-1 cell line (mouse ghrelinoma 3-1) is a ghrelin-producing cell line derived from stomach25. We confirmed co-localization of TRPA1 and ghrelin in these cells and studied the effect of CIN, on gene expression and ghrelin secretion.
First, to confirm the expression of TRPA1 in MGN3-1 cells, the relative amount of TRPA1 mRNA was measured by qRT-PCR, which was similar to the one of ghrelinO-acyltransferase (GOAT) responsible for adding an octanoyl group to pro-ghrelin at position Ser-3. Second, to investigate the possible roles of TRPA1 in MGN3-1 cells, the effect of CIN on the expression of several genes (TRPA1, Insulin receptor (InsR), Ghrelin and GOAT) was measured. Addition of 100 µM CIN up-regulated the expression of two genes: TRPA1 and InsR (Fig. 3A and B). Upregulation of InsR at the protein level was also confirmed by Western Blot (Supplementary Fig. 1A). No significant differences were found for Ghrelin and GOAT mRNA (see Fig. 3C and D).
Additionally, MGN 3-1 cells were used to study the effect of CIN on ghrelin secretion. Active and total ghrelin secreted in the cell culture medium 4 hours after stimulation with 100 µM of CIN were measured and we observed that octanoyl ghrelin and total ghrelin secreted were significantly lower (44%) than in control conditions. When 25 µM of the TRPA1 inhibitor HC-030031 was added at the same time than CIN to the cells, 12% of the effect of CIN on ghrelin secretion was blocked and no effect was observed with HC-030031 alone (Fig. 4A). Total ghrelin secreted in the media was affected exactly in the same manner than octanoyl ghrelin by CIN and HC-030031 (Fig. 4B). By Western Blot analysis, the reduction of ghrelin after CIN treatment has been confirmed (Supplementary Figure 1B), suggesting with regards to the qRT-PCR results (Fig. 3C) that CIN impacts ghrelin at a post-transcriptional level.
Regulation of ghrelin secretion by CIN in MGN 3-1 cells.
Four hours treatment of MGN 3-1 cells with CIN (100 μM) decreases both octanoyl ghrelin (A) and total ghrelin (B) secreted in the medium. This effect is partially recovered by the inhibition of TRPA1 with HC-030031 (25 µM). No effect is observed on the secretion of ghrelin when the inhibitor is applied alone (A and B). (*, p<0.05; **, p<0.01*; ***, p<0.005 n = 3; error bars: SEM).
Acute administration of cinnamaldehyde reduces food intake (FI) and gastric emptying
Since in vivo co-localization of ghrelin-secreting cells and TRPA1 expression was found and in vitro a decrease of ghrelin secretion in MGN3-1 cells when stimulated with CIN, we tested, first, if TRPA1 activation has any effect on FI after an acute treatment with CIN. To this end, the impact of a single dose of CIN on FI was measured. Mice received a gavage with 250 mg/kg (bw) of CIN and food intake was measured every two hours during the subsequent 24 hours. Strong and significant reduction of cumulative food intake was found 2 hours after the treatment. In addition, we observed that 10 hours after the gavage, mice treated with CIN, compensated the total amount of food ingested reaching the same quantity as control mice (Fig. 5A).
Effect on short term food intake and gastric emptying of mice receiving a CIN gavage.
Mice, after overnight fasting, received a single gavage of CIN (250 mg/kg bw) or an equivalent volume of water for control animals. Subsequently, either food intake (A) or intestinal transit was measured (B). A: CIN reduces significantly cumulative food intake during the 2 hours after the gavage, compared to the control group. (*, p<0.05; n = 25) B: CIN does not affect the intestinal transit time: geometric center being 6.21 ± 0.25 for the CIN group and 6.32 ± 0.26 for the control group. However, gastric emptying is slowed with CIN compared to the control group, in stomach and the two first segments of the intestine there is significantly more fluorescent dye in CIN group, SI: small intestine (*, p<0.05; **, p<0.01*; ***, p<0.005; n = 6; error bars: SEM).
Secondly, it was tested if CIN affects gastric emptying and intestinal transit. Mice were gavaged with a non-absorbable fluorogenic marker (FITC-dextran; MW 70,000) with or without 250 mg/kg bw of CIN. No significant differences in the calculation of the geometric center were observed in animals treated with or without CIN: 6.205 ± 0.253 versus 6.325 ± 0.259 (geometric center: mean segment number ± SEM). The gastric emptying rate however was significantly slower in mice gavaged with CIN (Fig. 5B). Indeed, in mice gavaged with CIN, 19.2% of the FITC-dextran remained in the stomach in contrast to 4.6% in mice gavaged with control solution. To verify the role of TRPA1 in the effect of CIN on gastric emptying the same experiment was performed using TRPA1 (-/-) mice9 and the proper control mice. In TRPA1 (-/-) mice the difference in gastric emptying was not significant (Fig. 6).
