Abstract
Glazing for adaptive solar control is the most promising for energy efficient development, because the use of this technology in buildings can be expected to significantly impact energy use and efficiency by screening sunlight that enters a building in summer. To achieve autonomous adjustable transparency, we have developed photothermotropic material system by combining photothermal materials with thermotropic hydrogels. We found that graphene oxide dispersed within a hydrogel matrix effectively converts the photo energy of sunlight into thermal energy, providing the efficient means to trigger transparency of thermotropic hydrogels. Therefore, we could develop switchable glazing of novel photothermotropic mechanism that screen strong sunlight and heat radiation in response to the sunlight intensity, as well as the temperature. Furthermore, in this study, a prototype device was manufactured with developed materials and successfully operated in outdoor testing.
Similar content being viewed by others
Introduction
Saving an energy consumption of the world is the first and best step to overcome global warming by reducing of carbon dioxide emission that causes Greenhouse effect1. Eliminating energy waste also provides financial profit for the sustainable growth of strong economies around the world. The energy consumption for the construction, operation and rehabilitation of buildings corresponds to 30–40% of the primary energy use in developed countries2. Because space cooling and heating is one of the key energy expenditures for building operations, many energy efficient concepts for buildings such as well-sealed windows and doors3, thermal insulation of walls4, wet roof5 and solar control glazings6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25 have been proposed in recent years. In particular, glazing for adaptive solar control is the most promising for energy efficient development, because the use of this technology in buildings can be expected to significantly impact energy use and efficiency. By screening sunlight that enters a building, air conditioning operating costs can be reduced in summer, while passive solar heating can minimize energy use for space heating in winter.
Conventional solar control glazing is usually achieved by attaching sun protection films on windows6. These films lead to a noticeable reduction in the heat during summer by reflecting and absorbing sunlight; however, their transparency cannot be tuned in response to weather conditions.
To overcome the problem of fixed optical properties for traditional protection films, many research efforts have focused on developing switchable solar control glazing with adjustable transparency. To render switchable solar control glazing, two types of materials are placed between two glass or plastic sheets, modulating the optical transparency in response to an external environment. The first material is an electrochromic material7 that controls the optical transparency by changing its color8,9,10,11 or reorienting the liquid crystal director12,13 when an electric field is applied, leading to control of the light permittivity. While this is effective for adaptive solar control, external electrical energy resources and human labor are necessary for switching control; therefore, this approach is an active switching method.
The second relies on the temperature-induced phase separation or phase transition of thermotropic materials14. In this system, thermotropic materials such as hydrogels15,16,17,18,19, polymer blends20,21 and block copolymers22,23 are homogeneously dispersed in a matrix, minimizing light scattering. As the temperature is increased to the switching threshold, a phase separation between the thermotropic domains and matrix abruptly occurs. Hence, the phase-separated domains work as scattering centers that affect the reflection of the incident solar radiation. Because the increase in heat or light automatically causes a decrease in the heat or light permittivity, the glazing with thermotropic materials results in “smart” or “intelligent” windows. However, in this system, the switching behavior depends solely on the temperature regardless of the intensity of the sunlight. If the temperature is below the switching threshold, despite intense solar radiation, the thermotropic gel does not heat up sufficiently to induce a phase change.
To enhance the monotonous switching mechanism of the thermotropic smart windows, hybrid window systems consisted of thermotropic hydrogels and electron-conducting indium-tin oxide glass which generates heat by Joule heating were reported24,25. While the transparent-opaque transition could be induced by applying an electric field as well as by temperature, this is not a fully passive method.
For adapting to various environmental conditions, we describe herein a novel mechanism for the fully passive switching of photothermotropic smart windows that are sensitive to the intensity of sunlight as well as to temperature.
Results and Discussion
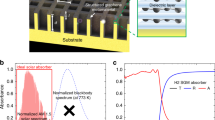
Our approach to switchable glazing for adaptive solar control windows using photothermotropic hydrogels is illustrated in Figure 1. We previously reported that an aqueous graphene oxide (GO) dispersion converts the photoenergy of visible light into thermal energy, increasing the temperature of water26. In this study, we exploit their photothermal conversion ability to generate heat depending on the sunlight intensity and thereby achieve phase separation of thermotropic hydrogels containing GO. Based on the photothermal GO dispersed in crosslinked poly(N-isopropylacrylamide) (PNIPAm), which is a typical temperature-responsive hydrogel with a low critical solution temperature (LCST) of approximately 32–33°C27, we fabricated a switchable glazing for adaptive solar control that can be passively switched on either by temperature or sunlight.
