Abstract
We tested the hypothesis that leukocyte telomere length (LTL) is associated with birth weight in both extremes of abnormal fetal growth: small (SGA) and large for gestational age newborns (LGA). Clinical and laboratory variables of the mothers and the neonates were explored; 45 newborns with appropriate weight for gestational age (AGA), 12 SGA and 12 LGA were included. Whether the differences might be explained by variation in OBFC1 (rs9419958) and CTC1 (rs3027234) genes associated with LTL was determined. A significant association between birth weight and LTL was observed; LTL was significantly shorter in LGA newborns (1.01 ± 0.12) compared with SGA (1.73 ± 0.19) p < 0.005, mean ± SE. Maternal (Spearman R = −0.6, p = 0.03) and neonatal LTL (R = −0.25, p = 0.03) were significantly and inversely correlated with maternal history of arterial hypertension in previous gestations. Neonatal LTL was not significantly associated with either rs9419950 or rs3027234, suggesting that the association between neonatal LTL and birth weight is not influenced by genetic variation in genes that modify the interindividual LTL. In conclusion, telomere biology seems to be modulated by abnormal fetal growth; modifications in telomere length might be programmed by an adverse environment in utero.
Similar content being viewed by others
Introduction
The thrifty phenotype/genotype hypothesis for explaining the origin of metabolic syndrome-associated phenotypes was introduced several years ago. Since the first description of David Barker and coworkers about the fetal metabolic programming of adult chronic diseases1, there has been a large amount of epidemiological data confirming the strong association between an impaired intrauterine environment and adult metabolic and cardiovascular diseases (CVD)2,3,4,5,6.
The concept of fetal programming has two important emerging putative consequences: the transmission of the phenotype from the mother to the progeny and even through generations7 and the permanent change of the metabolism of the newborn exposed to an adverse intrauterine environment even in the absence of the original stimulus8. In line with the last point, all the robust epidemiological data supporting the concept of fetal programming was specifically focused on birth weight and its relation with adult chronic diseases2,4,5,9.
In this regard, the most suitable hypothesis to explain the interplay between the in utero environment and the later development of chronic diseases is the concept of epigenetic modifications10, which illustrates how the in utero life modulates the adult phenotype and suggests the induction of permanent genomic marks. For instance, some interesting evidence from experimental studies showed that twinning and undernutrition in the mother around the time of conception alter the epigenetic status of genes involved in the regulation of metabolism in hypothalamus11. Specifically, an adverse in utero environment is associated with epigenetic changes in the methylation status of proopiomelanocortin and the glucocorticoid receptor (GR) that might have an impact on the regulation of food intake in offspring when they reach adulthood, leading to the development of obesity11.
In line with these data, Lillycrop and coworkers showed that feeding a protein-restricted diet to pregnant rats causes hypomethylation of GR in umbilical cord and a reduced Dnmt1 expression12. Moreover, we demonstrated that maternal diet during pregnancy has an impact on fetal metabolic programming through changes in liver mitochondrial DNA content and liver transcriptional activity of the peroxisome proliferator-activated receptor gamma, coactivator (Ppargc1a)13.
Furthermore, evidence from human studies examining changes in the global methylation status of cord blood DNA identified differential methylation at 19 cytosine-guanine dinucleotides associated with either decreased or increased birth weight14. We also reported that changes in methylation of genes related with mitochondrial biogenesis such as PPARGC1A is associated with small and large in comparison with normal for gestational age15. Finally, remarkable findings about offspring exposed to gestational diabetes mellitus support the concept of fetal programming of adult metabolic diseases16.
Recent evidence suggests that telomere biology is potentially plausible of modification during fetal life17 and modifications in telomere length might be thus programmed by an adverse environment in utero18.
For instance, a recent study showed that maternal estradiol concentration during early pregnancy might modulate telomere length of their infants19. In addition, Toutain et al. showed that placental telomere length in ongoing pregnancies is associated with intrauterine growth restriction secondary to placental insufficiency20. Moreover, it was shown that maternal psychological stress during pregnancy might exert a programming effect on the telomere biology system that directly impact on the newborn telomere length21. The putative mechanisms associated with telomere homeostasis during the intrauterine life and its impact on adult disease was comprehensive reviewed by Entringer and colleagues earlier21.