Effect of CIN gavage on gastric emptying of TRPA1 (-/-) mice.
After a overnight fasting, WT and TRPA1 (-/-) animals received a single gavage of CIN (250 mg/kg bw) or an equivalent volume of water containing a fluorescent tracer. One hour after the gavage the presence of the dye in the stomach was measured. There was no significant difference in the content of dye in the stomach of TRPA1(-/-) animals gavaged or not with CIN, whereas we observed an increase of the presence of dye in WT animals gavaged with CIN compared to those gavaged with water. (*, p<0.05; n.s., not significant; n = 6; error bars: SEM).
Repeated ingestion of Cinnamaldehyde reduces body weight gain in HFD-induced obese mice (DIO-mice)
To better understand the effect of CIN administrated in a chronic manner during 5 weeks, daily FI and BW were measured in DIO-mice fed with high fat food (60% of calories coming from fat) containing 0.2% of CIN. Contrary to the acute treatment, daily food intake was not affected by CIN (Fig. 7B) but surprisingly CIN had a significant impact reducing cumulative body weight gain starting at day 24 of treatment (Fig. 7A).
Effect of chronic intake of 0.2% CIN included in the diet of obese mice on daily food intake and body weight gain.
0.2% of CIN was included in the high fat diet of DIO mice for a period of 5 weeks and body weight gain and food intake was measured daily. We did not observe any effect on food intake, whereas a significant effect on cumulative body weight gain was observed from day 24. (*, p<0.05; n = 33; error bars: SEM).
Consistent with the reduced body weight, CIN-treated mice had reduced body fat mass gain compared with controls (HFD: 5.2 g ± 0.3 g; HFD + 0.2% CIN: 4.5 ± 0.3 g; p-value: 0.06) (Table 1, NMR results). However, no significant differences were observed in the plasma cathecholamine levels. As a marker for liver injury the levels of liver transaminases, have been measured and no differences were found between group control and CIN-treated mice (Table 1).
Moreover, we found no differences in plasma levels of creatinine, cholesterol and triglycerides between the mice treated with and without CIN. Also for the plasma hormone levels of leptin and adiponectine (Table 1). CIN did not affect lipid absorption in HFD-obese mice. No significant differences in lipid content of the feces were found between CIN and control treated.
Repeated ingestion of Cinnamaldehyde improves insulin sensitivity in HFD-induced obese mice
To test whether the ability to use glucose was changed after the treatment with CIN, an oral glucose tolerance test (OGTT) was performed in fasting conditions during the last week of the chronic treatment. Mice treated with CIN showed statistically significant decrease of blood glucose levels after a glucose load. These mice showed already a reduction of 10.6% of blood glucose levels in fasting conditions compared to the control group. During the OGTT, CIN treated mice showed a reduction of 21%, 29.8%, 25% and 8.25% of blood glucose levels after 15′, 30′, 60′ and 120′ respectively after the ingestion of glucose (Fig. 8). However, plasma insulin levels in the fasting state (Table 1) and during the OGTT (data not shown) were unchanged by the CIN treatment.
Effect of chronic intake of 0.2% CIN included in the diet of obese mice on blood glucose after a gavage with glucose.
After 5 weeks of consumption of high fat diet containing 0.2% of CIN, an oral glucose tolerance test (OGTT) was performed by gavaging the animal with 2 g/kg bw of 20% D-glucose in water. Glucose was assessed with a glucometer before and 15, 30, 60 and 120 min after glucose administration. Blood glucose levels are significantly lower for CIN treated animals as compared to the controls for each time point before and after glucose gavage. (*, p<0.05; **, p<0.01; n = 12; error bars: SEM).
This improvement in glucose tolerance without a change in the plasma insulin concentration suggests enhanced sensitivity to insulin in the periphery in response to a glucose load following treatment with CIN.
Effect of repeated ingestion of Cinnamaldehyde on gene expression related to fat metabolism and glucose transport of HFD-induced obese mice
From the different tissue samples collected at the end of the chronic treatment, gene expression analysis was performed to measure the relative mRNA amount of different sets of genes in each specific tissue (supplementary data).
Brown adipose tissue (BAT): After CIN treatment, a significant up-regulation of enzyme acyl-CoA synthetase 4 (ACSL4) was found (Table 2). This enzyme acitvates fatty acids destined for β-oxidation. Other genes were measured (see supplementary data) but no significant differences were found.
White adipose tissue (WAT): Up-regulation of several glucose transporters GLUT1, GLUT12 and GLUT 8, Cpt1a involved in stimulation of fatty acid oxidation and insulin receptor has been observed after CIN treatment compared to the control group (Table 2).
Muscle: No difference was found for any of the following genes: GLUT1; GLUT4; GLUT8; GLUT10; GLUT11; GLUT12; IL-6; MCP-1; insulin receptor; very low expression for GLUT5 and no expression of TRPA1 and SGLT3.
