Abstract
RNA contains a large number of modified nucleosides. In the metabolic re-exchange of RNA, modified nucleosides cannot be recycled and are thus excreted from cells into biological fluids. Determination of endogenous modified nucleosides in biological fluids may serve as non-invasive cancers diagnostic methods. Here we prepared boronate-affinity organic-silica hybrid capillary monolithic column (BOHCMC) that exhibited excellent selectivity toward the cis-diol-containing compounds. We then used the prepared BOHCMC as the on-line solid-phase microextraction (SPME) column and developed an on-line SPME-LC-MS/MS method to comprehensively profile cis-diol-containing nucleosides and ribosylated metabolites in human urine. Forty-five cis-diol-containing nucleosides and ribosylated metabolites were successfully identified in human urine. And five ribose conjugates, for the first time, were identified existence in human urine in the current study. Furthermore, the relative quantification suggested 4 cis-diol-containing compounds (5′-deoxy-5′-methylthioadensine, N4-acetylcytidine, 1-ribosyl-N-propionylhistamine and N2,N2,7-trimethylguanosine) increased more than 1.5 folds in all the 3 types of examined cancers (lung cancer, colorectal cancer and nasopharyngeal cancer) compared to healthy controls. The on-line SPME-LC-MS/MS method demonstrates a promising method for the comprehensive profiling of cis-diol-containing ribose conjugates in human urines, which provides an efficient strategy for the identification and discovery of biomarkers and may be used for the screening of cancers.
Similar content being viewed by others
Introduction
RNA contains a large number of modified nucleosides that are formed post-transcriptionally by various modification enzymes1. In the metabolic re-exchange of RNA, hydrolytic enzymes such as ribonucleases and phosphatases release normal and modified nucleosides during RNA regeneration. And normal nucleosides undergo reuse and degradation; whereas, modified nucleosides cannot be recycled as normal nucleosides for RNA synthesis and are thus excreted from cells into biological fluids2. The abnormal level of modified nucleosides has been reported to be associated with carcinogenesis3, dyskeratosis congenital4, diabetes5,6 and Alzheimer's disease7. In this respect, determination of endogenous modified nucleosides in biological fluids have attracted considerable interest in recent years owing to their usefulness as non-invasive diagnostic and/or follow-up methods for certain pathologies.
So far, some analytical methods have been developed for the analysis of nucleosides and their derivatives in biological fluids, such as high-performance liquid chromatography (HPLC) with UV8, radioactivity9, or mass spectrometry (MS) detection10,11 and capillary electrophoresis (CE) with UV12 or MS detection13. UV absorbance-based detection makes the identification of compounds difficult. HPLC with radioactivity detection is sensitive, but involves in the use of radioactive materials. Mass spectrometry provides structural information and has been used for analyses of purine and pyrimidine nucleoside antiviral agents and naturally occurring nucleosides14. However, due to the low abundance of modified nucleosides present in biological fluids as well as the serious matrix interferences of biological samples, comprehensive profiling of modified nucleosides in biological fluids is still challenging in clinical research.
Boronic acids are important ligands for the isolation and sensing of cis-diol-containing biomolecules, such as saccharides15, glycoproteins16 and nucleosides17. In this respect, boronic acids are widely used in the construction of functional materials, including nanoparticles18, nanotubes19, polymer brushes20 and monoliths21. The primary mechanism of the affinity capturing of cis-diol-containing compounds by boronic acids mainly relies on the covalent binding of cis-diol moieties with boronic acids to form five- or six membered cyclic esters in an alkaline aqueous solution; while the esters dissociate when surrounding pH is changed to acidic. Duo to the specific capturing towards cis-diol-containing biomolecules and relative easy desorption of target compounds, affinity chromatography with boronic acids ligands could be a promising strategy for the selective enrichment of low abundant modified nucleosides present in biological fluids.
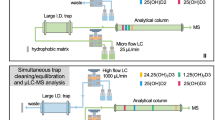
Herein, using the sol-gel combined with “thiol-ene” click reaction, we prepared boronate-affinity organic-silica hybrid capillary monolithic column (BOHCMC) that exhibited excellent selectivity toward the cis-diol-containing compounds. We then used the prepared BOHCMC as the on-line solid-phase microextraction (SPME) column and developed an on-line SPME-LC-MS/MS method to profile cis-diol-containing nucleosides and ribosylated metabolites in human urine (Figure 1). And 45 cis-diol-containing nucleosides and ribosylated metabolites were successfully identified. Furthermore, we performed the relative quantification of these identified cis-diol-containing nucleosides and ribosylated metabolites in urines of healthy controls and other 3 types of cancer patients, including lung cancer, colorectal cancer and nasopharyngeal cancer (Table S1 in Supporting Information).
