Abstract
Amino acids play key roles in the function of the central nervous system and their alterations are implicated in psychiatric disorders. In the search for a biomarker for major depressive disorder (MDD), we used high-performance liquid chromatography to measure amino acids and related molecules in the cerebrospinal fluid (CSF) of 52 patients with MDD (42 depressed and 10 remitted; DSM-IV) and 54 matched controls. Significant differences were found in four amino acid concentrations between the depressed patients and controls. After Bonferroni correction, only ethanolamine (EA) levels remained significantly reduced in depressed patients (nominal P = 0.0000011). A substantial proportion of the depressed patients (40.5%) showed abnormally low CSF EA levels (<12.1 μM) (P = 0.000033; OR = 11.6, 95% CI: 3.1–43.2). When patients with low EA and those with high EA levels were compared, the former had higher scores for overall depression severity (P = 0.0033) and ‘Somatic Anxiety’ symptoms (P = 0.00026). In unmedicated subjects, CSF EA levels showed a significant positive correlation with levels of homovanillic acid (P = 0.0030) and 5-hydroxyindoleacetic acid (P = 0.019). To our knowledge, this is the first study showing that patients with MDD have significantly lower CSF EA concentrations compared with control subjects. CSF EA could be a state-dependent biomarker for a subtype of MDD.
Similar content being viewed by others
Introduction
Major depressive disorder (MDD) is a common disease with a prevalence rate estimated at 4.4% worldwide1. Since the pathophysiology of MDD remains elusive, no established biochemical marker is available for everyday use in the clinical setting and the diagnosis of MDD largely depends on the clinical interview2. Although many candidate molecules are present in peripheral blood3, no study has successfully found a biomarker that is of practical use in the diagnosis, subtyping, or symptomatic assessment of MDD.
Since cerebrospinal fluid (CSF) contacts the interstitial fluid in the central nervous system (CNS)4 and is mostly segregated from the peripheral circulation by the blood-brain barrier, CSF reflects molecular dynamics in the brain. The composition of CSF (electrolytes5, amino acids6 and proteins7) differs substantially from that of peripheral blood. Total tau and phosphorylated tau protein in CSF have been established as biomarkers for Alzheimer's disease8, but are not detectable in peripheral blood. It is therefore feasible to search for a biomarker for MDD in the CSF. The proteomics approach to CSF samples seems to be promising9.
We have focused on amino acids and related molecules in the CSF to identify a biomarker for MDD, because alterations in the serotonin, noradrenaline, dopamine, glutamate and γ-amino-butyric acid (GABA) systems are implicated in MDD10. These neurotransmitters are themselves amino acids or are synthesized from amino acids. Previous studies examined amino acid levels in the peripheral blood of MDD patients, although their results are equivocal11,12,13,14. We have recently reported a meta-analysis demonstrating that the plasma L-tryptophan concentration is significantly lower in MDD patients than healthy controls (P = 0.000059)15. In this context, we chose to examine amino acids and related molecules in the CSF of MDD patients.
Although many studies compared CSF amino acid concentrations between MDD patients and healthy controls16,17,18,19,20,21,22,23,24,25,26,27,28, the majority examined a single amino acid or a few amino acids. Some researchers found reduced CSF GABA levels in depressed patients compared with controls18,19,21,28, while others reported contradictive negative results17,20,22. Increased glutamine levels in depressed patients24 and reduced glutamate and glycine concentrations in refractory patients were reported25, however, no differences in CSF glutamate and glutamine levels between 2 groups were inconsistently found27. No significant difference between patients and controls was found for tryptophan21,23, tyrosine21 or alanine24. To our knowledge, only two studies examined comprehensive amino acid profiles in CSF. An early study examined 32 amino acids and related molecules including ethanolamine (EA); however, that sample included only 8 subjects with unipolar depression and 2 controls16. A metabolomics-based approach in 14 currently-depressed patients, 14 remitted patients and 18 controls found that CSF methionine levels were significantly increased in remitted patients compared with controls26. Thus, previous information on CSF amino acid levels in MDD patients is surprisingly limited.
We measured CSF amino acids and related molecules in a relatively large sample to search for a biomarker for MDD. We also analyzed the correlations between CSF amino acid levels and depression severity, psychotropic medication and monoamine metabolites.