Liver: No difference was found for any of the genes analyzed (GLUT1; GLUT10; GLUT2; GLUT9; very low expression for IL-6 and no expression for SGLT3 and TRPA1).
Discussion
Over many years cinnamon has been described and used in traditional medicine to moderate postprandial glycemia and positively change body composition, although little was known about the mechanism of action for this spice26,27,28. The results obtained here contribute to clarify which are the active molecules and potential pathways responsible for these effects. Our results demonstrate, that ghrelin and TRPA1 co-localize in the same EC of the duodenum and that CIN decreases ghrelin secretion partially via TRPA1 activation.
Ghrelin is a potent orexigenic peptide that stimulates food intake and bodyweight gain, indicating that this hormone plays an important role in energy homeostasis. Moreover, recently several studies have demonstrated that gluconeogenesis and glycogenolysis are increased and insulin sensitivity is improved by the ablation of the ghrelin or growth hormone secretagogue receptor in mice29 and that ghrelin suppresses glucose-induced insulin release and that it deteriorates glucose tolerance23,24. Therefore, ghrelin antagonists are promising targets for pharmacological intervention in the treatment of obesity and type 2 diabetes.
MGN3-1 cells represent an ideal in vitro tool to study the potential effects of different ingredients on ghrelin secretion. Iwakura et al.25 demonstrate that ghrelin secretion is suppressed by somatostatin and insulin, indicating that MGN3-1 cells preserve the intrinsic characteristics of ghrelin-producing cells. The level of TRPA1 expression in these cells is considerably high, with the same relative amount of expression as GOAT enzyme. This could support the importance of this channel in ghrelin secreting cells.
The fact that TRPA1 activation (with CIN) decreases ghrelin secretion and up-regulates insulin receptors (in vitro), supports that CIN works as anti-hyperglycemic drug and, potentially, as an enhancer of insulin sensitivity through regulation of ghrelin secretion. We could not block 100% of the CIN effect in MGN3-1 cells, so we hypothesize about the existence of a different receptor activated by CIN or the poor efficiency of HC-030031 in this specific cell type. Our in vivo experiments also provide data supporting the effect of CIN on ghrelin secretion. After long-term treatment with HFD + 0.2% CIN, glucose plasma levels decrease significantly in fasting conditions and during OGTT, whereas insulin levels are similar. In fact, CIN demonstrates to have the same effect as the already described effect of insulin decreasing ghrelin secretion30,31.
Improved oral glucose tolerance values normally are the result of two phenomena, either enhanced peripheral tissue (muscle and adipose tissues) sensitivity to insulin or an improvement in hepatic insulin sensitivity. Our RT-PCR results show, after 36 days feeding HFD supplemented with 0.2% CIN, an up-regulation of 3 different glucose transporters (GLUT1, 8 and 12) and InsR in WAT. However, it still remains to be demonstrated if the observed improvement of insulin sensitivity is a cause or a consequence of this up-regulation.
TRPA1 has been detected in rat pancreatic β-cells18. Using in vitro assays, it was observed that TRPA1 ligands induce basal insulin release by Ca2+ influx. This path could also participate to the overall enhanced glucose tolerance, although in this study we do not observe any changes in insulin plasma levels.
Taken together, all these observations indicate that CIN has a strong potential as an anti-hyperglycemic drug to be used in diabetes type 2 treatment.
In addition, a significant delay in gastric emptying is observed after a single gavage of CIN in mice (Fig. 5B) but we do not observe any increase of the motility along the rest of the gastrointestinal tract as it has been shown in dogs and isolated guinea pig ileum14,16. The geometric center of the dye distribution in the gut was calculated as well and no difference is found between treated and untreated mice. This delay of gastric emptying after application of TRPA1 agonists such as CIN and allyl isothyocyanate (AITC) has been shown already before14,15,16, due to an increase of 5-hydroxytryptamine (5-HT) released. The authors could also show high expression of TRPA1 mRNA by ISH in enterochromaffin cells secreting 5-HT in the small intestine. A more recent study describes the expression of TRPA1 mostly in the myenteric ganglia on the stomach and in the myenteric and submucosal ganglia of the small and large intestine32 with a low level of expression in epithelial cells of the small intestine and colon. Surprisingly, the authors specified that TRPA1 immunoreactivity was not concentrated in discrete populations of epithelial cells such as EC32 and no effect of AITC by gavage in gastric emptying and small intestinal transit has been observed.
