Abstract
Some sea slugs are capable of retaining functional sequestered chloroplasts (kleptoplasts) for variable periods of time. The mechanisms supporting the maintenance of these organelles in animal hosts are still largely unknown. Non-photochemical quenching (NPQ) and the occurrence of a xanthophyll cycle were investigated in the sea slugs Elysia viridis and E. chlorotica using chlorophyll fluorescence measurements and pigment analysis. The photoprotective capacity of kleptoplasts was compared to that observed in their respective algal source, Codium tomentosum and Vaucheria litorea. A functional xanthophyll cycle and a rapidly reversible NPQ component were found in V. litorea and E. chlorotica but not in C. tomentosum and E. viridis. To our knowledge, this is the first report of the absence of a functional xanthophyll cycle in a green macroalgae. The absence of a functional xanthophyll cycle in C. tomentosum could contribute to the premature loss of photosynthetic activity and relatively short-term retention of kleptoplasts in E. viridis. On the contrary, E. chlorotica displays one of the longest functional examples of kleptoplasty known so far. We speculate that different efficiencies of photoprotection and repair mechanisms of algal food sources play a role in the longevity of photosynthetic activity in kleptoplasts retained by sea slugs.
Similar content being viewed by others
Introduction
The capacity of some sea slugs to retain photosynthetically active chloroplasts from their algal food sources continues to puzzle and attract the attention of researchers. These sacoglossan sea slugs are able to suck the algal cytoplasm and retain intact chloroplasts (kleptoplasts) within the cells of their digestive glands for variable periods of time. The retention time of photosynthetically active kleptoplasts in animals deprived of a food source (starvation) seems to be dependent on the sea slug species, with retention times varying from only a few days (e.g. Thuridilla sp., Elysia atroviridis and E. trisinuata), to some weeks or a few months (E. viridis, E. crispata, E. timida, Costasiella ocellifera), or over several months (E. chlorotica and Plakobranchus occellatus)1,2,3,4,5,6,7. The longevity of functional kleptoplasts is also dependent on several other conditions such as seasonal differences of wild-collected specimens8,9, wild vs. laboratory breeding origin of specimens and the length of time spent feeding to promote incorporation of the kleptoplasts10,11 and temperature1,9,11 and light regimes12 employed in laboratory protocols for the husbandry of these organisms. Moreover, the algal source of the kleptoplasts can play a key role in the longevity of functional kleptoplasts13,14,15.
The mechanisms supporting long-term retention and function of kleptoplasts are still largely unknown. Several chloroplast proteins are either encoded by the nuclear genome or their synthesis requires nuclear-encoded regulatory signals16. Mujer et al.17 described de novo transcription and translation of light harvesting components in kleptoplasts of E. chlorotica over a period of 8 months. When kleptoplasts are maintained in the animal cells, the algal nucleo-chloroplast communication is disrupted. Horizontal gene transfer from the algal nucleus to the animal cells was hypothesised to explain, to a certain degree, the long-term survival and functioning of V. litorea plastids in E. chlorotica18,19,20. However, recent works in E. chlorotica21,22 and other sacoglossan sea slugs23 do not support this hypothesis. The answer to functional long-term kleptoplasty may reside in the plastid robustness itself15,24,25 and/or “result from a combination of yet-to-be characterized physical and molecular mechanisms”21.
Oxygen-evolving photosynthetic organisms regulate light harvesting in photosystems (PS) in response to rapid changes in the light environment. Energy is absorbed by light harvesting pigments in antenna proteins and transferred to PSI and PSII reaction centres (RCs) in the thylakoid membrane. The excitation energy is then converted to chemical energy through a charge separation event that begins a chain of electron transport reactions. This ultimately leads to the reduction of NADP+ to NADPH and the production of ATP26. When energy absorption exceeds the rate at which energy can be used in the electron transport chain, highly reactive intermediates and by-products will be produced which will potentially cause photo-oxidative damage and inhibit photosynthesis. To reduce photo-oxidative damage, land plants and algae evolved mechanisms for dissipation of excessive energy27. When photoprotection mechanisms fail to protect chloroplasts and photodamage occurs, mechanisms that repair damaged proteins are crucial for maintaining photosynthetic performance. Genome-encoded photodamage repair mechanisms could be key to plastid longevity25. Therefore, investigating photoprotection mechanisms28 and photodamage repair capacities25 of kleptoplasts retained by sea slugs is crucial to understand if such mechanisms play a role in kleptoplast robustness and longevity.
Pulse Amplitude Modulated (PAM) fluorometry has become a key technique for the investigation of photoprotection and photoinhibition mechanisms, collectively referred to as non-photochemical fluorescence quenching (NPQ) because the quenching does not result in the productive storage of energy29. In photosynthetic organisms, one of the most important parts of NPQ is the energy-dependent chlorophyll (Chl) fluorescence quenching (qE). Generally, qE requires the irradiance-dependent establishment of a transthylakoidal proton gradient (ΔpH) and the activation of the xanthophyll cycle30,31,32. The xanthophyll cycle can be defined as the interconversion of xanthophylls that involves one or two de-epoxidation reactions occurring in high-light and a reverse epoxidation reaction in limiting light31. In the Xanthophycea, such as Vaucheria sp., the xanthophyll cycle comprises the de-epoxidation step conversion of diadinoxanthin (Dd) to diatoxanthin (Dt) in high-light and the reverse reaction in dark or dim light33. The Dd cycle is equivalent to the violaxanthin (Viola), antheraxanthin (Anth) and zeaxanthin (Zea) xanthophyll cycle present in vascular plants, green and brown algae30,34. To date, a functional xanthophyll cycle was only demonstrated to occur in kleptoplasts of E. timida28, but it is still unknown if this photoprotection mechanism remains functional throughout prolonged periods of starvation. In the present work, NPQ and the presence of a functional xanthophyll cycle were investigated in two sacoglossan sea slugs, E. viridis and E. chlorotica, with distinctly different retention-times of functional kleptoplasts.