Nevertheless, current data are still inconclusive, as previous work showed that leukocyte telomere length (LTL) is not much different in small for gestational babies (SGA) as compared with that in those who are appropriate for gestational age (AGA)22. Thus, whether differences exist in the two extremes of fetal growth is still unknown.
Hence, we tested the hypothesis that LTL may be associated with birth weight in both extremes of abnormal fetal growth: SGA and large for gestational age newborns (LGA). We also explored the clinical features and anthropometric and laboratory variables associated with LTL and whether the observed differences were explained by variation in two genes associated with LTL.
Results
Clinical features, anthropometrical variables and laboratory findings of the pregnant women (at the time of delivery) and the neonates according to the offspring birth weight can be seen in Table 1. Many features of the mother and newborn associated with birth weight are consistent with those already described23, that is, pregestational body mass index (BMI) of the mother is associated with neonatal sex and gestational age-adjusted body weight. As expected, newborn leptin correlated with neonatal sex and gestational age-adjusted body weight.
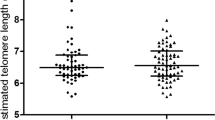
Interestingly, we observed a significant association between sex and gestational age-adjusted birth weight and LTL (Figure 1A); LTL was significantly shorter in the LGA newborns than in the SGA ones. Accordingly, LTL was significantly and inversely correlated (Spearman R: −0.21, p = 0.008) with newborn's body weight Z-score (Figure 1B). In addition, LTL was significantly and inversely associated with other developmental characteristics of the newborns, such as neonatal head circumference (Spearman correlation R = −0.3 p = 0.03). There were no sex differences in LTL among the neonates.
(A)—Neonatal leukocyte telomere length (LTL)/ single-copy gene (HBB, human β-globin) ratio in small for gestational age (SGA), appropriate for gestational age (AGA) and large for gestational age (LGA) neonates. P values stands for the post hoc Newman-Keuls test after ANOVA with log-transformed variables. (B)—Pearson correlation test of log transformed values of LTL sex and and age-adjusted neonatal birth weight. R: −0.344, p = 0.0046.
We also explored the LTL in the mothers and observed that there was no statistically significant association with the newborns birth weight (Figure 2A). Nevertheless, we found that maternal LTL was significantly and inversely correlated with history of arterial hypertension in previous pregnancies (Spearman R = −0.6, p = 0.03); differences in mothers' LTL according to history of elevated arterial blood pressure in previous pregnancies are shown in Figure 2B. Significant differences in mothers' systolic and diastolic blood pressure according to previous history of arterial hypertension were observed and are shown in Supplementary Figure 1.
(A)—Mother LTL/single-copy gene (HBB, human β-globin) ratio according to birth weight (small for gestational age (SGA), appropriate for gestational age (AGA) and large for gestational age (LGA). (B)—Mother LTL/single-copy gene (HBB, human β-globin) ratio according to their previous history of arterial hypertension. (C)—Neonatal LTL/single-copy gene (HBB, human β-globin) ratio according to the current status of maternal blood pressure.
Neonatal LTL was also significantly and inversely correlated with maternal history of arterial hypertension in previous pregnancies (Spearman R = −0.25, p = 0.03) and significantly influenced by the arterial blood pressure status of their mothers in current pregnancy (Figure 2C). These findings are not surprising because of the association of hypertension in successive pregnancies: 33% of mothers with previous antecedents of hypertension developed hypertension in the current pregnancy against 5% in those who do not (chi square, p < 0.02).
Neonatal LTL and gene variants
Because previous genome-wide association studies (GWASs) have identified some single nucleotide polymorphisms (SNPs) associated with LTL variation, such as the rs9419958 at OBFC1 oligonucleotide/oligosaccharide-binding fold locus24,25 and the rs3027234 at CTC1 telomere maintenance complex locus25, we decided to explore whether these genetic variants accounted for the observed differences in the neonatal LTL.