Results
Characterization of BOHCMC
Recently, our group successfully developed a novel method to prepare organic-silica hybrid capillary monolithic column by sol-gel combined with “thiol-ene” click reaction, which allows higher yield and less by-products compared to other methods and can be performed under mild conditions22. Using the “thiol-ene” click reaction, here we successfully prepared BOHCMC in one-pot synthesis with Tetramethoxysilane (TMOS) and 3-mercaptopropyltrimethoxysilane (MPTMS) as functional silane coupling reagents (Figure 2A). The preparation strategy also notably simplified the construction of the monolith.
Preparation and characterizations of the BOHCMC.
(A) Schematic procedure for the preparation of BOHCMC by sol-gel method combined with “thiol-ene” click reaction. (B) Analysis of eight normal nucleosides by LC-MS/MS. (C) Analysis of eight normal nucleosides by on-line-SPME-LC-MS/MS under optimized conditions. BOHCMC: 10 cm-long, 250 μm i.d. × 360 μm o.d. Sample concentration, 0.05 μg/mL for each nucleoside.
The morphology of the prepared BOHCMC was firstly examined by scanning electron microscopy (SEM). As shown in Figure S1A in Supporting Information, the formed monolith was homogeneous and attached well to the inner wall of the capillary, which can provide effective mass transfer and high stability. The specific surface area of the capillary monolithic column was 247 m2/g with 4.9 nm mesoporous distribution examined by nitrogen adsorption-desorption experiments (Figure S1B in Supporting Information).
Performance of the BOHCMC toward cis-diol-containing compounds
The selectivity, extraction efficiency, extraction capacity and stability of the BOHCMC were investigated.
The selectivity of the BOHCMC was evaluated using 2′-deoxyadenosine (dA) and adenosine (rA) as the analytes. The results showed that rA retained well on the BOHCMC since the cis-diol of rA can readily react with 3-acrylamidophenylboronic acid on BOGCMC to form cyclic boronate esters at pH 8; while dA had no retention and was eluted out directly (Figure S1C in Supporting Information). After switching pH to acidity (0.1% formic acid in water), the structure of cyclic boronate esters was destroyed and rA was fast eluted out (Figure S1C in Supporting Information). Therefore, the prepared BOHCMC exhibited good selectivity toward the cis-diol-containing compound of rA; while the compound that lacks of cis-diol structure (dA) cannot retain.
The extraction capacity of the BOHCMC was evaluated by stepwise increase of the sample volume of rA (100 μg/mL). A curve was made by plotting the peak area of rA versus the injection volume (Figure S2 in Supporting Information). The results showed that the peak area proportionally increased as the injection volume increased from 0.1 to 2 μL. While further increase of the injection volume of rA did not result in the increase of the peak area of rA. Therefore, the extraction capacity was estimated to be 500 μg/g based on the adsorbed amount of rA and the weight of the monolith.
The BOHCMC was used three times per week for consecutive four weeks to examine the stability, which was evaluated by the relative standard deviations (RSDs) of retention times and the peak areas of rA. The results showed that the RSDs of the retention time and peak areas of rA was 2.4% and 4.3%, respectively, demonstrating BOHCMC maintained good stability (Figure S3 in Supporting Information).
Optimization of on-line SPME conditions
The detailed experiments for the optimization of on-line SPME conditions can be found in Supporting Information. Under optimized conditions (loading flow rate, 5 μL/min; washing volume, 35 μL; desorption volume, 25 μL. Figure S4 in Supporting Information), we evaluated the extraction efficiency of BOHCMC using dA, T, dC, dG, rA, rU, rC and rG as analytes. As shown in Figure 2B and 2C, all the cis-diol-containing rA, rU, rC and rG can be well recovered after enrichment, while dA, T, dC and dG that don't contain cis-diol were efficiently removed. The calculated recoveries of 14 ribose conjugate standards at three different concentrations (25 ng/mL, 100 ng/mL and 500 ng/mL) were between 81.3% and 99.7% (Table S2 in Supporting Information), demonstrating good extraction efficiency of BOHCMC towards cis-diol-containing compounds were achieved.
Method validation
The reproducibility of the method was evaluated by the measurement of intra- and inter-day precisions. The intra- and inter-day RSDs were calculated by measuring 14 standards at the concentration of 0.2 mg/mL by on-line SPME-LC-MS/MS method. Three parallel analysis over a day gave the intra-day RSDs and the inter-day RSDs were determined on 3 consecutive days. The results showed that both intra- and inter-day RSDs were less than 12.4% (Table S3 in Supporting Information), demonstrating good reproducibility were achieved.