Subjects and methods
Subjects
Subjects were 52 patients with MDD and 54 healthy controls matched for age and sex. All participants were biologically-unrelated Japanese. Patients were recruited at the National Center of Neurology and Psychiatry (NCNP) Hospital (Tokyo, Japan), or through advertisements in free local magazines and by our website. Healthy controls were from the same geographical area (i.e., western Tokyo metropolitan) via advertisements in magazines and website. Trained psychologists or psychiatrists conducted a structured Mini-International Neuropsychiatric Interview (M.I.N.I.)29, Japanese version with all participants. A consensus diagnosis was made according to the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) criteria30 based on the M.I.N.I., an additional unstructured interview and information from medical records if available. Patients with any comorbid axis I disorder were excluded, as were individuals with a prior medical history of CNS disease, severe head injury, or substance abuse/dependence. After the nature of the study procedures had been fully explained, written informed consent was obtained from all subjects. MDD symptoms were assessed using the Japanese version of the 17-item Hamilton Depression Rating Scale (HAMD-17)31 and the cut-off score for remission was ≤732. Ten of the 52 patients were remitted. Among all MDD patients (depressed + remitted), 39 patients were medicated and the remaining 13 (11 depressed and 2 remitted) were not on psychotropic medication. Daily doses of benzodiazepine derivatives, antidepressants and antipsychotics were converted to equivalent doses of diazepam, imipramine and chlorpromazine respectively, using published guidelines33 When each class of drugs was not medicated, the dose was considered to be zero. The present experiments on our participants were conducted in accordance with the Declaration of Helsinki. The study protocol was approved by the ethics committee at the NCNP (No. 305).
Sample collection
Between 10:00 h and 16:00 h, CSF samples were obtained by lumbar puncture from the L4–5 or L3–4 interspace of participants, in the left decubitus position. CSF samples were immediately placed on ice and centrifuged at 4000 × g. Supernatants were aliquoted and stored at −80°C until assays were performed. All samples were collected from August 2010 to August 2013. Initial 2 mL of CSF was used for measuring glucose, chloride, total protein levels and monoamine metabolites. Homovanillic acid (HVA), 3-methoxy-4-hydroxyphenylethyleneglycol (MHPG) and 5-hydroxyindoleacetic acid (5-HIAA) were measured by high-performance liquid chromatography (HPLC) at SRL Co., Inc. (Tokyo, Japan).
Determination of CSF levels of amino acids and related molecules by HPLC
CSF sample was mixed with 4% 5-sulfosalicylic acid dihydrate (WAKO, Tokyo, Japan) and centrifuged for 10 min at 12,000 × g and 4°C. Each supernatant was transferred to a micro-tube, filtered using a 0.22-μm pore-diameter syringe-filter (AS ONE, Osaka, Japan) and subjected to HPLC (JASCO, Tokyo, Japan). Acquired data were processed and quantified on the chromatography data station ChromNAV (JASCO). A detailed description of the HPLC protocol is available in the Supplementary Methods.
Validation by capillary electrophoresis time-of-flight mass spectrometry (CE-TOF-MS)
Amino acids and related molecules were also measured using CE-TOF-MS for a subset of the subjects (24 depressed, 3 remitted patients and 27 controls). The CE-TOF-MS measurements were performed at Human Metabolome Technologies, Inc. (Yamagata, Japan), by coauthors who were blinded to the HPLC data obtained at the NCNP. Compounds were identified by their peaks using annotated tables with m/z values and normalized by migration times. Detailed CE-TOF-MS procedures are described elsewhere34.
Statistical analysis
Data are reported as means ± standard deviation (SD). Means were compared using t-tests or analysis of variance. Categorical variables were compared using the χ2 test with exact probability. Analysis of covariance (ANCOVA), controlling for age and sex, was performed to compare CSF levels of amino acids and related molecules in the patients and controls and between subgroups of the patients. Partial correlation analysis, controlling for age and sex, was performed to examine the correlations between equivalent doses of psychotropic drugs and concentrations of amino acids and related molecules and between EA levels and other CSF substances including biogenic amine metabolites. The Mann-Whitney U test with exact probability was used to compare clinical symptoms (HAMD-17 scores) between MDD subgroups (Low-EA vs. High-EA). We obtained 95% CI for the difference between two medians for Mann-Whitney U using Hodges-Lehmann estimate. HAMD-17 items were assigned to the following subscales: ‘Core’ (items 1, 2, 7, 8, 10, 13), ‘Sleep’ (items 4, 5, 6), ‘Activity’ (items 7, 8), ‘Psychic Anxiety’ (items 9, 10) and ‘Somatic Anxiety’ (items 11, 12, 13), according to Serretti et al.35. We used non-parametric estimate of receiver operating characteristic (ROC) curves for the assessment of specificity and sensitivity of EA to discriminate between MDD patients and controls and between depressed and remitted MDD groups. Statistical significance for a two-tailed P-value was <0.05 and was corrected for multiple comparisons using the Bonferroni method. All analyses were performed using IBM SPSS Statistics 22.0 Japanese version (IBM Japan, Tokyo, Japan).