Previous reports support the selection of these loci for exploration in our sample. For example, variation in OBFC1 showed a replicated association with mean LTL in a large scale GWA meta-analysis of 37,684 individuals from 15 cohorts26. In addition, a large population study on the risk of CVD showed that the rs9419958 was associated with CVD-related death, particularly among women27. The OBFC1 gene also appears to function in a telomere-associated complex of proteins required to protect telomeres from DNA degradation28. Finally, functional experiments demonstrated that OBFC1 represents a new player in the telomere interactome for telomere maintenance29.
Regarding to CTC1 gene, there is biological evidence that supports the selection of this locus for exploration of gene variants. For instance, this gene encodes a component of the CST complex, which plays an essential role in protecting telomeres from degradation28. Furthermore, CTC1 may have a function in DNA metabolism30 and in regulating telomere homeostasis25; the CST complex seems also to play critical roles in coordinating telomerase elongation and fill-in synthesis to complete telomere replication31.
Allelic frequencies and genotype distributions of the rs9419958 and rs3027234 were in Hardy-Weinberg equilibrium. For the analysis, we used a recessive model of inheritance because of the low frequency of the minor allele of each SNP in our sample.
We observed that the neonatal LTL was not significantly associated with either rs9419950 or rs3027234 (Table 2), suggesting that the association between neonatal LTL and birth weight is not influenced by genetic variation in genes that modify the interindividual LTL (Table 2). The rs9419958 was significantly correlated with neonatal plasma homocysteine levels (Spearman R = 0.26, p < 0.02 and Table 2) and significantly and negatively correlated with the mother systolic arterial blood pressure (Spearman R = −0.24, p < 0.04).
To better understand these unexpected findings associated with the gene variants, we performed a functional association analysis of interactions pathways of OBFC1 and CTC1 genes and its relation with arterial hypertension and homocysteine levels allowing the exploration of disease-gene interactions. The functional analysis showed a predicted disease-gene node around OBFC1 but not CTC1 that integrates an interrelated meta-node composed of two genes/proteins: 1) HNF1 homeobox a (alias TCF1)—a transcription factor that is strongly associated with the angiotensinogen (AGT), which is associated with hypertension and 2) HNF4A (hepatocyte nuclear factor 4 alpha) that is connected with the methylenetetrahydrofolate dehydrogenase (MTHFD1) and was predicted to modulate homocysteine levels (Figure 3).
Analysis of interaction pathways of OBFC1 and CTC1 genes (pink nodes) and its relation with arterial hypertension and homocysteine levels.
The integrative analysis of biological networks was performed using “VisANT 4.0: Integrative network platform to connect genes, drugs, diseases and therapies” (a freely available and Web-based workbench at http://visant.bu.edu)47.
Discussion
In this study, we asked whether newborn telomere biology might be modulated by abnormal fetal growth and observed that birth weight was significantly associated with LTL. Our results show for the first time that babies who are born large have shorter telomeres in comparison with small babies. Accordingly, LTL was also significantly and inversely correlated with anthropometric variables associated with newborn's body size, such as head circumference. Consistent with previous human reports, LTL of SGA babies was not significantly different from that of AGA babies22,32, suggesting that the molecular mechanisms behind decreased fetal growth are unrelated to telomere biology. A note of caution clarifying that the term of SGA is not synonymous with “intrauterine growth retardation (restriction)” must be added3.
We might wonder about the factors that modulate the telomere biology in larger babies with shorter telomeres. LGA and SGA babies face many challenges during fetal life, but the reason the LGA babies are different in terms of telomere biology remains largely unknown. Nevertheless, telomere biology might be suggested as an underlying mechanism connecting fetal programming and chronic adult diseases33, as the telomere homeostasis is quite receptive of the in utero environment and is also highly sensitive to inflammation, oxidative stress and related adverse and stressful situations33. Although homocysteine levels may be one of them, we were not able to find any significant association.
Moreover, LGA infants are well known to suffer from some complications, including fetal hypoxia and hypoglycemia, which is a consequence of mother hyperinsulinemia3. Still, whether these or other factors might be the determining ones of the modifications of the telomere length in LGA babies remains to be explored. In agreement with the hypothesis of the in utero reprogramming toward senescence, previous evidence showed higher cord blood telomerase activity in Type 1 and gestational diabetes34.