In the present study, CIN induced reduction of gastric emptying correlates with a reduction of FI 2 hours after gavage. The high expression of TRPA1 found in the stomach, the co-localization of TRPA1 with ghrelin in the small intestine and, the high in vitro expression of TRPA1 in ghrelin secreting cells (MGN 3-1) with the decrease in ghrelin secretion partially through TRPA1 activation, allows us to explain the effect of the TRPA1 agonist in gastric emptying and FI through a regulation of ghrelin secretion. It is well known that ghrelin hormone stimulates gastric emptying and hunger, even in humans33. Therefore, based on all data collected here there is enough evidence to support that ghrelin secretion could be reduced through TRPA1 activation. The activation of ghrelin secretion through other tastants, more specifically different bitter tastants, has been already shown34. Janssen et al. have shown that several T2R agonists accelerated gastric emptying and that ghrelin localizes with G proteins of the gustatory complex (alpha-gustducin and alpha-transducin) in EC cells. Taken together these results open a new potential approach to investigate how sensory stimuli regulate endogenous release of ghrelin for therapeutic intervention.
After 23 days of HFD supplemented with 0.2% of CIN a significant decrease in cumulative body weight gain is observed without a decrease in daily food intake during the entire study. The NMR results collected at the end of the treatment indicate a tendency to reduce fat mass. To date, two theories could explain CIN effects on body weight and on adipose tissue. The first one proposes that TRPA1 activation increases thermogenesis via the β-adrenergic pathway19. However, in our study no differences in the plasma catecholamine levels are detected. The second explanation proposed in the literature is that CIN prevents adipocyte differentiation and adipogenesis, based on the in vitro results obtained with 3T3-1L via down-regulating PPARγ and up-regulating AMPK pathways35. The authors also found an improvement of insulin secretion and fasting glucose plasma levels, together with a decrease in triglycerides (TG), non-esterified fatty acids (NEFA) and cholesterol serum levels. In our study, not only a decreased glucose level is observed in fasting condition but also after a glucose gastric load in CIN-treated mice. Tamura et al.21, also found a decrease in adipose tissue as we did, but they explained it by an activation of UCP1 BAT. The reduction of fat mass we observed suggests also an increase of cellular metabolism; however, in our study we do not observe a difference in UCP activity (UCP1, 2 and 3). Nonetheless this hypothesis is supported by the up-regulation we observe of ACSL4 (in BAT) and Cpt1a (in WAT), which could indicate an increase of fatty acid oxidation36.
On the other hand, it is known that CIN is easily oxidized into cinnamic acid (CNA)37. This data brings up the possibility that the long-term effects we observe in our study are due to CNA instead of CIN but as hTRPA1 transfected cells were not activated by CNA (data not shown), the effects described during the chronic treatment cannot be due to TRPA1 activation by CNA. However, a different pathway could be involved in this decrease of fat mass as Kopp S.P. et al., 201438 recently described. They showed an increase of adiponectin and pAMPK/AMPK ratio through GPR109A activation by CNA in 3T3-L1 adipocytes.
Other TRP channels have also been associated to thermogenesis of BAT. It has been shown that mice receiving a chronic treatment with menthol, as a TRPM8 agonist, showed an increase of the core temperature and locomotor activity, plus elevated levels of UCP-1 in brown adipocytes (in vitro)39.
Taken together, we conclude that the TRPA1 pathway is a potential target to improve cellular metabolism, insulin sensitivity and to reduce fat mass.
Methods
Animals
C57BL6/J mice were obtained from Charles River (France), C57BL/6NTac Mice on High Fat Diet (DIO-B6) from Taconic (Denmark) and TRPA1 KO (trpa1 -/-) (B6; 129P-Trpa1tm1kykw/J) from The Jackson Laboratory. Mice were maintained as heterozygotes and age- and sex-matched littermates were studied. Mice were maintained at 22°C with a 12 h dark-12 h light cycle.
All procedures were conducted in conformity with the Swiss Welfare Act and the Swiss National Institutional Guidelines on Animal Experimentation for the ethical use of animals. The project was approved by an Internal Experimentation Ethics Committee and the Swiss Cantonal Veterinary Office following its ethical review by the State Committee for Animal Experimentation.
Acute condition of treatment with Cinnamaldehyde
Dose of Cinnamaldehyde: The dose of 250 mg/kg/day of cinnamaldehyde was chosen based on the acceptable daily consumption (ADI) of 1.25 mg/kg of body weight40. A safety factor of 200 was adopted. allows for differences between test species and human beings (10 times) and differences between individuals (10 times)41.
Food intake measurements: To measure the impact of a single dose of CIN on food intake, single housed C57BL6 mice, fasted during 23 hours, received a gavage of 250 mg/kg bw of CIN and food intake was measured every two hours during the following 24 hours, starting at the beginning of the dark cycle. Mice were housed during the measurement without litter. A plate with a single pellet was placed in a cage and was weighed every 2 hours together with the food waste that was found in the bottom of the cage.