To confirm the photothermal effect of GO in response to sunlight, the temperature changes of aqueous GO and pure water were measured under sunlight irradiation. As seen in Figure 2a, the temperature of the aqueous GO dispersion increased from 30.2°C to 35.3°C when irradiated with sunlight for 8 min, whereas pure water did not undergo a significant temperature change under identical irradiation conditions. These results show that GO efficiently generates heat by absorbing sunlight. Sufficient heat is produced under sunlight irradiation to cause a substantial increase in the temperature of the aqueous GO dispersion, indicating that the photothermal conversion of GO could be triggered by sunlight as well as by visible or near infrared (NIR) light28.
We also measured the changes in the temperature of 0.5 mg/mL aqueous GO under irradiation with visible light of variable intensity to investigate whether the temperature increase due to photothermal effect could be controlled by the light intensity. Instead of sunlight, visible light from a high-pressure mercury short arc lamp fitted with a blue excitation filter (450–490 nm) was used to irradiate the sample in order to quantitatively and precisely control the light intensity. As seen in a 2b, when exposed to blue light of variable intensity, all the samples underwent significant temperature increases in proportion to the exposure time. For the sample irradiated for 8 min at 4.5 mW/cm2, the temperature increased by 0.7°C, while the temperature of the same sample increased by 2.2°C after it was irradiated for 8 min at 24.8 mW/cm2. Irradiation with visible light of 45.0 mW/cm2 for 8 min led to a temperature increase of 3.2°C. Based on the measured temperatures, we further analyzed the degree of the temperature change depending on the light intensity and plotted the results in Figure S1. We found that the increase in temperature is linearly proportional to the light intensity, suggesting that the temperature of the medium can be controlled by the intensity of the sunlight. We also found that a greater loading of GO induces a greater increase in the temperature of the medium (Figure S2). These results indicate that the degree of temperature increase can be controlled by the concentration of GO and/or the light intensity, suggesting that the phase separation of the thermotropic hydrogel containing GO can be induced by sunlight as well as by external temperature. Even though the external temperature is below the switching temperature of the hydrogels, they can reach the switching temperature with the aid of heat generated from the GO under sunlight. As the sunlight intensity increases, more heat is generated and the temperature increases further.
To develop photothermotropic hydrogels, we attempted to prepare a composite of a thermotropic PNIPAm hydrogel and aqueous GO that shows photothermal features through free-radical polymerization. By combining the thermotropic hydrogel network with photothermal materials, a transparent-opaque transition can be expected in response to sunlight even below transition temperature.
Because of the hydrophilic oxygen-containing surface functional groups, GO can be stably dispersed in water in its exfoliated form of monolayer sheets29. The composite solutions were formed by mixing aqueous GO with the NIPAm-monomer solution, thus entrapping the GO sheets within the hydrogel matrix after polymerization. To prevent freezing of the swelling medium in winter, 5% (v/v) of ethanol was added to the pre-gel solution.
To analyze the temperature-dependent optical properties of the prepared hydrogel composites, the UV-Vis spectra were recorded at 25°C and 40°C and are plotted in Figure 3a. The transmittance of both samples at 25°C dramatically decreased as the temperature increased to 40°C. At 25°C, both the composite and the pure hydrogel solution display a transparent state of high transmittance. Because of the light absorption of GO, the composite solution weakly absorbs light from 400 nm to 600 nm, but is fully transparent to light of other wavelengths. At 40°C, the composite solution became completely opaque over the entire range of wavelengths measured, while the pure hydrogel solution partially transmits from 650 nm to 900 nm. This result suggests that the PNIPAm/GO hydrogel has a great advantage in reducing energy consumption because it fully blocks NIR transmittance from sunlight in the cloudy state, which is the critical wavelength of sunlight that generates heat.
(a) Transmittance spectra measured at 25°C and 40°C for the PNIPAm hydrogel and PNIPAm/GO hydrogel. (b) Temperature-dependent transmittance for the PNIPAm hydrogel and PNIPAm/GO hydrogel during heating and cooling cycles. The error bars are the standard deviations for 7 independent measurements. (c) Reversible and repeatable transparent-opaque transition of the PNIPAm/GO hydrogel.
To investigate the transparent-opaque transition for the PNIPAm gel containing GO in more detail, we plotted the transmittances at 600 nm as a function of temperature in Figure 3b. As shown in Figure 3b, the hydrogel containing GO shows nearly the same switching behavior as that of the pure hydrogel with same chemical composition. Both systems exhibit an abrupt transparent-opaque transition near the lower critical solution temperature of 33.0°C because of the phase separation. This result reveals that the GO concentrations used in this work have little influence on the transition temperature of the composite hydrogel. The temperature-dependent switching for PNIPAm/GO took place between a clear state with a transmittance of 97.8% and a cloudy state, owing to light scattering, with a transmittance of 3.0%. The transparency contrast ratio of the hydrogel film seems sufficient in order to apply switchable glazing for screening sunlight. We note that the transmittance of the PNIPAm/GO composite below the LCST is slightly smaller than that of pure PNIPAm due to the absorption of GO, but it is still enough to use clear glazing.