Furthermore, when we explored whether maternal factors might be able to modify babies' LTL at birth, we observed that arterial hypertension was a critical factor in the modulation of the newborn LTL. We found that LTL at birth was significantly associated not only with arterial blood pressure of their mothers during current pregnancy but also with the history of arterial hypertension in previous pregnancies, despite the fact that most mothers who developed hypertension in the current pregnancy were devoid of personal history of hypertension. This finding supports the notion about the maternal disease-associated programming effect of the newborn telomere length. Although this finding is expected because CVDs, such as hypertension, are associated with short LTL35, the programming effect of the mother's hypertension is a novel finding and suggests that the arterial blood pressure control is an important goal to achieve in women during fertile age, although prospective studies are necessary to further investigate this issue.
On the other hand, we asked whether genetic factors might influence LTL at birth and then explored two SNPs in genes associated either with telomere biology (OBFC1) or with LTL (OBCF1 and CTC1). Interestingly, we observed that genetic variation does not account for LTL at birth. However, an unexpected result that needs further confirmation, such as the significant association between the rs9419958 and fetal plasma homocysteine levels and maternal systolic blood pressure, was observed. Functional association analysis supports the biological plausibility of our findings; specifically, it was shown that OBCF1 interacts with HNF4A as explained in the results section. Notably, a GWAS in the community-based Framingham Heart Study on risk factors of CVD showed an association between a variant in OBFC1 gene (the rs3814219) and flow-mediated dilation, a surrogate of impaired endothelial function36; this variant was among the top SNPs associated with brachial artery vascular function-related traits (p value for association 9.48 × 10−7)36. These results were replicated in a large sample of subjects from Europe, showing that variants located in OBFC1 are associated with higher risk of CVD, including coronary heart disease37; remarkably, higher expression of OBFC1 in adventitial aortic tissue was demonstrated in this study37. Taken together, theses findings support our results and suggest a strong biological plausibility of the association between the OBFC1 locus and the risk of CVD in humans.
The association between the variant in OBFC1 locus and fetal plasma homocysteine levels might represent a CVD-risk related-associated trait in consonance with the association found with maternal systolic blood pressure, as it was previously shown that there is a strong linear association between the maternal and fetal levels of homocysteine in circulation38 and that elevated homocysteine levels are a strong and independent risk factor for vascular disease and hypertension in adult39 and adolescents40.
Finally, we would like to discuss the putative clinical implications of our results. Although the design of our study prevents against any further speculation about adult health outcomes of the studied babies, some concluding remarks might be added. For instance, although long-term consequences of abnormal fetal growth are already known, specifically those related with LGA infants who are at increased risk of obesity, type 2 diabetes and CVD in adolescence and adulthood3, some novel data emerged. Our findings might suggest a “programming senescence effect” of adult chronic diseases associated with the metabolic syndrome, particularly arterial hypertension. As neonatal peripheral blood LTL is shown to be highly correlated with telomere length in tissues, we might speculate that both abnormal fetal growth and maternal stress have an impact on offspring telomere biology, leading to a “short-telomere phenotype” that renders them prone to cardiovascular complications in adulthood. This shorter telomere phenotype at birth indeed is imprinting an accelerated aging phenotype in target tissues that might compromise cellular function and integrity, affecting normal physiology and incrementing disease risk.
A limitation of our study should be highlighted as a larger-sized sample and availability longitudinal clinical and biochemical measurements in the infants would considerable strengthen our investigation. In addition, we could have limited statistical power to detect modest genetic effects, given the small sample size of our sample; finally, the extrapolation of our genetic findings to other ethnicities remains to be explored.
In conclusion, in this study, we tested the hypothesis that leukocyte telomere length (LTL) may be associated with birth weight in both extremes of abnormal fetal growth: small for gestational age and large for gestational age newborns. Our results show that babies who are born large have shorter telomeres in comparison with small babies. Furthermore, when we explored whether maternal factors might be able to modify babies' LTL at birth, we observed that arterial hypertension was a critical factor in the modulation of the newborn LTL. Our findings might suggest a “programming senescence effect” of adult chronic diseases associated with the metabolic syndrome, particularly arterial hypertension.
Methods
Ethics statements
This study was performed according to the principles of the Declaration of Helsinki and was approved by the institutional review board and the bioethical committee of our institution. Furthermore, written consent was obtained from all the patients.