Gastric emptying and gastrointestinal transit time was measured after 18 h overnight fast. FITC-labeled dextran (70,000 MW; Molecular Probes) was administered by gavage (100 μl of a 5-mg/ml solution prepared in water). Sixty minutes later, the entire GI tract from stomach to rectum was removed and placed in ice for 30 s to inhibit motility. The stomach, small intestine (divided into 10 equal length segments), cecum and colon (subdivided into two equal-length segments) were each placed in a separate tube containing PBS. The segments were coarsely chopped with scissors and luminal contents suspended using a combination of vigorous washing and vortexing. The fluorescent signal was quantified in a multiwell fluorescence plate reader (Flexstation II fluorescent plate reader; excitation at 485 nm; emission at 530 nm; Molecular Devices). The geometric center was determined as: (SUM [% of total fluorescence per segment × segment number])/100)42. Gastric emptying was calculated based on the amount of FITC-dextran left in the stomach compared with the total amount of fluorescence in the gastrointestinal tract.
Long term treatment with CIN included in the diet
Dose of Cinnamaldehyde: The dose of 0.2% CIN is based on the same daily consumption used for the acute treatment (250 mg/kg bw) assuming a minimal body weight of 25 gr/mice and a daily food intake of 3 gr.
Sixty mice were fed with a high fat diet (HFD) containing 60% of the calories as fat (D12492, Research Diet) for 10 weeks. The mice became obese (min. 40 g bw) and were randomized in two groups. The control group was fed with HFD and the group “treated with CIN” was fed with HFD ad libitum supplemented with 0.2% of CIN over the following 36 days. Food intake and body weight gain were recorded daily and glucose tolerance was assessed at the end of the treatment. At the end of the experiment the mice were sacrificed after a 6-h fasting period; blood and tissue samples were collected and stored at −80°C for further analyses as detailed below.
Histology (Tissue preparation immunohistochemistry/In situ hybridization (IHC/ISH))
Mice were anesthetized and intracardiacally perfused with a saline solution to remove blood from the vessels and small capillaries followed by a perfusion of 4% paraformaldehyde (PFA) in PBS. Different tissue samples were collected to perform in-situ hybridization and immune-staining. The tissue was rinsed with PBS and immersed in 4% PFA in PBS overnight at 4°C, cryoprotected in a solution of 20% sucrose in PBS and embedded in Tissue-Tek optimal cutting temperature compound (Sakura) frozen in dry ice. Sections of 16 μm were cut in the cryostat for ISH/IHC.
In Situ Hybridization (ISH): Sections were fixed in 4%PFA in PBS for 10 minutes and washed in PBS. The sections were then treated with Proteinase K solution (10 ng/mg in 100 nM Tris-HCl (pH 8), 50 mM EDTA for 5 minutes at 37°C and acetylated as follows: washed in PBS for 1 minute, 2 minutes in 100 mM triethanolamine (pH 8), 10 min with 0.25% acetic anhydride in 100 mM triethanolamine (pH 8) and finally washed with PBS for 1 minute and air dried. Afterward, the hybridization solution (50% formamide, 600 mM NaCl, 10 mM tris-HCl (pH 8), 1 mM EDTA, 1X Denhardt's solution, 0.25% SDS, 10% dextran sulfate, 200 ug/ml yeast RNA) was applied at 85°C for 10 minutes and finally digoxigenin-labeled probes for TRPA1 were added to in the solution pre-heated at 85°C for 3 minutes. The hybridization was done at 60°C overnight in a humidified chamber containing 50% formamide.
The following day sections were washed in 2xSSC containing 50% formamide at 60°C for 30 minutes, twice in 2xSSC at 60°C for 20 minutes and twice in 0.2xSSC at 60°C for 20 minutes.
Immunohistochemistry (TSA biotin system IHC). First, to quench endogenous peroxidase activity, a solution of 0.3% H2O2 was applied in PBS for 30 minutes and slides were blocked in TNB blocking buffer (0.1 M Tris-HCl, pH 7.5, 0.15 M NaCl, 0.5% Blocking Reagent) at room temperature (RT).
In a next step, slides were incubated with horse-radish peroxidase-conjugated antibody, anti-DIG-POD (1:100) at room temperature for 30 minutes and slides were washed in PBS.
Slides were then incubated in Biotinyl Tyramide amplification reagent and thereafter washed again. Following, incubation in streptavidin-Alexa Fluor 488 (SA-488; Invitrogen) (1:100) during 30 minutes at RT. Sections were blocked in blocking buffer for 1 hour at RT and the primary anti- ghrelin antibody (sc-10368; Santa Cruz Biotechnology) was added (1:1000) in the blocking buffer overnight at 4°C. To finish, the slides were incubated with a secondary antibody anti-goat alexa 555 (1:1000; Invitrogen).