Results
Changes in variable Chl fluorescence in response to high-light stress
Chl fluorescence traces showing the responses to high-light stress and the subsequent recovery in darkness (dark-recovery) were generally similar between sea slugs and their respective food source (Figure 1). When comparing the different associations tested, C. tomentosum and E. viridis (Figures 1a, b) and V. litorea and E. chlorotica (Figures 1c, d), Chl fluorescence traces showed distinctive trends in the dark-recovery phase. Only a small recovery in Fm' was observed for C. tomentosum (Figure 1a) and E. viridis (Figure 1b) during the dark-recovery phase, whereas in both V. litorea (Figure 1c) and E. chlorotica (Figure 1d) the recovery was more significant. In the former organisms, the dark-recovery of the variable fluorescence (Fm' – Fs) was mostly due to a decrease in the minimal fluorescence (Fs) rather than an increase in the maximum fluorescence (Fm'). On the contrary, in both V. litorea and E. chlorotica, the dark-recovery of variable fluorescence was due to an increase in the Fm' rather than a decrease in Fs.
Representative examples of chlorophyll (Chl) fluorescence measurements as a response to different light conditions in (a) Codium tomentosum thallus samples, (b) immobilized Elysia viridis individuals, (c) Vaucheria litorea filaments and (d) immobilized E. chlorotica individuals.
Grey bar: 20 μmol photons m−2 s−1 (low light); White bar: 920 (C. tomentosum and E. viridis) or 1800 (V. litorea and E. chlorotica) μmol photons m−2 s−1 (high-light stress); Dark bar: dark. Samples were dark-adapted for 60 min before the start of Chl fluorescence measurements.
The capacity of the chloroplasts in the macroalgae and kleptoplasts in the sea slugs to recover from a high-light stress was evaluated by measuring Chl fluorescence PSII quantum yield and NPQ (Figure 2). As a result of exposure to high-light, the PSII quantum yield significantly decreased followed by a significant increase (in all cases P < 0.001) in the dark-recovery phase in both macroalgae and sea slugs (Figures 2a, b). In C. tomentosum, 60 min in darkness was not sufficient for a full recovery from the high-light exposure (PSII quantum yield was 67% of the Fv/Fm after 60 min in dark-recovery). Similar results were found for E. viridis with no significant differences (P = 0.862) in the PSII quantum yield found between this species and C. tomentosum (Figure 2a). Generally, a similar trend occurred in V. litorea and E. chlorotica. In V. litorea, within 5 min of dark-recovery, 88% of the PSII quantum yield was recovered. In E. chlorotica PSII quantum yield recovered to 81% of the maximal capacity within the first 5 min, although it then decreased to 68% in the following 25 min (Figure 2b).
Chlorophyll (Chl) fluorescence maximum and effective quantum yield of PSII (Fv/Fm or ΔF/Fm', in dark-adapted or light conditions, respectively) and non-photochemical quenching (NPQ) of Chl fluorescence as a response to different light conditions in (a, c) Codium tomentosum thallus samples and immobilized Elysia viridis individuals and in (b, d) Vaucheria litorea filaments and immobilized E. chlorotica individuals.
Values represent average ± standard error (n = 5). Grey bar: 20 μmol photons m−2 s−1 (low light); White bar: 920 (C. tomentosum and E. viridis) or 1800 (V. litorea and E. chlorotica) μmol photons m−2 s−1 (high-light exposure, HL); Dark bar: dark. Samples were dark-adapted for 60 min before the start of Chl fluorescence measurements. No significant differences (P > 0.05) were found between measurement made on C. tomentosum and E. viridis at each time point. Significant differences (P < 0.05) were found between V. litorea and E. chlorotica during HL and dark-recovery periods.
Generally, NPQ reached higher values in the sea slugs then in their corresponding algal food source (Figures 2c, d), although differences were only significant (P < 0.001) between V. litorea and E. chlorotica (Figure 2d). In both macroalgae and sea slugs, NPQ significantly increased (in all cases P < 0.001) in response to exposure to high-light. Surprisingly, in C. tomentosum and E. viridis NPQ was still sustained after 60 min in darkness following exposure to high-light (Figure 2c). On the contrary, in V. litorea and E. chlorotica NPQ significantly decreased (P < 0.001) when organisms were transferred to dark conditions (Figure 2d).
Changes in pigment composition in response to light-stress and dark-recovery experiments
Samples collected at the different stages of the light-stress and dark-recovery experiments (dark-adapted state, after exposure to high-light and after recovery in darkness) were analysed for pigment content. In C. tomentosum and E. viridis the pigments Anth and Zea were not present in all four treatments, while Viola was found in trace amounts (Figure 3). Thus, it was concluded that the typical xanthophyll cycle pigments found in green algae as a response to high-light (conversion of Viola into Anth and Zea) were not present in C. tomentosum. Lutein, another pigment commonly involved in photoprotection from high-light, was also absent in both C. tomentosum and E. viridis (Figure 3). We tested if decapitation of sea slugs interfered with pigment composition, but again no significant differences (in all cases P > 0.05) were found between pigments extracted from motile and immobilized sea slugs exposed to the higher light level tested (2500 μmol photons m−2 s−1; Figure S2).
Pigment per chlorophyll (Chl) a ratios under different light conditions in (a) Codium tomentosum thallus and (b) immobilized Elysia viridis individuals.