The reproducibility of BOHCMC was evaluated by the measurement of batch-to-batch precisions. The RSDs of retention times and peak area ratios of 14 standards using 3 different batches of BOHCMC were calculated. The results showed that RSDs of retention times and peak area ratios were less than 3.2% and 13.1% respectively (Table S4 in Supporting Information), demonstrating good reproducibility of BOHCMC.
Determination of ribose conjugates in urine
We first profiled the cis-diol-containing nucleosides and ribosylated metabolites in urine by the developed on-line SPME-LC-MS/MS method. In this respect, a pooled sample that included urines from 10 lung cancer patients, 10 colorectal cancer patients, 10 nasopharyngeal cancer patients and 10 healthy controls was used. The results showed that 45 cis-diol-containing nucleosides and ribosylated metabolites were identified by tandem mass spectrometry analysis (Table 1). Among the 45 identified compounds, 14 cis-diol-containing ribose conjugates were further confirmed using the commercial standards through comparing the retention times and fragmentation ions (Figure S5 in Supporting Information). The fragmentation ions of the other 31 cis-diol-containing ribose conjugates were shown in Figure S6 in Supporting Information.
By comparison, we also profiled the cis-diol-containing ribose conjugates in urine without using on-line SPME. The results showed that the quantities of the peaks obtained using on-line SPME are much more than that without on-line SPME (Figure S7 in Supporting Information). In addition, only 13 cis-diol-containing ribose conjugates were identified by direct analysis without on-line SPME enrichment (Table 1), which may be attributed to the serious matrix interference of urine as well as the low abundance of the modified nucleosides present in urine.
Contents change of ribose conjugates in urine between healthy controls and cancer patients
Using the developed method, we further compared the amounts of the 45 cis-diol-containing nucleosides and ribosylated metabolites in urine between healthy controls and 3 types of cancers, including lung cancer, colorectal cancer and nasopharyngeal cancer. In this respect, pooled samples of healthy controls (n = 10) or each type of cancer (n = 10 for each type of cancer) were prepared to minimize the variation between individuals. Normally 5 to 15 individual samples are used to form one pooled sample23,24,25, therefore here we put 10 individual samples to form a pooled sample. In addition, to avoid the effects of drugs treatment on the alterations of ribose conjugates, all the cancer patients were diagnosed for the first time and hadn't been given any treatments. Before analysis, we quantified creatinine in each pooled sample according to previously described method26 (Table S5 in Supporting Information). The nucleosides and ribosylated metabolites are related to the urinary creatinine concentration, which is a standard manner to normalize urinary metabolites since the excretion of creatinine is rather constant over a longer time interval. To examine the contents change of ribose conjugates between healthy controls and cancer patients, DHzR was used as an internal standard to compensate the instrumental variation. Then the relative content ratio of each identified cis-diol-containing compound between each type of cancer and healthy controls can be obtained (Figure 3 and Table 2).
Relative amounts ratio of cis-diol-containing nucleosides and ribosylated metabolites in (A) lung cancer, (B) colorectal cancer and (C) nasopharyngeal cancer compared to heathy controls. (D) Bar graphs for the distribution of the relative amounts ratio of cis-diol-containing nucleosides and ribosylated metabolites.
To further validate the results obtained by the developed on-line SPME-LC-MS/MS method, we also examined the contents change of 13 ribose conjugates between healthy controls and cancer patients by direct LC-MS/MS (AB 3200 QTRAP mass spectrometer) analysis without on-line SPME under multiple reaction monitoring (MRM) mode (detailed experimental procedure can be found in Supporting Information). The results showed that the relative errors (REs) between these two different methods were under 13.3% (Table S6 in Supporting Information), demonstrating the contents change of the ribose conjugates measured by the two different methods are comparable.
The results showed that compared to healthy controls, 13, 8 and 14 identified ribose conjugates increased more than 1.5 folds in the urine of lung cancer, colorectal cancer and nasopharyngeal cancer, respectively. On the contrary, 3, 8 and 6 identified ribose conjugates decreased more than 1.5 folds in the urine of lung cancer, colorectal cancer and nasopharyngeal cancer, respectively.