Subjects
Sixty-nine consecutive healthy pregnant women and their babies were included in this study, selecting around two AGA babies for each SGA or LGA groups. According to the offspring birth weight normalized by sex and gestational age, there were 45 newborns with appropriate weight for gestational age (AGA) and 24 with abnormal weight for gestational age: 12 SGA and 12 LGA.
There were no multiple pregnancies in the study population. None of the mothers had either preeclampsia-eclampsia or gestational diabetes or were under any medication. At the time of delivery, an aliquot of cord blood was stored for further analysis.
All the investigations performed in this study were conducted in accordance with the guidelines of the Declaration of Helsinki. Written consent from the patients had been granted, in accordance with the procedures approved by the ethical committee of our institution. Complete medical, obstetrical and perinatal data were recorded as shown below.
Obstetric Variables
Gestational age was defined on the basis of the last menstrual period, physical examination and ultrasonographic biometry. Length of gestation at delivery was measured as the number of weeks of gestation. The ultrasound measure during the first trimester took precedence if these measures did not agree in terms of dating gestational age at birth.
Maternity data including age, geographical origin, pregestational BMI, education, smoking (number of cigarettes/day), history of abortion, history of preterm birth and previous abnormal fetal weight, preeclampsia-eclampsia, hypertension in pregnancy, hyperlipidemia or diabetes, pregnancy weight gain, weight of the previous offspring and gender of the newborn were evaluated. Besides, family history of disease was also recorded.
Resting systolic and diastolic arterial blood pressure (SABP and DABP, respectively) was measured in all the mothers after they had been sitting for at least 30 min. A mercury sphygmomanometer was used to measure blood pressure thrice at the right arm. Eleven mothers were diagnosed with hypertension (blood pressure equal to or greater than 140/90 mm Hg) during pregnancy, without clinical evidence of edema or proteinuria, after 20 weeks of gestation.
Birth outcomes
Infants were classified as SGA, LGA, or AGA according to the following criteria: SGA was defined as birth weight for gestational age less than the specific 10th percentile cutoff of a published Argentinian fetal growth reference41 and LGA was defined as birth weight above the 90th percentile for gestational age and sex3,42. The rest of the newborns were considered as AGA.
Evaluation of the newborn also included physical examination, search for any features suggestive of a malformation syndrome, supine lengths, Apgar score evaluation and type of delivery.
Biochemical Measurements
Blood was drawn from pregnant women who were in a supine resting position for at least 30 min and from the umbilical cord at delivery. Routine laboratory variables were measured using standard clinical laboratory techniques by the time of the delivery. A commercial ELISA kit was used to measure plasma leptin concentrations (Assay Designs, Inc, U.S.A.) in blood samples collected with sodium EDTA; concentrations of plasma total homocysteine were determined using immunochemoluminiscence methods according to the manufacturer's instructions (Immulite 1000, DPC, Los Angeles, CA, USA).
DNA isolation and measurements of telomere length
Genomic DNA was extracted from white blood cells from a blood sample of the mothers and their babies using a previously described standard method43.
An assay based on real-time quantitative PCR (iCycler thermocycler; Bio-Rad, Hercules, CA) was used for relative LTL measurement based on a method that uses a ratio of telomere DNA signal intensity to human β-globin single-copy gene signal intensity44; SYBR Green was used as a fluorescent dye (Invitrogen, Buenos Aires, Argentina).
All samples for both the telomere and single-copy gene (HBB, human β-globin) reactions were done in triplicate in the same plate and a control DNA sample was added to each plate to account for interassay variability. The Ct value corresponds to the cycle number at which the fluorescence generated within a reaction well exceeds a predefined threshold value (100 in each run). We used the software LinRegPCR45 (version 2012.0) for efficiency computation and computed the EffCt of each replicate. We determined the mean value of the replicates for each sample and each gene. The relative T/S ratio is the ratio of the telomere to HBB in one sample, set in relation to the same ratio in the control DNA. The formula for calculation of the relative T/S ratios can be stated as follows: T/S ratio = [Eff telomere (control)Ct telomere (control)/Eff telomere (sample)Ct telomere (sample)]/[Eff HBB (control)Ct HBB (control)/Eff HBB (sample)Ct HBB (sample)]46. Specificity of amplification and absence of primer dimers were confirmed by melting curve analysis at the end of each run. Primer sequences and resulting PCR product lengths are shown in Table 3.