Ghrelin secretion in MGN3-1 cells: measurements in culture media and gene expression analysis
MGN 3-1 cells were grown as indicated by Iwakura H. et al., 2010, in DMEM (41966-029; Life technologies) supplemented with 10% FBS, 100 U/ml penicillin and 100 g/ml streptomycin at 37°C in 10% CO2. MGN3-1 cells were seeded and cultured overnight in 12-well microtiter plates (7.5 × 105 cells/well). Subsequently, cells were incubated at 37°C for 4 h in DMEM supplemented with 0.5% BSA, as previously described by Iwakura H. et al., 2010 and with or without CIN before collecting supernatants. To measure ghrelin the culture medium was centrifuged and the resulting supernatants were immediately applied to Sep-Pak C18 cartridges (Waters Corp.) pre-equilibrated with 0.9% saline. Cartridges were washed in saline and 5% CH3CN/0.1% trifluoroacetic acid and eluted with 60% CH3CN/0.1% trifluoroacetic acid. Ghrelin concentrations in the supernatant were measured by ELISA (kit EZRGRA-90K, Millipore).
To determine the expression levels of ghrelin (taqman primers: Mm00445450_m1), GOAT(Mm01200389_m1), InsR (Mm01211875_m1) and TRPA1 (Mm01227437) mRNA, cells were incubated at 37°C for 24 h in DMEM supplemented with 0.5% BSA and the indicated additional reagents. Total RNA was extracted using a RNeasy Micro Kit (Qiagen). Reverse transcription was performed with a high-capacity RNA-to-cDNA kit (Applied Biosystems). Real-time quantitative PCR was performed using an ABI PRISM 7900HT sequence detection system (Applied Biosystems) using appropriate primers and TaqMan probes (Supplementary information table 1). The mRNA expression of each gene was normalized to levels of β-actin.
Oral glucose tolerance test
Mice were fasted for six hours during the light cycle and the fasting glycemia baseline was measured with a glucose meter (Ascensia Elite XL, Bayer). Then, a gavage containing a 30% glucose solution (D-Glucose 2 mg/g BW) was carried out and several blood samples were collected 15′, 30′, 60′ and 120′ after glucose load. Blood collections for insulin measurements are only at time points 0′,15′ and 60′. Insulin levels were measured according to the provided protocol of the company (90060:96test; Chrystal Chem).
Measurement of body fat mass and lean mass
Body composition was determined, at day 0 and at the end of treatment, by nuclear magnetic resonance (NMR) using Echo MRI TM (Echo Medical Systems).
Biochemical blood assays
Blood levels of aspartate transaminase (AST) (#04657543 190;Roche) and alanine aminotransferase (ALT) (#04718569 190; Roche,), creatinine (#05401755 190; Roche,), total cholesterol (#04718917 190; Roche), triglycerides (#04657594 190; Roche,), free fatty acids (#434-91795, 436-91995; WAKO) were measured using the Cobas c111 spectrophotometer (Roche Diagnostic GmbH). Plasma levels of adrenaline, noradrenaline and dopamine plasma levels were assayed by ELISA following manufacturer's instructions (BA E-5100: Adrenaline; BA E-5200: Noradrenaline; BA E-5300: Dopamine (Labor Diagnostika Nord, GmbH & Co.KG).
Fecal Calorie content
At the end of the treatment, over 24 hours, mouse feces were collected and were frozen at -80°C. The feces were dried by vacuum oven-drying (50°C, 24 hours). Dried feces were then homogenized with a mortar and compacted into two pellets using a Pellet Press 2811 (Parr Instrument Company). The dried materials were added to 0.4 g benzoic acid and burned in a 6100 Oxygen Bomb Calorimeter (Parr Instrument Company) to measure their caloric content. Measurements were done in duplicate.
Gene Expression Analysis
Tissue was collected at the end of the treatment. 10 mice per group were euthanized and samples of inguinal white adipose tissue and interscapular brown adipose tissue, muscle from quadriceps and liver were dissected and frozen immediately in liquid nitrogen.
Gene expression analysis: changes in gene expression were measured by qRT-PCR in dissected tissue. Samples were homogenized using Lysing Matrix D tubes (MPbio) and total RNA was extracted using the Agencourt RNAdvance Tissue system. The mRNA expression of each gene was normalized to levels of beta-actin. cDNA was synthesized using the High Capacity RNA-to-cDNA Kit (Applied Biosystems) and Real-time PCR was performed using an ABI PRISM 7900 HT Sequence Detection System (Applied Biosystems). The expression levels of the different genes and two different reference genes were measured with pre-designed Taqman gene expression assays, each containing two gene specific primers and an internal FAM™ dye-labeled TaqMan® MGB probe. See taqman probes used for the different tissues in supplementary information table 1.
References
Anderson, R. A. et al. Isolation and characterization of polyphenol type-A polymers from cinnamon with insulin-like biological activity. J. Agric. Food Chem. 52, 65–70 (2004).
Jia, Q. et al. Hypoglycemic activity of a polyphenolic oligomer-rich extract of Cinnamomum parthenoxylon bark in normal and streptozotocin-induced diabetic rats. Phytomedicine. 16, 744–750 (2009).