Next we evaluated the relative contents change of urinary ribose conjugates between different types of cancer and healthy controls. In our current study, we found N4-acetylcytidine increased in urines of all the examined types of cancer, which was consistent with previous reports that the level of N4-acetylcytidine dramatically increased in multiple cancers, including lung, breast, kidney, colon and colorectal cancers27,28. It was worth noting that here we also identified, for the first time, another three ribose conjugates of 5′-deoxy-5′-methylthioadensine, 1-ribosyl-N-propionylhistamine and N2,N2,7-trimethylguanosine that were increased in urines of all the tested types of cancers (Table 2 and Figure 4).
The ribose conjugates that increased or decreased more than 1.5 folds in different cancer types compared to healthy controls.
(A) The identified ribose conjugates whose contents increased more than 1.5 folds in urines of cancer patients compared to healthy controls. (B) The identified ribose conjugates whose contents decreased more than 1.5 folds in urines of cancer patients compared to healthy controls. Each number represents one compound listed in Table 2. And the numbers listed in the overlap region between cycles represent the common compounds that either increased (A) or decreased (B) in different cancer types compared to healthy controls.
As in lung cancer, we identified 13 increased ribose conjugates, among which 1-methylinosine and N4-acetylcytidine were previously reported to be increased in urine of lung cancer patients27. And in colorectal cancer, we identified 8 increased ribose conjugates, among which 1-methyladenosine and N4-acetylcytidine were previously reported to be increased in urine of colorectal cancer patients29,30. While so far there is no study reporting the identification of modified nucleosides in urine of nasopharyngeal cancer; and our study presented the first report for the identification of 14 increased and 6 decreased ribose conjugates, respectively, in urine of nasopharyngeal cancer.
Discussion
RNA molecules contain various modified nucleosides in addition to normal nucleosides. During RNA turnover, free nucleosides are generated by the hydrolytic action of ribonucleases and phosphatases. Normal nucleosides can be re-utilized to form nucleotide triphosphates which are incorporated into nucleic acids, or further degraded to form uric acid and β-alanine31. Modified nucleosides, in contrast, cannot be reused in de novo RNA synthesis or further metabolized32; therefore, modified nucleosides circulate in blood stream and are then excreted into urine. Consequently, the levels of the modified nucleosides reflect RNA turnover in organism33,34. It has been postulated that diseases may influence the rate of RNA turnover and thus be seen in the levels of excreted modified nucleosides35. Based on these biochemical findings, modified nucleosides have been proposed and evaluated as tumor biomarkers.
Here we developed a on-line SPME-LC-MS/MS method for the comprehensive profiling of cis-diol-containing nucleosides and ribosylated metabolites in urine. The BOHCMC exhibited excellent performance on the selective capturing of cis-diol-containing compounds. The unique property of the boronate-affinity on-line SPME notably improved the detection of the cis-diol-containing compounds by enriching target analytes as well as removing matrix inteference during LC-MS/MS analysis. Using the developed on-line SPME-LC-MS/MS method, 45 cis-diol-containing compounds were succesfully enriched and identified in a single LC-MS/MS analysis, which is much better than previous reports8,10,27,28,30,36,37.
In these identified cis-diol-containing compounds, 5 modified nucleosides and ribosylated metabolites were first discorved in human urine, including 3-hydroxychavicol 1-glucoside, 5-carbamoylmethyluridine, 6-hydroxyl-1,6-dihydropurine ribonucleoside, 1-ribosyl-N-acetylhistamine and 4-((1H-imidazol-2-yl)methyl)phenol-1-glucoside, which extends the divisity of the modified nucleosides and ribosylated metabolites present in human urine. It is worth noting that many ribose conjuates were also found decrease in urine of cancer patients, which may reflect the abnormal metabolism of nucleic acids. However, further exploration is needed to elucidate the mechanism.
We found that different contents of ribose conjugates were associated with different types of cancers (Table 2). The variable pattern of ribose conjugates in patients with various kinds of cancer may be due to the heterogeneity of different cancers. Nevertheless, 4 compounds, 5′-deoxy-5′-methylthioadensine, N4-acetylcytidine, 1-ribosyl-N-propionylhistamine and N2,N2,7-trimethylguanosine, were found more than 1.5 folds increase in urines of all the examined types of cancers, which may be employed as potential indicator for the screening of cancers. From a clinical standpoint, the information contained in the human urine should provide clinicians and clinical chemists with a convenient, centralized resource from which to learn more about human urine and its unique chemical constituents. And additional research should provide an insight into the better use of urinary nucleosides as indicators of cancers.