Results
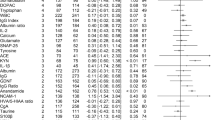
Demographic and clinical characteristics of the subjects are shown in Table 1. There were no significant differences in sex ratio, age distribution, time of CSF sampling, or number or days from sample collection among the depressed, remitted and control subjects. No significant difference was observed in psychotropic drug doses between depressed and remitted MDD groups. CSF concentrations of 25 of the 41 amino acids and related molecules were successfully determined in the majority of subjects (Table 2). There was nominally a significant difference in the concentrations of threonine, glutamine, EA and carnosine between the depressed MDD and control groups. When the critical P = 0.002 (0.05/25) was conservatively applied after the Bonferroni correction, only EA remained significant (nominal P = 0.0000011).
We then performed detailed analyses on the possible relationships between CSF EA levels and clinical variables. In controls, there was no significant difference in CSF EA levels between men and women controlled for age (F = 0.045, df = 1, P = 0.83; 95% CI: −1.10 to 1.36) and no significant correlation between EA and age controlled for sex (r = −0.063, df = 51, P = 0.66; 95% CI: −0.33 to 0.21), between EA and time of CSF sampling controlled for sex and age (r = 0.061, df = 50, P = 0.67: 95% CI: −0.22 to 0.33), or between EA and number of days from sample collection controlled for sex and age (r = 0.0062, df = 50, P = 0.97; 95% CI: −0.27 to 0.28), in a partial correlation analysis. Figure 1a shows dot plots of CSF EA concentrations in depressed MDD patients and controls. Among all MDD patients, no significant difference in EA levels was observed between the medicated and unmedicated patients using ANCOVA, controlling for sex and age (F = 0.0030, df = 1, P = 0.96; 95%CI: −1.57 to 1.66) (Figure 1b) and there were no significant differences in age, sex, or total HAMD-17 score. When unmedicated MDD patients and controls were compared, there was a significant difference in CSF EA based on ANCOVA (F = 7.0, df = 1, P = 0.010; 95% CI: −3.28 to −0.46). Remitted MDD patients had significantly higher EA levels than depressed MDD patients (ANCOVA; F = 8.1, df = 1, P = 0.0066; 95%CI: 0.67 to 3.90) (Figure 1c) and they showed no significant differences in age or sex. There was no significant difference in CSF EA levels between remitted patients and controls (F = 0.0092, df = 1, P = 0.92; 95%CI: −1.68 to 1.53). These comparisons for CSF EA concentrations between subgroups are summarized in Table 3. Area under curve (AUC) by the ROC curve to discriminate between depressed (non-remitted) MDD patients and controls was 0.77 (Supplementary Fig. S1a), that between depressed and remitted MDD groups was 0.75 (Supplementary Fig. S1b), indicating ‘fair test’ for these discriminations. When “abnormally low CSF EA levels” were defined as the 5th percentile value of the controls (<12.1 μM), 17 depressed patients (40.5%) and 3 controls fell within this range (χ2 = 17.5, df = 1, P = 0.000033; OR = 11.6, 95% CI: 3.1 to 43.2).
Dot plots of CSF EA concentrations in patients with MDD and controls.
Horizontal bars represent mean values of the groups. Statistical analyses were performed by ANCOVA, controlling for age and sex. (a) Comparison between dMDD patients and HC, (b) that between mMDD and uMDD and (c) that between rMDD and dMDD. Abbreviations: CSF, cerebrospinal fluid; EA, ethanolamine; HC, healthy controls; dMDD, depressed (non-remitted) patients with major depressive disorder; rMDD, remitted patients; mMDD, medicated patients; uMDD, unmedicated patients; ANCOVA, analysis of covariance.
To investigate the relationship between patient's symptoms and CSF EA levels, we defined MDD patients (depressed + remitted) whose levels fell below the 1st quartile of CSF EA values as ‘Low-EA’ (N = 13) and above the 3rd quartile as ‘High-EA’ (N = 13). The Low-EA group had higher HAMD-17 total scores (P = 0.0033, Mann-Whitney U test) and ‘Core’ (P = 0.034) and ‘Somatic Anxiety’ (P = 0.00026) subscale scores (Figure 2). Other HAMD subscales (‘Sleep’, ‘Activity’ and ‘Psychic Anxiety’) showed no significant differences between Low-EA and High-EA groups.
Dot plots of HAMD-17 total score and subscale scores in High and Low-EA patients.