The PCR efficiencies for telomere and HBB amplification were 1.76 and 1.95 on average. As individual samples were analyzed in triplicate and accepted only if the standard deviation of the Ct values was <1 Ct, the inter- and intra- experimental coefficient of variation of the qPCR was less than 8% and 3%, respectively.
Polymorphism genotyping
Two SNPs (rs9419958 at 10: 105675945 in OBFC1 and rs3027234 at 17: 8136092 in CTC1) were analyzed in the neonatal sample. Genotypes of these genetic polymorphisms were examined using PCR-based restriction fragment length polymorphism (RFLP); primers used in PCR-RFLP for amplifying genomic DNA are shown in Table 3. The restriction digestion of rs9419958 and rs3027234 and PCR products was carried out with restriction enzymes HpaII and DdeI (New England Biolabs Inc.), respectively, at 37°C for about 12 h. The digested products were loaded and visualized on 2.5% agarose gel after staining with ethidium bromide. Genotyping accuracy was assessed using inclusion of duplicates and negative.
The integrative analysis of biological networks was performed by the VisANT 4.0 platform (a freely available and Web-based workbench at http://visant.bu.edu)47.
Statistical Analysis
Otherwise indicated, quantitative data were expressed as mean ± SE and differences between groups were assessed using ANOVA or ANCOVA. Because some variables, particularly, the LTL/HBB ratio, do not have a normal distribution, we assessed differences in this variable using Mann-Whitney nonparametric test, or post hoc Newman-Keuls after ANOVA or ANCOVA, after a Log transformation if applicable. Correlation between two variables was performed using Spearman rank correlation or Pearson tests. To perform these analyses, we used the CSS/Statistica program package, StatSoft V 6.0 (Tulsa, USA).
References
Barker, D. J., Bull, A. R., Osmond, C. & Simmonds, S. J. Fetal and placental size and risk of hypertension in adult life. BMJ 301, 259–262 (1990).
Curhan, G. C. et al. Birth weight and adult hypertension, diabetes mellitus and obesity in US men. Circulation 94, 3246–3250 (1996).
Das, U. G. & Sysyn, G. D. Abnormal fetal growth: intrauterine growth retardation, small for gestational age, large for gestational age. Pediatr. Clin. North Am. 51, 639–654 (2004).
Harder, T., Rodekamp, E., Schellong, K., Dudenhausen, J. W. & Plagemann, A. Birth weight and subsequent risk of type 2 diabetes: a meta-analysis. Am. J. Epidemiol. 165, 849–857 (2007).
Osmond, C., Barker, D. J., Winter, P. D., Fall, C. H. & Simmonds, S. J. Early growth and death from cardiovascular disease in women. BMJ 307, 1519–1524 (1993).
Poston, L. Gestational weight gain: influences on the long-term health of the child. Curr. Opin. Clin. Nutr. Metab Care 15, 252–257 (2012).
Patel, M. S. & Srinivasan, M. Metabolic programming: causes and consequences. J. Biol. Chem. 277, 1629–1632 (2002).
Barker, D. J. A new model for the origins of chronic disease. Med Health Care Philos. 4, 31–35 (2001).
Forsen, T., Eriksson, J. G., Tuomilehto, J., Osmond, C. & Barker, D. J. Growth in utero and during childhood among women who develop coronary heart disease: longitudinal study. BMJ 319, 1403–1407 (1999).
Sookoian, S., Gianotti, T. F., Burgueno, A. L. & Pirola, C. J. Fetal metabolic programming and epigenetic modifications: a systems biology approach. Pediatr. Res. 73, 531–542 (2013).
Begum, G. et al. Epigenetic changes in fetal hypothalamic energy regulating pathways are associated with maternal undernutrition and twinning. FASEB J. 26, 1694–1703 (2012).
Lillycrop, K. A. et al. Induction of altered epigenetic regulation of the hepatic glucocorticoid receptor in the offspring of rats fed a protein-restricted diet during pregnancy suggests that reduced DNA methyltransferase-1 expression is involved in impaired DNA methylation and changes in histone modifications. Br. J. Nutr. 97, 1064–1073 (2007).