Methods
Reagents
Fused-silica capillary (250 μm i.d. × 360 μm o.d.) was purchased from Yongnian Optic Fiber Plant (Hebei, China). Tetramethoxysilane (TMOS) and 3-mercaptopropyltrimethoxysilane (MPTMS) were purchased from Wuhan University Silicone New Material (Wuhan, China). Azobisisobutyronitrile (AIBN) and poly(ethylene glycol) with the molecular weight of 6000 (PEG-6000) were all purchased from Shanghai Chemical Reagent Corporation (Shanghai, China). AIBN was purified by recrystallization from ethanol at 40°C. 3-acrylamidophenylboronic acid (AAPBA) and creatinine were purchased from Sigma-Aldrich (Beijing, China). Organic solvents were all of HPLC grade. The water used throughout all experiments was purified using a Milli-Q apparatus (Millipore, Bradford, USA). All other reagents were obtained from various commercial sources and were of analytical grade unless otherwise indicated.
2′-Deoxycytidine (dC), 2′-deoxyguanosine (dG), 2′-deoxyadenosine (dA), thymidine (T), cytidine (rC), guanosine (rG), adenosine (rA), uridine (rU), 1-methyladenosine, N6-methyladenosine, 5′-deoxyadenosine, inosine, xanthosine, 3-methylcytidine, N4-acetylcytidine, 5-methyluridine, 3-methyluridine, pseudouridine, double hydrogen zeatin-riboside (DHzR) were purchased from Sigma-Aldrich (Beijing, China). The standard solution of each analyte was prepared at 1.0 mg/mL in H2O and stored at −20°C.
Urine samples
The urine samples from 10 lung cancer patients, 10 colorectal cancer patients, 10 nasopharyngeal cancer patients and 10 healthy controls were collected from Hubei Cancer Hospital, China. Detailed information can be found in Table S1 in Supporting Information. Healthy controls were selected based on medical history and physical examination. All the patients were diagnosed with cancer for the first time and had not been given any treatment at the time point of urine samples collection. The healthy controls and cancer patients were not detected with other diseases. Written informed consent was obtained from the study subjects and an approval was granted by the Hubei Cancer Hospital Ethics Committee and met the declaration of Helsinki. All the experiments were performed in accordance with Hubei Cancer Hospital Ethics Committee's guidelines and regulations.
Urine sample collection and pretreatment were performed according to previous report38,39 and the detailed procedure can be found in Supporting Information.
Preparation of BOHCMC
To activate the silanol groups, the fused-silica capillaries were sequentially washed with 1 M NaOH for 2 h, H2O for 30 min, 1 M HCl for 1 h, H2O for 30 min and methanol for 30 min followed by drying under nitrogen flow at 160°C for 6 h. For the preparation of BOHCMC, a polymerization mixture containing acetic acid (HAc) (0.01 M, 500 mg), PEG-6000 (45 mg), TMOS (185 mg), MPTMS (15 mg), AAPBA (15 mg) and AIBN (1 mg) was completely mixed and degassed by ultra-sonication for 5 min. The mixture was then manually introduced into the activated fused silica capillary (250 μm i.d. × 360 μm o.d.) by a syringe. After both ends of the capillary were sealed with two pieces of silicone rubber, the mixture was incubated at 40°C for 12 h for simultaneous polymerization and “thiol-ene” click reaction. The resulting monolith was completely flushed with water and ACN sequentially to remove the PEG-6000 and other residuals.
Characterization of BOHCMC
The specific surface area of prepared boronate-affinity organic-silica hybrid monolithic materials were measured by nitrogen adsorption-desorption experiments using a JW-BK specific surface area and pore size analyzer (JWGB Sci& Tech Co., Ltd., Beijing, China). Before measurement, the monolithic cubic pieces were evacuated in vacuum and heated to 120°C for 4 h to remove the physically adsorbed substances. Specific surface area values were determined by the Brunauer-Emmett-Teller (BET) equation at P/P0 between 0.05 and 0.340. The microscopic morphology of the monoliths was examined by scanning electron microscopy (SEM) using a Quanta 200 scanning electron microscope (FEI Company, Holland).
Boronate-affinity monolithic capillary liquid chromatography
The boronate-affinity monolithic capillary liquid chromatography experiments were performed on a SHIMADZU capillary LC system consisting of a Shimadzu LC-20AB binary pump (Tokyo, Japan), one FVC nano valve of two positions (Tokyo, Japan), a 5 μL sample loop, one GL Sciences MU 701 UV-vis detector with a 6 nL detection cell (Tokyo, Japan). To achieve micro-flow rate of 5 μL/min for separation, a T-union with one end connected to the FVC nano valve and the other end connected to a capillary (10 cm-long, 250 μm i.d.) was employed as a flow splitter between the pump and FVC nano valve. All the experiments were performed at 25°C. The chromatograms were recorded at a wavelength of 254 nm. Mobile phase A consisted of 20 mM ammonium formate (pH 8.0). Mobile phase B was a mixture of 0.1% formic acid in water. The mobile phase gradient was 0–6 min, 0% B, 6–15 min, 100% B.