We defined MDD patients with CSF EA values below the 1st quartile as ‘Low-EA’ (N = 13) and MDD patients with CSF EA values above the 3rd quartile as ‘High-EA’ (N = 13) to compare the CSF EA levels with symptoms. Horizontal bars in dot clusters represent mean values of the groups. P-values were obtained by the Mann-Whitney U test. (a) Low-EA showed a significantly higher total score for HAMD-17 than High-EA (U = 29.0, P = 0.0033; 95% CI: 3.0 to 15.0). (b) Low-EA showed a significantly higher score for the ‘Core’ subscale than High-EA (U = 43.5, P = 0.034; 95% CI: 1.0 to 7.0). There were no significant differences between the two groups for (c) ‘Sleep’ (U = 71.5, P = 0.51; 95% CI: −1.0 to 2.0), (d) ‘Activity’ (U = 48.0, P = 0.059; 95% CI: 0.0 to 3.0) and (e) ‘Psychic Anxiety’ (U = 57.5, P = 0.17; 95% CI: 0.0 to 2.0). (f) Low-EA showed a significantly higher score for ‘Somatic Anxiety’ than High-EA (U = 18.5, P = 0.00026; 95% CI: 1.0 to 4.0). Abbreviations: HAMD-17, 17-item Hamilton Depression Rating Scale; CSF, cerebrospinal fluid; EA, ethanolamine; Low-EA, patients with CSF EA values below the 1st quartile; High-EA, patients with CSF EA values above the 3rd quartile; CI, confidence interval for the difference between two medians.
Although there was no significant difference in CSF EA levels between medicated and unmedicated MDD patients, we examined the possible correlation between the equivalent dose of psychotropic drugs and CSF amino acid levels in the depressed MDD group (Table S1). CSF EA levels did not significantly correlate with benzodiazepine derivatives (P = 0.29), antidepressants (P = 0.34), or antipsychotics (P = 0.21). With respect to other molecules, several amino acid concentrations nominally showed significant correlations with medications (Supplementary Table S1). The correlations between isoleucine and antidepressants (P = 0.00056) (Supplementary Fig. S2), remained significant even after correcting for multiple comparisons [critical P-value of 0.05/(25 × 2) = 0.001].
We further investigated the correlations between CSF EA levels and other CSF substances in unmedicated patients with MDD and controls (total N = 67), based on reports that levels of CSF biogenic amine metabolites are affected by psychotropic drugs36. HVA, a catabolite of dopamine (P = 0.0030) and 5-HIAA, a catabolite of serotonin (P = 0.019), showed significant correlations, while other substances (total protein, glucose, chloride and MHPG) did not (Figure 3 and Table 4).
Scatter plots and regression lines for the relationships of EA with HVA and 5-HIAA in CSF.
Partial correlation test controlling for age and sex was performed in healthy controls (N = 54) and unmedicated patients with major depressive disorder (N = 13). Significant correlations of EA with (a) HVA and (b) 5-HIAA in CSF are shown. Abbreviations: EA, ethanolamine; CSF, cerebrospinal fluid; HVA, homovanillic acid; 5-HIAA, 5-hydroxyindoleacetic acid.
To validate the HPLC measurements, CSF EA levels were measured in a subset of the subjects using CE-TOF-MS. The EA values obtained using CE-TOF-MS and HPLC methods showed a near-perfect correlation (r = 0.89, P < 5 × 10−18) in partial correlation analysis, with age and sex as covariates (Supplementary Fig. S3a). Similar to Figure 1a, a significant difference in CSF EA values based on CE-TOF-MS data was observed between the depressed MDD patients and controls (P = 0.0052, ANCOVA) (Supplementary Fig. S3b).
Discussion
Several of our findings are potentially of clinical significance. The levels of some CSF amino acids differed between MDD patients and controls. EA (also known as monoethanolamine or 2-aminoethanol), in particular, remained significantly lower in patients than controls after correcting for multiple comparisons (P = 0.0000011). Notably, as many as 40% of the depressed patients showed abnormally low levels and EA levels were significantly lower in depressed patients than remitted patients. When relationships with clinical variables were examined in the patients, the Low-EA group showed higher HAMD-17 and subscale scores compared with the High-EA group. CSF EA did not correlate with the dose of psychotropic drugs, although isoleucine showed a correlation with antidepressants even after correcting for multiple testing. Lastly, CSF EA values were significantly correlated with CSF HVA and 5-HIAA levels in unmedicated subjects.