Burgueno, A. L., Cabrerizo, R., Gonzales, M. N., Sookoian, S. & Pirola, C. J. Maternal high-fat intake during pregnancy programs metabolic-syndrome-related phenotypes through liver mitochondrial DNA copy number and transcriptional activity of liver PPARGC1A. J. Nutr. Biochem. 24, 6–13 (2012).
Engel, S. M. et al. Neonatal genome-wide methylation patterns in relation to birth weight in the Norwegian Mother and Child Cohort. Am. J. Epidemiol. 179, 834–842 (2014).
Gemma, C. et al. Maternal pregestational BMI is associated with methylation of the PPARGC1A promoter in newborns. Obesity. (Silver. Spring) 17, 1032–1039 (2009).
Ruchat, S. M. et al. Gestational diabetes mellitus epigenetically affects genes predominantly involved in metabolic diseases. Epigenetics 8, 935–943 (2013).
Tarry-Adkins, J. L. & Ozanne, S. E. Mechanisms of early life programming: current knowledge and future directions. Am. J. Clin. Nutr. 94, 1765S–1771S (2011).
Entringer, S., Buss, C. & Wadhwa, P. D. Prenatal stress, telomere biology and fetal programming of health and disease risk. Sci. Signal. 5, t12 (2012).
Entringer, S. et al. Maternal estriol (E) concentrations in early gestation predict infant telomere length. J. Clin. Endocrinol. Metabjc20142744 (2014).
Toutain, J. et al. Reduced placental telomere length during pregnancies complicated by intrauterine growth restriction. PLoS. One. 8, e54013 (2013).
Entringer, S. et al. Maternal psychosocial stress during pregnancy is associated with newborn leukocyte telomere length. Am. J. Obstet. Gynecol. 208, 134–137 (2013).
Akkad, A. et al. Telomere length in small-for-gestational-age babies. BJOG. 113, 318–323 (2006).
Gemma, C. et al. Mitochondrial DNA depletion in small- and large-for-gestational-age newborns. Obesity. (Silver. Spring) 14, 2193–2199 (2006).
Levy, D. et al. Genome-wide association identifies OBFC1 as a locus involved in human leukocyte telomere biology. Proc. Natl. Acad. Sci. U. S. A. 107, 9293–9298 (2010).
Mangino, M. et al. Genome-wide meta-analysis points to CTC1 and ZNF676 as genes regulating telomere homeostasis in humans. Hum. Mol. Genet. 21, 5385–5394 (2012).
Codd, V. et al. Identification of seven loci affecting mean telomere length and their association with disease. Nat. Genet. 45, 422 (2013).
Burnett-Hartman, A. N. et al. Telomere-associated polymorphisms correlate with cardiovascular disease mortality in Caucasian women: the Cardiovascular Health Study. Mech. Ageing Dev. 133, 275–281 (2012).
Miyake, Y. et al. RPA-like mammalian Ctc1-Stn1-Ten1 complex binds to single-stranded DNA and protects telomeres independently of the Pot1 pathway. Mol. Cell 36, 193–206 (2009).
Wan, M., Qin, J., Songyang, Z. & Liu, D. OB fold-containing protein 1 (OBFC1), a human homolog of yeast Stn1, associates with TPP1 and is implicated in telomere length regulation. J. Biol. Chem. 284, 26725–26731 (2009).
Anderson, B. H. et al. Mutations in CTC1, encoding conserved telomere maintenance component 1, cause Coats plus. Nat. Genet. 44, 338–342 (2012).
Chen, L. Y. & Lingner, J. CST for the grand finale of telomere replication. Nucleus 4, 277–282 (2013).
Friedrich, U., Schwab, M., Griese, E. U., Fritz, P. & Klotz, U. Telomeres in neonates: new insights in fetal hematopoiesis. Pediatr. Res. 49, 252–256 (2001).
Entringer, S., Buss, C. & Wadhwa, P. D. Prenatal stress, telomere biology and fetal programming of health and disease risk. Sci. Signal. 5, t12 (2012).