Evaluation of the stability and extraction capacity of BOHCMC
To evaluate the stability, the BOHCMC was used three times per week for four weeks. After each analysis, the column was rinsed by water for 30 min and then stored at 4°C. To evaluate the extraction capacity, the extraction equilibrium profile was assessed by increasing the sample injection volume according to previously described method41.
On-line SPME-LC-MS/MS
The on-line SPME-LC-MS/MS analysis system consisted of a MicrOTOF-Q orthogonal-accelerated TOF mass spectrometer (Bruker Daltonics, Bremen, Germany) with an ESI source (Turbo Ionspray) and a Shimadzu LC-20AB binary pump HPLC (Tokyo, Japan), a SIL-20AC auto sampler and a DGU-20A3 degasser (Figure 1). A 10-cm long BOHCMC (250 μm i.d. × 360 μm o.d.) was employed as the on-line SPME column. The analytical column was performed on a Waters SunfireTM C18 column (150 mm × 1.0 mm i.d., 3.5 μm, Waters, MA) with a flow rate of 0.05 mL/min at 25°C. Formic acid in water (0.1%, v/v, solvent A) and formic acid in methanol (0.1%, v/v, solvent B) were employed as mobile phase. A gradient of 0–5 min 5% B, 5–10 min 5% to 30% B, 10–20 min 30% to 50% B, 20–60 min 50% B and 60–70 min 5% B was used.
Data acquisition and processing were performed using Bruker Daltonics Control 3.4 and Bruker Daltonics Data analysis 4.0 software. The mixture of the nucleoside standards sample was employed to optimize the mass spectrometry conditions under positive ion mode (detailed mass spectrometry parameters can be found in Supporting Information).
Determination of cis-diol-containing nucleosides and ribosylated metabolites in urine
For the identification of the cis-diol-containing compounds in urine, a pooled sample that included urines from 10 lung cancer patients, 10 colorectal cancer patients, 10 nasopharyngeal cancer patients and 10 healthy controls was used. The pooled sample was lyophilized to dryness and then reconstituted 20 mM ammonium formate (pH 8.0). And then, 50 μL was analyzed by on-line SPME-LC-MS/MS. The prospective molecular formulas of the cis-diol-containing compounds were generated based on the accurate molecular mass, MS/MS fragment information and isotope patterns of elemental composition using Bruker Daltonics Data analysis 4.0 software. A mass tolerance of 5.0 mDa was set and a maximum elemental composition of C = 50, H = 100, N = 50, O = 50, S = 10 was used. The molecular formulas and MS/MS fragment information obtained by TOF were further searched in the database of METLIN (http://metlin.scripps.edu) for putative identification. As for the relative quantification of cis-diol-containing compounds between cancer patients and healthy controls, four pooled samples from three types of cancers as well as healthy controls were made. And then each pooled sample from one type of cancer patients was compared to the pooled sample of healthy controls.
References
Machnicka, M. A. et al. MODOMICS: a database of RNA modification pathways--2013 update. Nucleic Acids Res 41, D262–267 (2013).
Schram, K. H. Urinary nucleosides. Mass Spectrom Rev 17, 131–251 (1998).
Seidel, A., Brunner, S., Seidel, P., Fritz, G. I. & Herbarth, O. Modified nucleosides: an accurate tumour marker for clinical diagnosis of cancer, early detection and therapy control. Br J Cancer 94, 1726–1733 (2006).
Ruggero, D. et al. Dyskeratosis congenita and cancer in mice deficient in ribosomal RNA modification. Science 299, 259–262 (2003).
Wei, F. Y. et al. Deficit of tRNA(Lys) modification by Cdkal1 causes the development of type 2 diabetes in mice. J Clin Invest 121, 3598–3608 (2011).
Shen, F. et al. Decreased N-methyladenosine in peripheral blood RNA from Diabetic Patients Is Associated with FTO Expression Rather than ALKBH5. J Clin Endocrinol Metab, 10.1210/jc.2014-1893. (2014).
Abe, T., Tohgi, H., Isobe, C., Murata, T. & Sato, C. Remarkable increase in the concentration of 8-hydroxyguanosine in cerebrospinal fluid from patients with Alzheimer's disease. J Neurosci Res 70, 447–450 (2002).
Liebich, H. M. et al. Age-dependence of urinary normal and modified nucleosides in childhood as determined by reversed-phase high-performance liquid chromatography. J Chromatogr B 814, 275–283 (2005).