An early study by Goodnick et al.16 reported that CSF tyrosine levels differed across diagnostic groups (bipolar, unipolar depression and controls). The authors reported the CSF EA levels; however, there were only two controls in their sample and it is difficult to compare their results with ours. Frye et al.25 reported that CSF glutamate and glycine levels were decreased in patients with mood disorder. By contrast, we found no significant difference in the levels of either amino acid between depressed patients and controls. One of the reasons for the inconsistency may be that the majority of the patients in Frye et al.'s study had bipolar disorder. Another reason may be that their subjects were all unmedicated; however, we could not confirm the results of Frye et al. even when our unmedicated patients were compared with controls (data not shown). Levine et al. reported that depressed patients (MDD and bipolar disorder) showed increased CSF glutamine levels than controls24. CSF glutamine levels in our MDD patients were also significantly increased than in controls. However, there was no significant difference between our unmedicated patients (N = 11) and controls (629.5 ± 72.9 vs. 644.3 ± 108.8 μM, F = 0.046, df = 1, P = 0.83, 95% CI: −69.33 to 55.89). Therefore, the observed increase in glutamine in our total subjects may be attributable to medication. Regarding CSF GABA, several previous reports18,19,21,28 showed its decrease in depressed subjects; however, we observed no significant differences between patients and controls, which is in line with other studies17,20,22. Recently, Kaddurah-Daouk et al., who employed a metabolomics-based approach, reported that methionine was increased in remitted MDD patients compared with depressed patients and healthy controls26. Our subjects showed similar results. Although CSF methionine levels did not differ significantly between the currently depressed patients and controls, our remitted patients had significantly higher CSF methionine levels than depressed patients (4.1 ± 1.2 vs. 3.3 ± 0.9 μM, F = 9.1, df = 1, P = 0.0041; 95% CI: 0.31 to 1.57, ANCOVA) and controls (vs. 3.5 ± 0.8, F = 5.7, df = 1, P = 0.020; 95% CI: 0.11 to 1.29). Methionine might be involved in the recovery processes of MDD and could be a biomarker for remission.
The significantly higher levels of CSF EA in remitted patients compared with depressed patients suggest that CSF EA levels might be state-dependent. In line, our Low-EA patients showed a higher HAMD-17 total score than the High-EA group. Vagus nerve stimulation (VNS), which is effective for MDD patients and alters the metabolites of neurotransmitters37, was reported to elevate CSF EA levels in epileptic patients38. VNS may exert its effect through mechanisms that increase central EA. Longitudinal studies are warranted to examine whether antidepressant treatments increase CSF EA.
When we defined abnormally low EA levels based on the 5th percentile of the controls, approximately 40% of the depressed patients fell into this range, suggesting that a substantial proportion of subjects with MDD could be distinguished from normal subjects based on CSF EA levels, which would be useful for diagnosis. MDD patients with low CSF EA levels may constitute a subtype of MDD. Indeed, the Low-EA group was characterized by higher ‘Core’ and ‘Somatic Anxiety’ symptoms. In addition, we found a significant positive correlation between CSF EA and HVA and 5-HIAA levels, suggesting that MDD characterized by low CSF EA levels reflects impaired dopaminergic and serotonergic functions in the CNS, possibly due to synaptic dysregulation by an altered endocannabinoid system (see below).
Since most of our patients were medicated, the observed decrease in CSF EA may be attributable to medication. However, this possibility is unlikely because CSF EA was decreased in unmedicated patients compared with controls; there was no significant difference in CSF EA levels between medicated and unmedicated patients; and there was no significant correlation between CSF EA levels and the dose of any class of psychotropic drugs. Our results therefore suggest that CSF EA could be a useful biomarker even in medicated patients. With respect to the effect of antidepressants, we found a significant correlation with CSF isoleucine levels even after correcting for multiple comparisons. To our knowledge, no study has examined the effect of antidepressants on CSF amino acid levels; therefore, further studies are warranted.
EA is closely related to endocannabinoid signaling in the CNS (see Supplementary Fig. S4). EA is both a precursor to and a metabolite of, anandamide (N-arachidonoylethanolamine), a ligand for cannabinoid receptors (CBs) and transient receptor potential vanilloid type 1 (TRPV1). The endocannabinoid system is implicated in depression, suicide and stress-related affective disorders39. CB1 receptor density is high at presynaptic axon terminals, where it functions to inhibit neurotransmitter release40. This may substantiate our observation that CSF EA was correlated with HVA and 5-HIAA levels. In human studies, inconsistent results have been reported on serum endocannabinoid levels in MDD41,42,43. One study found no significant difference in the CSF anandamide level between MDD patients and controls, although it was elevated in unmedicated patients with schizophrenia44. Anandamide is synthesized on demand, binds with high affinity to extracellular CB1 receptors and is rapidly inactivated by active transport into neurons, followed by hydrolysis45. EA might be a stable surrogate marker for the anandamide system.