Siphonaxanthin (Siph), all-trans-Neoxanthin (t-Neo), 9′-cis-Neoxanthin (c-Neo), Violaxanthin (Viola), Siphonaxanthin dodecenoate (Sipho-do), ε,ε-Carotene (εε-Car) and β,ε-Carotene (βε-Car), Chl b and Chl a were quantified using HPLC (average ± standard deviation; n = 5). Samples for pigment analyses were freeze-dried after 60 min of dark-adaptation (“Dark”), after 30 min of a high-light (HL) exposure (“HL920” and “HL2500”: 920 and 2500 μmol photons m−2 s−1, respectively) and after 60 min of recovery in the dark following the 920 μmol photons m−2 s−1 HL exposure (“Recovery”). No significant differences (P > 0.05) were found between pigment ratios in the different light treatments in both C. tomentosum and E. viridis.
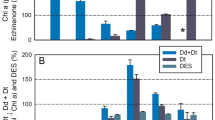
On the other hand, the xanthophyll cycle pigments Dd and Dt (equivalent to Viola and Zea in green algae) were found in both V. litorea and E. chlorotica (Figure S3). Using the Dd and Dt pigment concentrations, the de-epoxidation state (DES; changes in Dt/[Dt + Dd] ratio) was evaluated. A significant (P < 0.001) de-epoxidation of Dd into Dt was observed in response to exposure to high-light that was reversed in the dark-recovery phase (Figure 4a). A considerable amount of Dt was already present in dark-adapted V. litorea and E. chlorotica, with a significantly higher (P < 0.001) Dt/[Dt + Dd] ratio in E. chlorotica (Figure 4a). De novo synthesis of Dd was investigated by quantifying the total pool of pigments of the xanthophyll cycle ([Dd + Dt]/Chl a), with no significant differences (P = 0.460) found in response to changes in the light conditions (Figure 4b).
Xanthophyll cycle pigment ratios (average ± standard deviation; n = 5) in Vaucheria litorea and Elysia chlorotica in response to different light conditions: (i) “Dark”, dark-adapted samples for 60 min before the start of chlorophyll (Chl) fluorescence measurements; (ii) “HL”, high-light stress correspondent to exposure to 1800 μmol photons m−2 s−1 for 15 min; and (iii) “Recovery”, exposure to dark conditions during 30 min following the HL stress.
(a) Dt/(Dt + Dd) ratios (content of diatoxanthin [Dt] relative to the total pool of pigments of the xanthophyll cycle Dt + diadinoxanthin [Dd]). (b) Total pool of pigments of the xanthophyll cycle per Chl a. * indicates significant differences (P < 0.05) between light treatments (Dark, HL and Recovery).
Discussion
Immobilization of sea slugs for photobiology studies
A prerequisite for evaluation of fluorescence quenching during illumination is reliable determination of the minimal and maximal fluorescence after dark adaptation, Fo and Fm, respectively. These values serve as reference for the evaluation of photochemical and non-photochemical quenching in an illuminated sample by the saturation pulse method. To address Chl fluorescence quenching in kleptoplasts of sea slugs it is, therefore, crucial to immobilize individuals during measurements35. Since sea slugs used in Chl fluorescence measurements would be sacrificed for pigment analysis, rapid decapitation was the preferred immobilization method applied in this study. This methodology did not significantly affect the PSII quantum yield of the kleptoplasts (Figure S1), therefore, it can be assumed that NPQ measurements performed in both E. viridis and E. chlorotica sea slugs were also not affected by animal decapitation.
Comparing responses of different organisms to light exposure
The fraction of incident light absorbed will drastically differ in cells displaying different optical properties36. Factors such as species-specific morphology will affect the Chl-specific in vivo-absorption coefficient. For instance, lateral light transfer in coral tissues affects the irradiance reaching the algal symbionts, with consequences at the photophysiology level37. Chloroplasts in the algae may not be in the same optical environment as kleptoplasts in the sea slugs. Thus, even when the same photon flux is applied, responses of chloroplasts and kleptoplasts must be compared with care38,39. Moreover, pigments determine light absorption of a cell because only photons absorbed by pigments can work photochemically40. This implies that, without determination of the specific light absorption profile, the comparison of PSII quantum yield and NPQ may be biased by differences in the composition of light harvesting complexes of the different sea slugs and macroalgae.
NPQ and xanthophyll cycle functionality
In both V. litorea and E. chlorotica, Chl fluorescence dark-recovery after high-light exposure suggested the presence of typical reversible NPQ41,42. A functional xanthophyll cycle was also found, as demonstrated by the de-epoxidation of Dd to Dt during high-light exposure and the reversed reaction in dark conditions. Contrary to V. litorea and E. chlorotica, in the Chl fluorescence traces observed for C. tomentosum and E. viridis, Fm' did not increase significantly in the recovery phase but a pronounced decay of Fo was recorded. This translated into significant recovery of PSII quantum yield after high-light exposure, while NPQ evolving during that period was sustained in the dark-recovery phase.
Similar Chl fluorescence traces to those observed in C. tomentosum and E. viridis were found in the diatom Phaeodactylum tricornutum43. However, in this diatom, NPQ in the dark was sustained due to the presence of the xanthophyll Dt. Surprisingly, neither Anth or Zea (Dt equivalent in the xanthophyll cycle of green algae) were found in high-light exposed C. tomentosum or E. viridis and Viola was only a minor pigment. This indicates that one of the most important photoprotection mechanisms in plant cells, the xanthophyll cycle, was absent in chloroplasts of C. tomentosum. Consequently, no functional xanthophyll cycle was recorded in kleptoplasts of E. viridis. Lutein is also known to be involved in quenching of excessive energy and to have a photoprotective function32,34. However, in the present work, no lutein was detected in C. tomentosum and E. viridis. Furthermore, Raniello et al.44 showed that lutein and siphonaxanthin varied inversely through the day and suggested a photoprotective role for the interconversion of these two pigments. In search of a similar photoprotective role of siphonaxanthin in C. tomentosum, the interconversion between siphonaxanthin and siphonaxanthin-dodecenoate (also known as siphonein) was investigated with no significant changes between these pigments being found throughout the experimental trials. In order to exclude a potential artefact, promoted by the immobilization method employed (e.g., in the enzyme activity of the xanthophyll cycle), we performed additional pigment analysis in motile and immobilized animals exposed to high-light conditions and found no differences between them (Figure S2).