Hoggard, P. G., Manion, V., Barry, M. G. & Back, D. J. Effect of protease inhibitors on nucleoside analogue phosphorylation in vitro. Br J Clin Pharmacol 45, 164–167 (1998).
Kammerer, B. et al. Mass spectrometric identification of modified urinary nucleosides used as potential biomedical markers by LC-ITMS coupling. Anal Bioanal Chem 382, 1017–1026 (2005).
Cho, S. H. et al. Direct determination of nucleosides in the urine of patients with breast cancer using column-switching liquid chromatography-tandem mass spectrometry. Biomed Chromatogr 20, 1229–1236 (2006).
Zhao, R., Xu, G., Yue, B., Liebich, H. M. & Zhang, Y. Artificial neural network classification based on capillary electrophoresis of urinary nucleosides for the clinical diagnosis of tumors. J Chromatogr A 828, 489–496 (1998).
Liu, C. C., Huang, J. S., Tyrrell, D. L. & Dovichi, N. J. Capillary electrophoresis-electrospray-mass spectrometry of nucleosides and nucleotides: application to phosphorylation studies of anti-human immunodeficiency virus nucleosides in a human hepatoma cell line. Electrophoresis 26, 1424–1431 (2005).
Dudley, E. & Bond, L. Mass spectrometry analysis of nucleosides and nucleotides. Mass Spectrom Rev 33, 302–331 (2014).
Elstner, M., Weisshart, K., Mullen, K. & Schiller, A. Molecular logic with a saccharide probe on the few-molecules level. J Am Chem Soc 134, 8098–8100 (2012).
Bi, X. & Liu, Z. Facile preparation of glycoprotein-imprinted 96-well microplates for enzyme-linked immunosorbent assay by boronate affinity-based oriented surface imprinting. Anal Chem 86, 959–966 (2014).
Wang, H. et al. Separation and analysis of cis-diol-containing compounds by boronate affinity-assisted micellar electrokinetic chromatography. Anal Bioanal Chem 405, 8579–8586 (2013).
Schumacher, S. et al. Label-free detection of enhanced saccharide binding at pH 7.4 to nanoparticulate benzoboroxole based receptor units. J Mol Recognit 24, 953–959 (2011).
Vlandas, A., Kurkina, T., Ahmad, A., Kern, K. & Balasubramanian, K. Enzyme-free sugar sensing in microfluidic channels with an affinity-based single-wall carbon nanotube sensor. Anal Chem 82, 6090–6097 (2010).
Ivanov, A. E. et al. Evaluation of boronate-containing polymer brushes and gels as substrates for carbohydrate-mediated adhesion and cultivation of animal cells. Colloids Surf B Biointerfaces 75, 510–519 (2010).
Li, H., Wang, H., Liu, Y. & Liu, Z. A benzoboroxole-functionalized monolithic column for the selective enrichment and separation of cis-diol containing biomolecules. Chem Commun (Camb) 48, 4115–4117 (2012).
Chen, M. L. et al. Facile preparation of organic-silica hybrid monolith for capillary hydrophilic liquid chromatography based on “thiol-ene” click chemistry. J chromatogr. A 1284, 118–125 (2013).
Han, H. et al. Combination of UHPLC/Q-TOF-MS, NMR spectroscopy and ECD calculation for screening and identification of reactive metabolites of gentiopicroside in humans. Anal Bioanal Chem 406, 1781–1793 (2014).
Dai, W. et al. Comprehensive and highly sensitive urinary steroid hormone profiling method based on stable isotope-labeling liquid chromatography-mass spectrometry. Anal Chem 84, 10245–10251 (2012).
Christou, C., Gika, H. G., Raikos, N. & Theodoridis, G. GC-MS analysis of organic acids in human urine in clinical settings: a study of derivatization and other analytical parameters. J Chromatogr B 964, 195–201 (2014).
Huang, Y. Q., Ruan, G. D., Liu, J. Q., Gao, Q. & Feng, Y. Q. Use of isotope differential derivatization for simultaneous determination of thiols and oxidized thiols by liquid chromatography tandem mass spectrometry. Anal Biochem 416, 159–166 (2011).
Zheng, Y. F. et al. Study of urinary nucleosides as biological marker in cancer patients analyzed by micellar electrokinetic capillary chromatography. Electrophoresis 23, 4104–4109 (2002).
Liebich, H. M., Xu, G., Di Stefano, C. & Lehmann, R. Capillary electrophoresis of urinary normal and modified nucleosides of cancer patients. J Chromatogr A 793, 341–347 (1998).