Decreased CSF EA may be due to inflammatory responses that have been implicated in MDD46. We previously reported elevated CSF IL-6 levels in MDD47 suggesting the involvement of neuroinflammation. In the inflammatory process, activation of microglia and upregulation of cyclooxygenase-2 may facilitate conversion of EA to N-acylethanolamines or prostaglandin H2 ethanolamide48,49.
There are several limitations to this study. Firstly, the numbers of unmedicated patients with MDD (N = 13) and remitted individuals (N = 10) were small. However, we detected a significant difference in CSF EA levels between unmedicated patients and controls; and between depressed and remitted patients, which suggests large effect sizes. Secondly, the measurement of the CSF sample took place in a real-world setting; the majority of patients were medicated and sampling was not performed after fasting or at a fixed time. However, we did not observe any correlation of EA with psychotropic medication or CSF sampling time. This makes CSF EA a feasible biomarker for everyday use in the clinical setting. Nevertheless, studies are necessary to elucidate the possible effects of fasting. Thirdly, there were missing values for several amino acids (see Table 2), which were likely due to small values below the detection limit and might have caused false negative results. Fourthly, small proportion of patients (N = 13) received antipsychotic medication, which may have an effect on CSF EA levels. However, there was no significant correlation between daily chlorpromazine equivalent doses of antipsychotics and CSF EA levels; therefore, the possible effect might be minimal. Finally, we obtained data only for MDD patients and controls. Further studies on other neuropsychiatric disorders are necessary to determine whether low EA is specific to MDD.
In conclusion, we found, for the first time, that CSF EA levels were reduced independently of medication in a substantial proportion (40%) of depressed MDD patients. Such patients had characteristic symptomatology (i.e., ‘Somatic Anxiety’) and CSF monoamine metabolite profiles (i.e., reduced HVA and 5-HIAA) and thus constitute a subtype of MDD.
References
Ferrari, A. J. et al. The epidemiological modelling of major depressive disorder: application for the Global Burden of Disease Study 2010. PLoS One 8, e69637 (2013).
Smith, K. M., Renshaw, P. F. & Bilello, J. The diagnosis of depression: current and emerging methods. Compr Psychiatry 54, 1–6 (2013).
Schmidt, H. D., Shelton, R. C. & Duman, R. S. Functional biomarkers of depression: diagnosis, treatment and pathophysiology. Neuropsychopharmacology 36, 2375–2394 (2011).
Strittmatter, W. J. Bathing the brain. J Clin Invest 123, 1013–1015 (2013).
Segal, M. B. Transport of nutrients across the choroid plexus. Microsc Res Tech 52, 38–48 (2001).
Rainesalo, S. et al. Plasma and cerebrospinal fluid amino acids in epileptic patients. Neurochem Res 29, 319–324 (2004).
Reiber, H. Proteins in cerebrospinal fluid and blood: barriers, CSF flow rate and source-related dynamics. Restor Neurol Neurosci 21, 79–96 (2003).
Humpel, C. & Hochstrasser, T. Cerebrospinal fluid and blood biomarkers in Alzheimer's disease. World J Psychiatry 1, 8–18 (2011).
Ditzen, C. et al. Cerebrospinal fluid biomarkers for major depression confirm relevance of associated pathophysiology. Neuropsychopharmacology 37, 1013–1025 (2012).
Hasler, G. Pathophysiology of depression: do we have any solid evidence of interest to clinicians? World Psychiatry 9, 155–161 (2010).
Altamura, C. A. et al. Plasma and platelet excitatory amino acids in psychiatric disorders. Am J Psychiatry 150, 1731–1733 (1993).
Maes, M., Verkerk, R., Vandoolaeghe, E., Lin, A. & Scharpe, S. Serum levels of excitatory amino acids, serine, glycine, histidine, threonine, taurine, alanine and arginine in treatment-resistant depression: modulation by treatment with antidepressants and prediction of clinical responsivity. Acta Psychiatr Scand 97, 302–308 (1998).
Mauri, M. C. et al. Predictive value of amino acids in the treatment of major depression with fluvoxamine. Neuropsychobiology 44, 134–138 (2001).
Mitani, H. et al. Correlation between plasma levels of glutamate, alanine and serine with severity of depression. Prog Neuropsychopharmacol Biol Psychiatry 30, 1155–1158 (2006).
Ogawa, S. et al. Plasma L-tryptophan concentration in major depressive disorder: new data and meta-analysis. J Clin Psychiatry 75, e906–915 (2014).
Goodnick, P. J., Evans, H. E., Dunner, D. L. & Fieve, R. R. Amino acid concentrations in cerebrospinal fluid: effects of aging, depression and probenecid. Biol Psychiatry 15, 557–563 (1980).