The absence of a xanthophyll cycle is known in cyanobacteria and its presence is questionable in red algae30. An absent xanthophyll cycle in a macroalgae from the Chlorophyte lineage is here reported, to our knowledge, for the first time. Previous works have reported the absence of the xanthophyll Zea in Codium sp.45,46,47, but none have investigated the interconversion between the xanthophylls Viola, Anth and Zea as a photoprotection mechanism. NPQ and PSII quantum yield in C. tomentosum and E. viridis were similar to that observed in a mutant of Arabidopsis thaliana defective in the gene encoding Viola de-epoxidase (npq1 mutant)48 and a mutant of A. thaliana lacking PsbS protein (npq4 mutant)49,50,51. In vascular plants, the presence of activated PsbS (member of the LHC superfamily that seems to have appeared exclusively in the green lineage)31 together with a de-epoxidised xanthophyll in PSII seems essential for the formation of qE26. We speculate that, similar to the npq1 mutant of A. thaliana, C. tomentosum is defective in the Viola de-epoxidase gene, but further investigations are needed to confirm this assumption.
It is not possible to conclude from the present experimental data if the sustained NPQ in the dark-recovery phase observed for C. tomentosum and E. viridis is caused by photoinhibition, or is simply indicative of a sustained and possibly protective quenching mechanism52. Recent works have demonstrated that there are slower components in photoprotective quenching which are impossible to distinguish from true photoinhibitory quenching using analysis of reversible NPQ upon light treatment53,54. To overcome this issue, a new method allowing one to verify during the light treatment the amount of protective NPQ (pNPQ)55,56,57 and the point of the onset of the inactivation of the PSII reaction centres, should be applied.
Photoprotection mechanisms may enhance functional kleptoplast longevity
How the kleptoplasts remain functional for several months inside the animal cells is still not well understood. E. viridis has been shown to survive up to 3 months without feeding1,8,12,46, while E. chlorotica can maintain functional kleptoplasts for eight to ten months2,17. However, retention times of functional kleptoplasts in sacoglossan sea slugs can drastically change depending on factors such as husbandry conditions in the laboratory, life-history traits prior to their collection from the wild, or the algal source of kleptoplasts38. Therefore, the often-used definition of “short-” or “long-term” retention times must be applied with caution. In addition, different photoprotection capacity and/or repair mechanisms of algal food sources will have implications in the longevity of photosynthetic activity in kleptoplasts retained by the sea slugs. In the particular case of E. viridis feeding on C. tomentosum, the absence of a functional xanthophyll cycle in C. tomentosum could relate to the shorter longevity of kleptoplasts in E. viridis when compared to other sea slugs such as E. timida28 or E. chlorotica. For instance, Vieira and co-workers12 clearly showed that when E. viridis was deprived of a food source, exposure to higher light regimes resulted in a drastic reduction in the time kleptoplasts remained functional, indicating potential permanent damage due to photoinhibition limited kleptoplasty longevity.
Conclusion
Photoprotection capacities of algal food sources may largely determine the longevity of sequestered chloroplasts and it is important to further understand if such mechanisms are maintained in kleptoplasts isolated from their algal nucleus. For instance, although two recently fed sea slug species have been shown to possess a functional xanthophyll cycle (Elysia timida28 and E. chorotica [present work]), it is unknown if this ability is maintained throughout a prolonged starvation period. Genes for the xanthophyll cycle enzymes are nuclear encoded in many organisms. It is therefore possible that a functional xanthophyll cycle in kleptoplasts is limited by the abundance and lifetime of enzymes (co-factors must also be considered) present at the time the chloroplasts were sequestered. de Vries et al.25 suggests that the presence of a specific gene (ftsH, a D1 quality control protease that is essential for photosystem II repair) in the chloroplast genomes of Acetabularia acetabulum and V. litorea (sole food source of two “long-term” sacoglossa species, E. timida and E. chlorotica, respectively) might be a key to “long-term” plastid activity in sea slugs, stressing the importance of PSII maintenance for kleptoplast longevity. Algae possess multiple photoprotective mechanisms including adjustment of light-harvesting antenna size, synthesis of stress proteins, accumulation of lipophilic and water-soluble antioxidant molecules, enhancement of scavenging enzymatic systems and accumulation of sunscreen58. In this way, further investigation of those processes is required to gain an in-depth knowledge on how light harvesting, photoprotection and photodamage repair can condition the long-term retention of functional kleptoplasts in sacoglossan sea slugs.