Below the LCST of 33.0°C, water is a relatively good solvent for the PNIPAm chains; therefore, the hydrogel chains are extended in water and the solution appears transparent. In the transition regime near the LCST temperature, PNIPAm undergoes a conformational change, becoming hydrophobic. This results in water becoming a poor solvent for the polymer and polymer-water H-bonds are broken, inducing phase separation between the polymer chains and water. This means that the PNIPAm chains form heterogeneous scattering domains, resulting in an opaque state. Above the LCST, the polymer chains reach a stable equilibrium cloudy state, resulting in a leveling off of the optical transmittance. These transitions for the PNIPAm/GO composite and pure PNIPAm were found to be fully reversible, but a slight hysteresis of ~0.9°C was observed for both samples.
Furthermore, we found that this reversible transparent-opaque transition of the composite hydrogel could be repeated multiple times without significant variation. Figure 3c shows the response of the optical transparency as the GO-containing PNIPAm undergoes several heating and cooling cycles between 40°C and 25°C. The composite hydrogel is transparent at temperatures of 25°C and becomes opaque at 40°C and the transparent-opaque transition is fully reversible and repeatable.
On the basis of the highly responsive and reversible transparent-opaque transition of the GO-containing hydrogel studied above, we fabricated a prototype glass panel with a 140 μm of photothermotropic hydrogel layer. For control experiments, we fabricated a prototype glass panel filled with only PNIPAm hydrogels. Figure 4 shows the results of the outdoor switching test for the fabricated smart windows performed under sunlight at 30°C. At the initial state, shown in Figure 4a, both windows were transparent; thus, the background was clearly visible. When exposed to sunlight, the window fabricated with the GO-containing hydrogel became opaque and blocked the sunlight, while that made with only the hydrogel was still clear, as seen in Figure 4b.
During outdoor switching test, we measured temperatures of the fabricated prototype glass panels by taking thermal photographs with IR camera. As shown in Figure 4c, equilibrium temperatures of both glass panels were 30°C at initial state. When irradiated with sunlight for 5 min, the temperature of the glass panel containing photothermotropic hydrogel layer increased to 35°C. Sunlight irradiation for 15 minute led to an increase of the temperature to 38°C, whereas pure hydrogel layer did not undergo a significant temperature change under identical irradiation conditions. No more increase of temperature was observed after 15 min irradiation. These measurements are well matched with the optical switching test shown in Figure 4b, which is only glass panel containing photothermotropic hydrogel layer was opaque.
This switching process for the photothermotropic hydrogel window was found to be reversible, allowing the glass to autonomously screen sunlight. However, the smart window fabricated with only the pure hydrogel was still transparent, indicating that no phase transition occurred under these climate conditions.
The principle of the switching behavior for the smart window fabricated with the photothermotropic hydrogel is straightforward. Because the outdoor temperature is below the transition temperature, both systems are transparent in the initial state. When exposed to sunlight, the incorporated GO in the PNIPAm matrix absorbs sunlight and converts the photoenergy into thermal energy, which heats up the thermotropic hydrogel network. Consequently, the temperature of the hydrogel increases beyond the transition temperature, resulting in a change in transmittance from transparent to opaque. Under these conditions, the pure hydrogel exhibited no change in its temperature and therefore, no transition occurred. Photothermotropic glass consists of two panes of glass sandwiching a GO-containing hydrogel that undergoes a transition from transparent to opaque under sunlight, allowing the glass to autonomously screen solar radiation.
In this photothermotropic system, the switching conditions are flexible and can be varied by adjusting the concentration of GO and changing the transition temperature of the thermotropic hydrogels. Because the degree of the temperature increase caused by the photothermal effect is proportional to the concentration of GO, a thermotropic hydrogel containing a greater loading of GO is more sensitive to sunlight intensity. According to previous reports, the transition temperature of the thermotropic hydrogel can be tuned by varying the concentration of salts30 or surfactants23,31 in the hydrogel. Depending on the climate conditions of the installation area, the switching can be adjusted for optimal performance.