Cross, J. A. et al. Cord blood telomere length, telomerase activity and inflammatory markers in pregnancies in women with diabetes or gestational diabetes. Diabet. Med. 27, 1264–1270 (2010).
Fyhrquist, F. & Saijonmaa, O. Telomere length and cardiovascular aging. Ann. Med. 44 Suppl 1S138–S142 (2012).
Vasan, R. S. et al. Genome-wide association of echocardiographic dimensions, brachial artery endothelial function and treadmill exercise responses in the Framingham Heart Study. BMC. Med. Genet. 8 Suppl 1S2 (2007).
Maubaret, C. G. et al. Association of TERC and OBFC1 haplotypes with mean leukocyte telomere length and risk for coronary heart disease. PLoS. One. 8, e83122 (2013).
Molloy, A. M. et al. Maternal and fetal plasma homocysteine concentrations at birth: the influence of folate, vitamin B12 and the 5,10-methylenetetrahydrofolate reductase 677C-->T variant. Am. J. Obstet. Gynecol. 186, 499–503 (2002).
Refsum, H., Ueland, P. M., Nygard, O. & Vollset, S. E. Homocysteine and cardiovascular disease. Annu. Rev. Med. 49, 31–62 (1998).
Garfunkel, V. A. et al. Hyperhomocysteinemia but not MTHFR genotype is associated with young-onset essential hypertension. J Hum. Hypertens. 17, 361–364 (2003).
San, P. M., Grandi, C., Larguia, M. & Solana, C. [Standard of birth weight for gestational age in 55706 healthy newborns in a public maternity of Buenos Aires]. Medicina (B Aires) 61, 15–22 (2001).
Kitzmiller, J. L. et al. Diabetic pregnancy and perinatal morbidity. Am. J. Obstet. Gynecol. 131, 560–580 (1978).
Kawasaki, E. S. [Sample preparation from blood, cells and other fluids]. PCR Protocols. A guide to Methods and Applications [Innis M. A., Gelfand D. H., Sninsky J. J., & White T. J. (eds.)] [146–152] (Academic Press, INC.San diego, 1990).
McGrath, M., Wong, J. Y., Michaud, D., Hunter, D. J. & De, V. I. Telomere length, cigarette smoking and bladder cancer risk in men and women. Cancer Epidemiol. Biomarkers Prev. 16, 815–819 (2007).
Ramakers, C., Ruijter, J. M., Deprez, R. H. & Moorman, A. F. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 339, 62–66 (2003).
Ehrlenbach, S. et al. Influences on the reduction of relative telomere length over 10 years in the population-based Bruneck Study: introduction of a well-controlled high-throughput assay. Int. J. Epidemiol. 38, 1725–1734 (2009).
Hu, Z. et al. VisANT 4.0: Integrative network platform to connect genes, drugs, diseases and therapies. Nucleic Acids Res. 41, W225–31 (2013).
Acknowledgements
This study was partially supported by grants PICT 2012-159 and PICT 2010- 0441 (Agencia Nacional de Promoción Científica y Tecnológica) and UBACYT CM04 (Universidad de Buenos Aires). MT, SS and CJP are members of Consejo Nacional de Investigaciones Científicas.
Author information
Authors and Affiliations
Contributions
M.T. and T.F.G.: preformed molecular experiments in human samples, J.A. and C.G.: collected clinical information, C.J.P. and S.S.: conceived and designed the study, supervised the experiments, analyzed the data, wrote the MS.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary Figure 1
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Tellechea, M., Gianotti, T., Alvariñas, J. et al. Telomere length in the two extremes of abnormal fetal growth and the programming effect of maternal arterial hypertension. Sci Rep 5, 7869 (2015). https://doi.org/10.1038/srep07869
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep07869
This article is cited by
-
Variability in newborn telomere length is explained by inheritance and intrauterine environment
BMC Medicine (2022)
-
Leukocyte telomere length is associated with elevated plasma glucose and HbA1c in young healthy men independent of birth weight
Scientific Reports (2019)
-
Correlation of cord blood telomere length with birth weight
BMC Research Notes (2017)
-
Cord blood telomere length in Latino infants: relation with maternal education and infant sex
Journal of Perinatology (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.