Methods
Biological material: collection and maintenance
Adults of the sea slug E. viridis Montagu 1804 and specimens of the macroalgae C. tomentosum Stackhouse 1797 were collected in the intertidal rocky area of Barra beach, Aveiro, Portugal (40° 38' 37.67'' N, 8° 44' 56.75'' W). The sea slugs and the macroalgae were maintained in a recirculated life support system operated with artificial seawater (prepared by mixing Tropic Marin© salts with freshwater purified by reverse osmosis) at salinity 35 and 18°C temperature. Photoperiod was 14 h light:10 h dark at an irradiance of approximately 20 μmol photons m−2 s−1 (all light intensities measured at air-water interface). E. chlorotica Gould 1870 were raised in the laboratory at the University of Connecticut, USA, as described by Pelletreau et al.11. Specimens were originally collected from wild populations from Martha's Vineyard, MA (USA). Adults of the sea slug E. chlorotica were maintained in artificial seawater (prepared by mixing Instant Ocean© salts with freshwater purified by reverse osmosis) at salinity 32 and 10°C temperature on a 12 h light:12 h dark photoperiod at 10 μmol photons m−2 s−1. Unialgal cultures of the filamentous alga V. litorea Agardh 1823, originating from a collection from Martha's Vineyard, were cultured in modified f/2 media (Na2SiO3·9H2O was omitted; concentrations of vitamins in final media were as follows: biotin 0.16 μM; thiamine HCl 0.118 μM; vitamin B12 0.029 μM). V. litorea cultures were maintained at 24°C on a 12 h light:12 h dark photoperiod and at a light intensity of 23 μmol photons m−2 s−1.
Chl fluorescence measurements
Variable fluorescence of C. tomentosum and E. viridis was measured at room temperature using a PAM fluorometer comprising a computer-operated PAM-Control Unit (Walz GmbH, Effeltrich, Germany) and a WATER-EDF-Universal emitter-detector unit with a 6 mm diameter Fluid Light Guide fiberoptics bundle (Gademann Instruments GmbH, Würzburg, Germany). This fluorometer uses a modulated blue light (LED-lamp, emission maximum at 450 nm and a half-bandwidth of 20 nm) as source for measuring, actinic and saturating light, emitted at a frequency of 18 Hz when measuring Fo or 20 kHz when measuring other fluorescence parameters (see notation in Table 1). Variable fluorescence of V. litorea and E. chlorotica was measured at room temperature using a mini-PAM coupled with a 5.5 mm mini-PAM/F fiberoptics (Walz GmbH, Effeltrich, Germany). This fluorometer uses a modulated red light (LED-lamp, emission maximum at 650 nm) as source for measuring light, emitted at a frequency of 0.6 Hz when measuring Fo or 20 kHz when measuring other fluorescence parameters and a halogen lamp (8 V/20 W blue enriched, λ < 710 nm) as source for actinic and saturating light. Both fluorometers use fiberoptics to guide measuring, actinic and saturating light to the sample and to collect fluorescence. Variable fluorescence was measured by recording Fo and Fs (dark and light adapted samples, Table 1) in the presence of a weak measuring-light and applying a saturating-light pulse (SP) to obtain Fm and Fm' (dark and light adapted, Table 1).
Thalli of C. tomentosum measuring 6 mm in length, filaments of V. litorea and motile or immobilized E. viridis and E. chlorotica sea slugs were individually placed on a well of a 96-well plate filled with water and covered with a coverslip for subsequent measurements of Chl fluorescence. In the case of C. tomentosum and both sea slugs, the well was partially filled with polystyrene foam (up to 2 mm from the top). Chl fluorescence fiberoptics where placed directly on top of the coverslip covering the sample. Samples were previously dark-adapted for 60 min in a Petri dish before being transferred to the well plate for Chl fluorescence measurements.
Immobilization methodology
To measure Chl fluorescence in the sea slugs and enable accurate NPQ calculations, individuals were immobilized using rapid decapitation. The effect of decapitation of sea slugs on Chl fluorescence quantum yield of PSII (Fv/Fm and ΔF/Fm', in dark and light conditions respectively) was tested using E. viridis as described in the supplementary material (Figure S1). No differences were found between measurements taken in motile or immobilized sea slugs.
Light-stress and dark-recovery experiments
The effect of a high-light exposure and recovery in darkness was tested as follows: Chl fluorescence was measured in 15 samples of C. tomentosum and V. litorea and in 15 specimens of immobilized E. viridis and E. chlorotica as described above. All samples were dark-adapted for 60 min in a petri dish before being transferred to the well plate. After transfer to the well, a SP was applied to determine Fv/Fm (see notations in Table 1). Subsequently, samples were exposed to low light (LL; 20 μmol photons m−2 s−1 for 10 min in C. tomentosum and E. viridis or 5 min in V. litorea and E. chlorotica) followed by a high-light exposure (HL; 920 μmol photons m−2 s−1 for 30 min in C. tomentosum and E. viridis or 1800 μmol photons m−2 s−1 for 15 min in V. litorea and E. chlorotica). Following the exposure to high-light, samples were allowed to recover in the dark for 60 or 30 min (C. tomentosum and E. viridis or V. litorea and E. chlorotica, respectively). A SP was applied every 10 or 5 min (C. tomentosum and E. viridis or V. litorea and E. chlorotica, respectively) for calculation of ΔF/Fm' and NPQ (see notations in Table 1). Differences in the protocols applied to the different organisms were due to PAM fluorometer equipment-specific limitations. Preliminary tests (data not shown) allowed us to determine the level of high-light exposure that would return similar levels of NPQ and PS II quantum yield efficiencies between both associations. From each group of macroalgae or sea slug specimens, five replicates were collected and immediately frozen in liquid nitrogen for pigment analysis at the following steps: (i) after Fv/Fm determination; (ii) at the end of the high-light exposure and (iii) at the end of the dark-recovery period. In order (i) to test an alternative light source and intensity that could induce de-epoxidation of Viola to Anth and Zea in C. tomentosum and E. viridis and (ii) to exclude a possible negative effect of decapitation of E. viridis in the xanthophyll cycle activation, the following experiment was performed: five samples of C. tomentosum, five specimens of immobilized E. viridis and five specimens of motile E. viridis were exposed to 2500 μmol photons m−2 s−1 for 30 min using a halogen lamp coupled to a heat absorbing glass. At the end of exposure to high-light, each sample was immediately frozen in liquid nitrogen for pigment analysis.