Onderoglu, S., Sozer, S., Erbil, K. M., Ortac, R. & Lermioglu, F. The evaluation of long-term effects of cinnamon bark and olive leaf on toxicity induced by streptozotocin administration to rats. J. Pharm. Pharmacol. 51, 1305–1312 (1999).
Verspohl, E. J., Bauer, K. & Neddermann, E. Antidiabetic effect of Cinnamomum cassia and Cinnamomum zeylanicum in vivo and in vitro. Phytother. Res. 19, 203–206 (2005).
Subash, B. P., Prabuseenivasan, S. & Ignacimuthu, S. Cinnamaldehyde--a potential antidiabetic agent. Phytomedicine. 14, 15–22 (2007).
Zhang, W., Xu, Y. C., Guo, F. J., Meng, Y. & Li, M. L. Anti-diabetic effects of cinnamaldehyde and berberine and their impacts on retinol-binding protein 4 expression in rats with type 2 diabetes mellitus. Chin Med. J. (Engl.) 121, 2124–2128 (2008).
Bandell, M. et al. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 41, 849–857 (2004).
Bautista, D. M. et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 124, 1269–1282 (2006).
Kwan, K. Y. et al. TRPA1 contributes to cold, mechanical and chemical nociception but is not essential for hair-cell transduction. Neuron 50, 277–289 (2006).
Nagata, K., Duggan, A., Kumar, G. & Garcia-Anoveros, J. Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J. Neurosci. 25, 4052–4061 (2005).
Macpherson, L. J. et al. The pungency of garlic: activation of TRPA1 and TRPV1 in response to allicin. Curr. Biol. 15, 929–934 (2005).
Buch, T. R. et al. Functional expression of the transient receptor potential channel TRPA1, a sensor for toxic lung inhalants, in pulmonary epithelial cells. Chem. Biol. Interact. 206, 462–471 (2013).
Cho, H. J., Callaghan, B., Bron, R., Bravo, D. M. & Furness, J. B. Identification of enteroendocrine cells that express TRPA1 channels in the mouse intestine. Cell Tissue Res. 356, 77–82 (2014).
Nozawa, K. et al. TRPA1 regulates gastrointestinal motility through serotonin release from enterochromaffin cells. Proc Natl. Acad. Sci. U. S. A 106, 3408–3413 (2009).
Doihara, H. et al. TRPA1 agonists delay gastric emptying in rats through serotonergic pathways. Naunyn Schmiedebergs Arch. Pharmacol. 380, 353–357 (2009).
Doihara, H. et al. Molecular cloning and characterization of dog TRPA1 and AITC stimulate the gastrointestinal motility through TRPA1 in conscious dogs. Eur. J. Pharmacol. 617, 124–129 (2009).
Purhonen, A. K., Louhivuori, L. M., Kiehne, K., Kerman, K. E. & Herzig, K. H. TRPA1 channel activation induces cholecystokinin release via extracellular calcium. FEBS Lett. 582, 229–232 (2008).
Cao, D. S. et al. Expression of transient receptor potential ankyrin 1 (TRPA1) and its role in insulin release from rat pancreatic beta cells. PLoS. One. 7, e38005 (2012).
Iwasaki, Y., Tanabe, M., Kobata, K. & Watanabe, T. TRPA1 agonists--allyl isothiocyanate and cinnamaldehyde--induce adrenaline secretion. Biosci. Biotechnol. Biochem. 72, 2608–2614 (2008).
Masamoto, Y., Kawabata, F. & Fushiki, T. Intragastric administration of TRPV1, TRPV3, TRPM8 and TRPA1 agonists modulates autonomic thermoregulation in different manners in mice. Biosci. Biotechnol. Biochem. 73, 1021–1027 (2009).
Tamura, Y., Iwasaki, Y., Narukawa, M. & Watanabe, T. Ingestion of cinnamaldehyde, a TRPA1 agonist, reduces visceral fats in mice fed a high-fat and high-sucrose diet. J. Nutr. Sci. Vitaminol. (Tokyo) 58, 9–13 (2012).
Kojima, M., Hosoda, H., Matsuo, H. & Kangawa, K. Ghrelin: discovery of the natural endogenous ligand for the growth hormone secretagogue receptor. Trends Endocrinol. Metab 12, 118–122 (2001).
Tong, J. et al. Ghrelin suppresses glucose-stimulated insulin secretion and deteriorates glucose tolerance in healthy humans. Diabetes 59, 2145–2151 (2010).
Yada, T. et al. Ghrelin regulates insulin release and glycemia: physiological role and therapeutic potential. Curr. Diabetes Rev. 4, 18–23 (2008).
Iwakura, H. et al. Establishment of a novel ghrelin-producing cell line. Endocrinology 151, 2940–2945 (2010).
Khan, A., Safdar, M., Ali Khan, M. M., Khattak, K. N. & Anderson, R. A. Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care 26, 3215–3218 (2003).