Hsu, W. Y. et al. Urinary Nucleosides as Biomarkers of Breast, Colon, Lung and Gastric Cancer in Taiwanese. Plos One 8, e81701 (2013).
Feng, B. et al. Normal and modified urinary nucleosides represent novel biomarkers for colorectal cancer diagnosis and surgery monitoring. J Gastroenterol Hepatol 20, 1913–1919 (2005).
Simmonds, H. A., Fairbanks, L. D., Morris, G. S., Webster, D. R. & Harley, E. H. Altered erythrocyte nucleotide patterns are characteristic of inherited disorders of purine or pyrimidine metabolism. Clin Chim Acta 171, 197–210 (1988).
Borek, E., Sharma, O. K. & Waalkes, T. P. New applications of urinary nucleoside markers. Recent Results Cancer Res 84, 301–316 (1983).
Nakano, K. et al. Urinary excretion of modified nucleosides as biological marker of RNA turnover in patients with cancer and AIDS. Clin Chim Acta 218, 169–183 (1993).
Topp, H., Duden, R. & Schoch, G. 5,6-Dihydrouridine: a marker ribonucleoside for determining whole body degradation rates of transfer RNA in man and rats. Clin Chim Acta 218, 73–82 (1993).
Borek, E. et al. High turnover rate of transfer RNA in tumor tissue. Cancer Res 37, 3362–3366 (1977).
Hsu, W. Y. et al. Analysis of urinary nucleosides as potential tumor markers in human colorectal cancer by high performance liquid chromatography/electrospray ionization tandem mass spectrometry. Clin Chim Acta 402, 31–37 (2009).
Chen, M. L., Wei, S. S., Yuan, B. F. & Feng, Y. Q. Preparation of methacrylate-based monolith for capillary hydrophilic interaction chromatography and its application in determination of nucleosides in urine. J Chromatogr A 1228, 183–192 (2012).
Zhou, R., Guo, K. & Li, L. 5-Diethylamino-naphthalene-1-sulfonyl chloride (DensCl): a novel triplex isotope labeling reagent for quantitative metabolome analysis by liquid chromatography mass spectrometry. Anal chem 85, 11532–11539 (2013).
Peng, J., Chen, Y. T., Chen, C. L. & Li, L. Development of a Universal Metabolome-Standard Method for Long-Term LC-MS Metabolome Profiling and Its Application for Bladder Cancer Urine-Metabolite-Biomarker Discovery. Anal Chem 86, 6540–6547 (2014).
Brunauer, S., Emmett, P. H. & Teller, E. Adsorption of Gases in Multimolecular Layers. J Am Chem Soc 60, 309–319 (1938).
Chen, M., Lu, Y., Ma, Q., Guo, L. & Feng, Y. Q. Boronate affinity monolith for highly selective enrichment of glycopeptides and glycoproteins. The Analyst 134, 2158–2164 (2009).
Acknowledgements
The authors thank the financial support from the National Basic Research Program of China (973 Program) (2012CB720601) and the National Natural Science Foundation of China (21205091).
Author information
Authors and Affiliations
Contributions
B.F.Y., Y.Q.F. and H.P.J. conceived and designed the research, analyzed the data and wrote the paper; H.P.J., C.B.Q., J.M.C. performed the research; C.B.Q. collected the urine samples.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supporting information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Jiang, HP., Qi, CB., Chu, JM. et al. Profiling of cis-Diol-containing Nucleosides and Ribosylated Metabolites by Boronate-affinity Organic-silica Hybrid Monolithic Capillary Liquid Chromatography/Mass Spectrometry. Sci Rep 5, 7785 (2015). https://doi.org/10.1038/srep07785
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep07785
This article is cited by
-
Advances in monolithic silica columns for high-performance liquid chromatography
Journal of Analytical Science and Technology (2017)
-
Association between Oxidative DNA Damage and Risk of Colorectal Cancer: Sensitive Determination of Urinary 8-Hydroxy-2′-deoxyguanosine by UPLC-MS/MS Analysis
Scientific Reports (2016)
-
Determination of thiol metabolites in human urine by stable isotope labeling in combination with pseudo-targeted mass spectrometry analysis
Scientific Reports (2016)
-
DNA hydroxymethylation age of human blood determined by capillary hydrophilic-interaction liquid chromatography/mass spectrometry
Clinical Epigenetics (2015)
-
Quantitative and qualitative analysis of the novel antitumor 1,3,4-oxadiazole derivative (GLB) and its metabolites using HPLC-UV and UPLC-QTOF-MS
Scientific Reports (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.