Pigment analysis
Sea slugs and macroalgal samples were freeze-dried and pigments extracted in 95% cold buffered methanol (2% ammonium acetate). Samples were ground with a glass rod, sonicated for 30 s and briefly vortexed. Samples were then transferred to −20°C for 20 min in the dark. Extracts were filtered through 0.2 μm Fluoropore membrane filters (Millipore, Billerica, MA, USA) and immediately injected into an HPLC system (Shimadzu, Kyoto, Japan) with a photodiode array detector (SPD-M10AVP). Chromatographic separation was carried out using a Supelcosil C18 column (25 cm length; 4.6 mm diameter; 5 μm particles; Sigma-Aldrich, St. Louis, MO, USA) for reverse phase chromatography and a 35 min elution programme. The solvent gradient followed Kraay et al.59, with an injection volume of 100 μl and a flow rate of 0.6 ml min−1. Pigments were identified from absorbance spectra and retention times and concentrations calculated from the signals in the photodiode array detector in comparison with pure crystalline standards from DHI (Hørsolm, Denmark). The fucoxanthin standard was used for the quantification of siphonaxanthin and siphonaxanthin dodecenoate because no purified standard or specific absorption coefficients were available60.
Statistical analysis
Differences in PSII quantum yield and NPQ between sea slugs and respective food source during the light stress experiments were tested using repeated measures one-way analysis of variance (ANOVA). Differences in pigment ratios were tested using two-way ANOVA for effects of light treatments (dark, high-light and dark-recovery) and organisms (sea slugs and respective algal food source). Pairwise comparisons were performed using the Tukey HSD test. Statistical analyses were carried out using Statistica 10 (StatSoft Inc., USA).
References
Hinde, R. & Smith, D. C. Persistence of functional chloroplasts in Elysia viridis (Opisthobranchia, Sacoglossa). Nature 239, 30–31 (1972).
Green, B. J. et al. Mollusc-algal chloroplast endosymbiosis. Photosynthesis, thylakoid protein maintenance and chloroplast gene expression continue for many months in the absence of the algal nucleus. Plant Physiol. 124, 331–342 (2000).
Evertsen, J., Burghardt, I., Johnsen, G. & Wägele, H. Retention of functional chloroplasts in some sacoglossans from the Indo-Pacific and Mediterranean. Mar. Biol. 151, 2159–2166 (2007).
Händeler, K., Grzymbowski, Y. P., Krug, P. J. & Wägele, H. Functional chloroplasts in metazoan cells - a unique evolutionary strategy in animal life. Front. Zool. 6, 28–46 (2009).
Ventura, P., Calado, G. & Jesus, B. Photosynthetic efficiency and kleptoplast pigment diversity in the sea slug Thuridilla hopei (Vérany, 1853). J. Exp. Mar. Biol. Ecol. 441, 105–109 (2013).
Akimoto, A., Hirano, Y. M., Sakai, A. & Yusa, Y. Relative importance and interactive effects of photosynthesis and food in two solar-powered sea slugs. Mar. Biol. 161, 1095–1102 (2014).
Christa, G. et al. Functional kleptoplasty in a limapontioidean genus: phylogeny, food preferences and photosynthesis in Costasiella with focus on C. ocellifera (Gastropoda, Sacoglossa). J. Moll. Stud. 80, 499–507 (2014).
Hinde, R. & Smith, D. C. The role of photosynthesis in the nutrition of the mollusc Elysia viridis. Biol. J. Linn. Soc. 7, 161–171 (1975).
Marín, A. & Ros, J. D. Dynamics of a peculiar plant-herbivore relationship: the photosynthetic ascoglossan Elysia timida and the chlorophycean Acetabularia acetabulum. Mar. Biol. 112, 677–682 (1992).
Pelletreau, K. N., Worful, J. M., Sarver, K. E. & Rumpho, M. E. Laboratory culturing of Elysia chlorotica reveals a shift from transient to permanent kleptoplasty. Symbiosis 58, 221–232 (2012).
Schmitt, V. & Wägele, H. Chloroplast incorporation and long-term photosynthetic performance through the life cycle in laboratory cultures of Elysia timida (Sacoglossa, Heterobranchia). Front. Zool. 11, 5 (2014).
Vieira, S., Calado, R., Coelho, H. & Serôdio, J. Effects of light exposure on the retention of kleptoplastic photosynthetic activity in the sacoglossan mollusc Elysia viridis. Mar. Biol. 156, 1007–1020 (2009).
Wägele, H. Potential key characters in Opistobranchia (Gastropoda, Mollusca) enhancing adaptive radiation. Org. Div. Evol. 4, 175–188 (2004).
Christa, G., Wescott, L., Schäberle, T. F., König, G. M. & Wägele, H. What remains after 2 months of starvation? Analysis of sequestered algae in a photosynthetic slug, Plakobranchus ocellatus (Sacoglossa, Opisthobranchia), by barcoding. Planta 237, 559–572 (2013).
de Vries, J., Christa, G. & Gould, S. B. Plastid survival in the cytosol of animal cells. Trends. Plant. Sci. 19, 347–350 (2014).
Shi, L. X. & Theg, S. M. The chloroplast protein import system: from algae to trees. Biochim. Biophys. Acta 1833, 314–331 (2013).
Mujer, C. V., Andrews, D. L., Manhart, J. R., Pierce, S. K. & Rumpho, M. E. Chloroplast genes are expressed during intracellular symbiotic association of Vaucheria litorea plastids with the sea slug Elysia chlorotica. Proc. Natl. Acad. Sci. USA 93, 12333–12338 (1996).