Solomon, T. P. & Blannin, A. K. Changes in glucose tolerance and insulin sensitivity following 2 weeks of daily cinnamon ingestion in healthy humans. Eur. J. Appl. Physiol 105, 969–976 (2009).
Mang, B. et al. Effects of a cinnamon extract on plasma glucose, HbA and serum lipids in diabetes mellitus type 2. Eur. J. Clin. Invest 36, 340–344 (2006).
Chacko, S. K. et al. Effect of ghrelin on glucose regulation in mice. Am. J. Physiol Endocrinol. Metab 302, E1055–E1062 (2012).
Saad, M. F. et al. Insulin regulates plasma ghrelin concentration. J. Clin. Endocrinol. Metab 87, 3997–4000 (2002).
Murdolo, G. et al. Insulin is required for prandial ghrelin suppression in humans. Diabetes 52, 2923–2927 (2003).
Poole, D. P. et al. Transient receptor potential ankyrin 1 is expressed by inhibitory motoneurons of the mouse intestine. Gastroenterology 141, 565–75, 575 (2011).
Levin, F. et al. Ghrelin stimulates gastric emptying and hunger in normal-weight humans. J. Clin. Endocrinol. Metab 91, 3296–3302 (2006).
Janssen, S. et al. Bitter taste receptors and alpha-gustducin regulate the secretion of ghrelin with functional effects on food intake and gastric emptying. Proc. Natl. Acad. Sci. U. S. A 108, 2094–2099 (2011).
Huang, B., Yuan, H. D., Kim, D. Y., Quan, H. Y. & Chung, S. H. Cinnamaldehyde prevents adipocyte differentiation and adipogenesis via regulation of peroxisome proliferator-activated receptor-gamma (PPARgamma) and AMP-activated protein kinase (AMPK) pathways. J. Agric. Food Chem. 59, 3666–3673 (2011).
Ellis, J. M. et al. Adipose acyl-CoA synthetase-1 directs fatty acids toward beta-oxidation and is required for cold thermogenesis. Cell Metab 12, 53–64 (2010).
Flavor and Extract Manufacturers' Association of the United States. Scientific Literature Review of Cinnamyl Alcohol and Related Substances in Flavor Usage (The association, Washington, 1978).
Kopp, C. et al. Trans-Cinnamic Acid Increases Adiponectin and the Phosphorylation of AMP-Activated Protein Kinase through G-Protein-Coupled Receptor Signaling in 3T3-L1 Adipocytes. Int. J Mol. Sci. 15, 2906–2915 (2014).
Ma, S. et al. Activation of the cold-sensing TRPM8 channel triggers UCP1-dependent thermogenesis and prevents obesity. J. Mol. Cell Biol. 4, 88–96 (2012).
W.H.O. Evaluation of Certain Food Additives and Contaminants. Fifty-fifth report of the Joint FAO/WHO Expert Committee and Food Additives. Tech Rep Ser 901, 1–120 (2001).
W.H.O. Air quality guidelines for Europe. Reg Publ Eur Ser 23, 426 (1987).
Samuel, B. S. et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc. Natl. Acad. Sci. U. S. A 105, 16767–16772 (2008).
Acknowledgements
We thank Prof. Hiroshi Iwakura (Kyoto University Hospital, Kyoto University graduate School of Medicine) for providing us with MGN 3-1 cells.
Author information
Authors and Affiliations
Contributions
J. l C. and H.M., S.C. and S.M., conceived the project. S.C. and S.M. conceived and performed the experiments. J. l C. and S.M. supervised the project. C. d S., J. M., Ju.M., M.P. performed some of the experiments. S.C. and S.M. wrote the manuscript with help from all of the authors. J. l C. revised the complete manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary information
Supplementary data 1
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Camacho, S., Michlig, S., de Senarclens-Bezençon, C. et al. Anti-Obesity and Anti-Hyperglycemic Effects of Cinnamaldehyde via altered Ghrelin Secretion and Functional impact on Food Intake and Gastric Emptying. Sci Rep 5, 7919 (2015). https://doi.org/10.1038/srep07919
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep07919
This article is cited by
-
Association between serum aldehyde concentrations and metabolic syndrome in adults
Environmental Science and Pollution Research (2023)
-
Cinnamaldehyde supplementation acts as an insulin mimetic compound improving glucose metabolism during adolescence, but not during adulthood, in healthy male rats
Hormones (2023)
-
Combination of TRP channel dietary agonists induces energy expending and glucose utilizing phenotype in HFD-fed mice
International Journal of Obesity (2022)
-
Mechanistic and therapeutic insight into the effects of cinnamon in polycystic ovary syndrome: a systematic review
Journal of Ovarian Research (2021)
-
Evaluation of dietary supplementation with cinnamon products on growth, blood composition, liver structure, and meat quality of striped catfish (Pangasianodon hypophthalmus)
Aquaculture International (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.