Post, R. M. et al. Cerebrospinal fluid GABA in normals and patients with affective disorders. Brain Research Bulletin 5, Supplement 2, 755–759 (1980).
Gerner, R. H. & Hare, T. A. CSF GABA in normal subjects and patients with depression, schizophrenia, mania and anorexia nervosa. Am J Psychiatry 138, 1098–1101 (1981).
Kasa, K. et al. Cerebrospinal fluid gamma-aminobutyric acid and homovanillic acid in depressive disorders. Biol Psychiatry 17, 877–883 (1982).
Kuroda, H. et al. Cerebrospinal fluid GABA levels in various neurological and psychiatric diseases. J Neurol Neurosurg Psychiatry 45, 257–260 (1982).
Gerner, R. H. et al. CSF neurochemistry in depressed, manic and schizophrenic patients compared with that of normal controls. Am J Psychiatry 141, 1533–1540 (1984).
Roy, A., Dejong, J. & Ferraro, T. CSF GABA in depressed patients and normal controls. Psychol Med 21, 613–618 (1991).
Geracioti, T. D., Jr. et al. Uncoupling of serotonergic and noradrenergic systems in depression: preliminary evidence from continuous cerebrospinal fluid sampling. Depress Anxiety 6, 89–94 (1997).
Levine, J. et al. Increased cerebrospinal fluid glutamine levels in depressed patients. Biol Psychiatry 47, 586–593 (2000).
Frye, M. A., Tsai, G. E., Huggins, T., Coyle, J. T. & Post, R. M. Low cerebrospinal fluid glutamate and glycine in refractory affective disorder. Biol Psychiatry 61, 162–166 (2007).
Kaddurah-Daouk, R. et al. Cerebrospinal fluid metabolome in mood disorders-remission state has a unique metabolic profile. Sci Rep 2, 667 (2012).
Garakani, A., Martinez, J. M., Yehuda, R. & Gorman, J. M. Cerebrospinal fluid levels of glutamate and corticotropin releasing hormone in major depression before and after treatment. J Affect Disord 146, 262–265 (2013).
Mann, J. J. et al. Anxiety in major depression and cerebrospinal fluid free gamma-aminobutyric Acid. Depress Anxiety 31, 814–821 (2014).
Sheehan, D. V. et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59 Suppl 20, 22–33; quiz 34–57 (1998).
American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV, 4th edn. (American Psychiatric Association, Washington, D.C., 1994).
Hamilton, M. A rating scale for depression. J Neurol Neurosurg Psychiatry 23, 56–62 (1960).
Zimmerman, M., Martinez, J. H., Young, D., Chelminski, I. & Dalrymple, K. Severity classification on the Hamilton Depression Rating Scale. J Affect Disord 150, 384–388 (2013).
Inagaki, A., Inada, T., Fujii, Y. & Yagi, G. Equivalent dose of psychotropics. (Seiwa Shoten, Tokyo, 2013).
Soga, T. et al. Metabolomic profiling of anionic metabolites by capillary electrophoresis mass spectrometry. Anal Chem 81, 6165–6174 (2009).
Serretti, A. et al. Serotonin transporter gene (5-HTTLPR) is not associated with depressive symptomatology in mood disorders. Mol Psychiatry 4, 280–283 (1999).
Sheline, Y., Bardgett, M. E. & Csernansky, J. G. Correlated reductions in cerebrospinal fluid 5-HIAA and MHPG concentrations after treatment with selective serotonin reuptake inhibitors. J Clin Psychopharmacol 17, 11–14 (1997).
Carpenter, L. L. et al. Effect of vagus nerve stimulation on cerebrospinal fluid monoamine metabolites, norepinephrine and gamma-aminobutyric acid concentrations in depressed patients. Biol Psychiatry 56, 418–426 (2004).
Ben-Menachem, E. et al. Effects of vagus nerve stimulation on amino acids and other metabolites in the CSF of patients with partial seizures. Epilepsy Res 20, 221–227 (1995).
Vinod, K. Y. & Hungund, B. L. Role of the endocannabinoid system in depression and suicide. Trends Pharmacol Sci 27, 539–545 (2006).
Ohno-Shosaku, T. & Kano, M. Endocannabinoid-mediated retrograde modulation of synaptic transmission. Curr Opin Neurobiol 29C, 1–8 (2014).
Hill, M. N., Miller, G. E., Ho, W. S., Gorzalka, B. B. & Hillard, C. J. Serum endocannabinoid content is altered in females with depressive disorders: a preliminary report. Pharmacopsychiatry 41, 48–53 (2008).