Pierce, S. K., Curtis, N. E., Hanten, J. J., Boerner, S. L. & Schwartz, J. A. Transfer, integration and expression of functional nuclear genes between multicellular species. Symbiosis 43, 57–64 (2007).
Rumpho, M. E. et al. Horizontal gene transfer of the algal nuclear gene psbO to the photosynthetic sea slug Elysia chlorotica. Proc. Natl. Acad. Sci. USA 105, 17867–17871 (2008).
Pierce, S. K. et al. Transcriptomic evidence for the expression of horizontally transferred algal nuclear genes in the photosynthetic sea slug, Elysia chlorotica. Mol. Biol. Evol. 29, 1545–1556 (2012).
Rumpho, M. E., Pelletreau, K. N., Moustafa, A. & Bhattacharya, D. The making of a photosynthetic animal. J. Exp. Biol. 214, 303–311 (2011).
Bhattacharya, D., Pelletreau, K. N., Price, D. C., Sarver, K. E. & Rumpho, M. E. Genome analysis of Elysia chlorotica egg DNA provides no evidence for horizontal gene transfer into the germ line of this kleptoplastic mollusc. Mol. Biol. Evol. 30, 1843–1852 (2013).
Wägele, H. et al. Transcriptomic evidence that longevity of acquired plastids in the photosynthetic slugs Elysia timida and Plakobranchus ocellatus does not entail lateral transfer of algal nuclear genes. Mol. Biol. Evol. 28, 699–706 (2011).
Green, B. J., Fox, T. C., Manhart, J. R. & Rumpho, M. E. Stability of isolated chromophytic algal chloroplasts that participate in a unique molluscan/algal endosymbiosis. Symbiosis 40, 31–40 (2005).
de Vries, J. et al. Is ftsH the key to plastid longevity in sacoglossan slugs? Genome Biol. Evol. 5, 2540–2548 (2013).
Zaks, J., Amarnath, K., Sylak-Glassman, E. J. & Fleming, G. R. Models and measurements of energy-dependent quenching. Photosynth. Res. 116, 389–409 (2013).
Li, Z., Wakao, S., Fischer, B. B. & Niyogi, K. K. Sensing and responding to excess light. Annu. Rev. Plant Biol. 60, 239–260 (2009).
Jesus, B., Ventura, P. & Calado, G. Behaviour and a functional xanthophyll cycle enhance photo-regulation mechanisms in the solar-powered sea slug Elysia timida (Risso, 1818). J. Exp. Mar. Biol. Ecol. 395, 98–105 (2010).
Müller, P., Li, X.-P. & Niyogi, K. K. Non-photochemical quenching. A response to excess light energy. Plant Physiol. 125, 1558–1566 (2001).
Goss, R. & Jakob, T. Regulation and function of xanthophyll cycle-dependent photoprotection in algae. Photosynth. Res. 106, 103–122 (2010).
Niyogi, K. K. & Truong, T. B. Evolution of flexible non-photochemical quenching mechanisms that regulate light harvesting in oxygenic photosynthesis. Curr. Opin. Plant Biol. 16, 307–314 (2013).
Goss, R. & Lepetit, B. Biodiversity of NPQ. J. Plant Physiol. 172, 13–32 (2015).
Whittle, S. J. & Casselton, P. J. The chloroplast pigments of the algal classes Eustigmatophyceae and Xanthophyceae. II. Xanthophyceae. Br. Phycol. J. 10, 192–204 (1975).
Jahns, P. & Holzwarth, A. R. The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim. Biophys. Acta 1817, 182–193 (2012).
Cruz, S., Dionísio, G., Rosa, R., Calado, R. & Serôdio, J. Anesthetizing solar-powered sea slugs for photobiological studies. Biol. Bull. 223, 328–336 (2012).
Blache, U., Jakob, T., Su, W. & Wilhelm, C. The impact of cell-specific absorption properties on the correlation of electron transport rates measured by chlorophyll fluorescence and photosynthetic oxygen production in planktonic algae. Plant Physiol. Bioch. 49, 801–808 (2011).
Wangpraseurt, D., Larkum, A. W. D., Franklin, J., Szabó, M., Ralph, P. J. & Kühl, M. Lateral light transfer ensures efficient resource distribution in symbiont-bearing corals. J. Exp. Biol. 217, 489–498 (2014).
Cruz, S., Calado, R., Serôdio, J. & Cartaxana, P. Crawling leaves: photosynthesis in sacoglossan sea slugs. J. Exp. Bot. 64, 3999–4009 (2013).
Serôdio, J., Cruz, S., Cartaxana, P. & Calado, R. Photophysiology of kleptoplasts: photosynthetic use of light by chloroplasts living in animal cells. Phil. Trans. R. Soc. B 369, 20130242 (2014).
Terashima, I., Fujita, T., Inoue, T., Chow, W. S. & Oguchi, R. Green light drives leaf photosynthesis more efficiently than red light in strong white light: revisiting the enigmatic question of why leaves are green. Plant Cell Physiol. 50, 684–697 (2009).
Eberhard, S., Finazzi, G. & Wollman, F. A. The dynamics of photosynthesis. Annu. Rev. Genet. 42, 463–515 (2008).
Roháček, K. Method for resolution and quantification of components of the non-photochemical quenching (qN). Photosynth. Res. 105, 101–113 (2010).
Grouneva, I., Jakob, T., Wilhelm, C. & Goss, R. The regulation of xanthophyll cycle activity and of non-photochemical fluorescence quenching by two alternative electron flows in the diatoms Phaeodactylum tricornutum and Cyclotella meneghiniana. Biochim. Biophys. Acta 1787, 929–938 (2009).
Raniello, R., Lorenti, M., Brunet, C. & Buia, M. C. Photoacclimation of the invasive alga Caulerpa racemosa var.cylindracea to depth and daylight patterns and a putative new role for siphonaxanthin. Mar. Ecol. 27, 20–30 (2006).
Benson, E. E. & Cobb, A. H. The separation, identification and quantitative determination of photopigments from the siphonaceous marine alga Codium fragile. New Phytol. 88, 627–632 (1981).
Evertsen, J. & Jonhsen, G. In vivo and in vitro differences in chloroplast functionality in the two north Atlantic sacoglossans (Gastropoda, Opisthobranchia) Placida dendritica and Elysia viridis. Mar. Biol. 156, 847–859 (2009).
Cruz, S., Calado, R., Serôdio, J., Jesus, B. & Cartaxana, P. Pigment profile in the photosynthetic sea slug Elysia viridis (Montagu, 1804). J. Moll. Stud. 80, 475–481 (2014).
Niyogi, K. K., Grossman, A. R. & Björkman, O. Arabidopsis mutants define a central role for the xanthophyll cycle in the regulation of photosynthetic energy conversion. Plant Cell 10, 1121–1134 (1998).
Li, X.-P. et al. A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature 403, 391–395 (2000).
Li, X.-P., Muller-Moule, P., Gilmore, A. M. & Niyogi, K. K. PsbS-dependent enhancement of feedback de-excitation protects photosystem II from photoinhibition. Proc. Natl. Acad. Sci. USA 99, 15222–15227 (2002).
Zaks, J., Amarnath, K., Kramer, D. M., Niyogi, K. K. & Fleming, G. R. A kinetic model of rapidly reversible nonphotochemical quenching. Proc. Natl. Acad. Sci. USA 109, 15757–15762 (2012).
Ruban, A. V. Evolution under the sun: optimizing light harvesting in photosynthesis. J. Exp. Bot. 10.1093/jxb/eru400 (2014).
Johnson, M. P. & Ruban, A. V. Arabidopsis plants lacking PsbS protein possess photoprotective energy dissipation. Plant J. 61, 283–289 (2010).
Ruban, A. V., Johnson, M. P. & Duffy, C. D. P. The photoprotective molecular switch in the photosystem II antenna. Biochm Biophys Acta 1817, 167–181 (2012).
Ruban, A. V. & Murchie, E. H. Assessing the photoprotective effectiveness of non-photochemical chlorophyll fluorescence quenching: A new approach. Biochm Biophys Acta 1817, 977–982 (2012).
Murchie, E. H. & Lawson, T. Chlorophyll fluorescence analysis: a guide to good practice and understanding some new applications. J. Exp. Bot. 64, 3983–3998 (2013).
Ruban, A. V., & Belgio, E. The relationship between maximum tolerated light intensity and photoprotective energy dissipation in the photosynthetic antenna: chloroplast gains and losses. Phil. Trans. R. Soc. B 369, 20130222 (2014).
Havaux, M. & Kloppstech, K. The photoprotective functions of carotenoid and flavonoid pigments against excess visible radiation at chilling temperature investigated in Arabidopsis npq and tt mutants. Planta 213, 953–966 (2001).
Kraay, G. W., Zapata, M. & Veldhuis, M. Separation of chlorophylls c1, c2 and c3 of marine phytoplankton by reversed-phase C18 high-performance liquid chromatography. J. Phycol. 28, 708–712 (1992).
Egeland, E. S. et al. [Data sheets aiding identification of phytoplankton carotenoids and chlorophylls] .Phytoplankton Pigments: Characterization, Chemotaxonomy and Applications in Oceanography. [Roy, S., Llewellyn, C. A., Egeland, E. S. & Johnsen, G. (ed.)] [665–822] (Cambridge University Press, Cambridge, UK, 2011).
Acknowledgements
The authors thank Susan Devine and Gregory Mirando for help in the laboratory during the experimental work at the University of Connecticut (USA) and for growing and maintaining E. chlorotica specimens and the alga V. litorea used in this work. Sónia Cruz and Gisela Dionísio were supported by the Fundação para a Ciência e a Tecnologia, through the postdoctoral grant SFRH/BPD/74531/2010 (S. Cruz) and doctoral grant SFRH/BD/73205/2010 (G. Dionísio). This work was funded by the FP7 Marie Curie Career Integration grant PCIG11-GA-2012-322349 (S. Cruz) and by National Science Foundation grant IOS-0726178 (M. Rumpho).
Author information
Authors and Affiliations
Contributions
S.C., P.C., R.C., J.S., K.N.P. and M.E.R. conceived and designed research. S.C., P.C., G.D. and R.N. conducted experiments. S.C. and P.C. analyzed data. S.C. wrote the manuscript. All authors read, commented and approved the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary Information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Cruz, S., Cartaxana, P., Newcomer, R. et al. Photoprotection in sequestered plastids of sea slugs and respective algal sources. Sci Rep 5, 7904 (2015). https://doi.org/10.1038/srep07904
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep07904
This article is cited by
-
Photosynthetic animals and where to find them: abundance and size of a solar-powered sea slug in different light conditions
Marine Biology (2023)
-
Kleptoplast distribution, photosynthetic efficiency and sequestration mechanisms in intertidal benthic foraminifera
The ISME Journal (2022)
-
Ultraviolet screening by slug tissue and tight packing of plastids protect photosynthetic sea slugs from photoinhibition
Photosynthesis Research (2022)
-
Effects of photoperiod and light spectra on growth and pigment composition of the green macroalga Codium tomentosum
Journal of Applied Phycology (2021)
-
The ability to incorporate functional plastids by the sea slug Elysia viridis is governed by its food source
Marine Biology (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.