Hill, M. N., Miller, G. E., Carrier, E. J., Gorzalka, B. B. & Hillard, C. J. Circulating endocannabinoids and N-acyl ethanolamines are differentially regulated in major depression and following exposure to social stress. Psychoneuroendocrinology 34, 1257–1262 (2009).
Ho, W. S., Hill, M. N., Miller, G. E., Gorzalka, B. B. & Hillard, C. J. Serum contents of endocannabinoids are correlated with blood pressure in depressed women. Lipids Health Dis 11, 32 (2012).
Giuffrida, A. et al. Cerebrospinal anandamide levels are elevated in acute schizophrenia and are inversely correlated with psychotic symptoms. Neuropsychopharmacology 29, 2108–2114 (2004).
Di Marzo, V., Bifulco, M. & De Petrocellis, L. The endocannabinoid system and its therapeutic exploitation. Nat Rev Drug Discov 3, 771–784 (2004).
Licinio, J. & Wong, M. L. The role of inflammatory mediators in the biology of major depression: central nervous system cytokines modulate the biological substrate of depressive symptoms, regulate stress-responsive systems and contribute to neurotoxicity and neuroprotection. Mol Psychiatry 4, 317–327 (1999).
Sasayama, D. et al. Increased cerebrospinal fluid interleukin-6 levels in patients with schizophrenia and those with major depressive disorder. J Psychiatr Res 47, 401–406 (2013).
Muccioli, G. G. & Stella, N. Microglia produce and hydrolyze palmitoylethanolamide. Neuropharmacology 54, 16–22 (2008).
Prieto, D. Nitric oxide-mediated negative regulation of cyclooxygenase-2 induction in vascular inflammation. Am J Physiol Heart Circ Physiol 299, H600–601 (2010).
Acknowledgements
We would like to appreciate Ms. Tomoko Miyakawa, Misao Nakano and Mariko Iwaki, for their sample handling. We also thank Ms. Tomoko Kurashimo, Chie Kimizuka, Anna Nagashima, Yukiko Kinoshita and Mr. Ikki Ishida, for their assistance in recruitment of participants and psychiatric assessments. We are grateful to Ms. Aya Hoshi for her technical assistance. This study was supported by the Intramural Research Grant (24-11) for Neurological and Psychiatric Disorders of NCNP, Health and Labour Sciences Research Grants (KHC1214; H22-seishin-ippan-001) and the Strategic Research Program for Brain Sciences by the Ministry of Education, Culture, Sports, Science and Technology of Japan (10102837).
Author information
Authors and Affiliations
Contributions
S.O. designed the study, managed the literature searches, profiled the CSF samples by HPLC, undertook the statistical analyses and wrote the draft of the manuscript. K.H. and Y.Y. recruited the participants. K.H. and D.S. diagnosed the participants and collected the CSF samples. K.H. selected the sample set. Y.Y. and R.M. made psychological assessments. R.M. created and maintained database system. M.O., H.H. and T.T. screened the participants and diagnosed the patients. Y.O. and H.S. performed the CE-TOF-MS measuring for data validation. N.M. and J.M. reviewed the draft and gave critical comments on the manuscript. T.H., S.Y. and T.N. contributed to the recruitment of clinical volunteers. H.K. supervised the entire project and gave critical comments on the manuscript. All authors contributed to and have approved the final manuscript.
Ethics declarations
Competing interests
Y.O. and H.S. were employees in the Human Metabolome Technologies, Inc., Tsuruoka, Yamagata, 997-0052, Japan. The other authors declare no conflict of interest.
Electronic supplementary material
Supplementary Information
Supplementary Materials
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Ogawa, S., Hattori, K., Sasayama, D. et al. Reduced cerebrospinal fluid ethanolamine concentration in major depressive disorder. Sci Rep 5, 7796 (2015). https://doi.org/10.1038/srep07796
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep07796
This article is cited by
-
Lycium barbarum glycopeptide alleviates neuroinflammation in spinal cord injury via modulating docosahexaenoic acid to inhibiting MAPKs/NF-kB and pyroptosis pathways
Journal of Translational Medicine (2023)
-
Biomarker profiling of postmortem blood for diabetes mellitus and discussion of possible applications of metabolomics for forensic casework
International Journal of Legal Medicine (2022)
-
Access to the CNS: Biomarker Strategies for Dopaminergic Treatments
Pharmaceutical Research (2018)
-
Effects of l-theanine on anxiety-like behavior, cerebrospinal fluid amino acid profile, and hippocampal activity in Wistar Kyoto rats
Psychopharmacology (2018)
-
Altered KYN/TRP, Gln/Glu, and Met/methionine sulfoxide ratios in the blood plasma of medication-free patients with major depressive disorder
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.