To further confirm the sunlight effect on optical switching of the window, the photomasks of predetermined patterns are placed over the windows to make shadows. As shown in Figure 5, we found that the transparent-opaque transition of the hydrogel layer is occurred at only exposed areas. We also found that the shape and size are identical to mask patterns the boundaries of transition areas are sharp and clean. These results mean that the optical switching of the photothermotropic hydrogel layer is induced from the sunlight irradiation and further suggest the potential application of the photothermotropic hydrogel layer on sunlight-induced micropatterning.
Conclusion
In conclusion, we have demonstrated passive switching of adaptive solar control glazing in response to sunlight irradiation as well as temperature. The smart window reported in this paper is mainly composed of a thermotropic hydrogel containing photothermal materials. When exposed to sunlight, GO absorb sunlight and convert it to heat, increasing the temperature of the thermotropic hydrogel network over the transition temperature. Consequently, the phase separation between the thermotropic domains and the matrix abruptly proceeds to form scattering centers that reflect incident solar radiation. The proposed glazing for solar control with a photothermotropic hydrogel layer could effectively screen solar radiation in response to sunlight as well as external temperature and is expected to have a significant impact on energy use and efficiency.
Methods
Materials
Aqueous GO (5 mg/mL, composition: Carbon (79%); Oxygen (20%), Flake size: 0.5–5 μm) was obtained from Graphene Supermarket (Calverton, NY, USA). N-isopropylacrylamide (NIPAm) was purchased from TCI (Nihonbashi-honcho, Chuo-ku, Japan). All other chemicals were obtained from Sigma-Aldrich (St Louis, MO, USA) and used as received without further purification.
Preparation of composite hydrogel and prototype windows
The photothermotropic hydrogel layer was prepared from a mixture of aqueous GO and monomer solution. GO sheets were entrapped by the hydrogel matrix after polymerization. A monomer solution (2.55 mL) containing 355.1 mg of NIPAm and 4.9 mg of N,N′-methylenebis(acrylamide) (BisAA) was mixed with 0.30 mL of a 5 mg/mL aqueous dispersion of GO and 0.15 mL ethanol. The final concentration of aqueous GO in the pre-gel solution was 0.5 mg/mL. Free-radical polymerization was initiated by adding 9.0 μL of N,N,N′,N′-tetramethylethylenediamine and 18.0 μL of 10 wt% aqueous ammonium persulfate to 3.0 mL of degassed pre-gel solution. The resulting solutions were immediately loaded into a capillary channel formed by two glass panes separated with spacers of thickness 140 μm. Gelation was carried out in a sealed chamber under a positive pressure of nitrogen for 1 h. After polymerization, the edges of the glass panes were sealed with butyl rubber to prevent water evaporation from the swollen hydrogel.
Outdoor switching test
Fabricated glass panels were attached side by side on external window of the building. After reaching the equilibrium temperature, prototype glass panels were exposed to the sunlight for 1 hour at the external temperature of 30°C. Thermal photographs were taken by infrared thermal camera (FLIR T425, FLIR Systems, USA).
Measurements
The temperature changes of the 0.5 mg/mL aqueous GO and pure water were measured under sunlight irradiation. Before being exposed to sunlight, each sample was placed outside in the shade until it was in equilibrium with the external temperature of 30°C. To measure the temperature changes of aqueous GO exposed to visible light of variable intensity, an intensity-adjustable light source was used instead of sunlight. The sample was irradiated with visible light generated from a high-pressure mercury short arc lamp (EL6000, Leica, Germany) fitted with a blue excitation filter (450–490 nm) while the temperature was recorded. The light intensity was regulated by an intensity filter on an epi-fluorescence microscope (DMI-3000B, Leica, Germany). The transmittance as a function of temperature was measured with a UV-Vis spectrometer (V-550, Jasco Inc., MD, USA). The temperature of the hydrogel layer was controlled by a bath circulator (RBC-10, Jeio tech, Korea) that connected to the sample holder in the UV-Vis spectrometer.
References
Bolin, B. Scope 29 - The greenhouse effect, climatic change and ecosystems (eds. Bolin B., Döös B. R., Jäger H.-J., & Warrick R. A.) (John Wiley & Sons, New York, NY, USA, 1986).
UNEP. Buildings and climate change - Status, challenges and opportunities (ed. UNEP) (United Nations Environment Programme, Paris, France, 2007).
Bell, M. & Lowe, R. Energy efficient modernisation of housing: a UK case study. Energy Build. 32, 267–280 (2000).
Papadopoulos, A. M. State of the art in thermal insulation materials and aims for future developments. Energy Build. 37, 77–86 (2005).
Rotzetter, A. C. C. et al. Thermoresponsive polymer induced sweating surfaces as an efficient way to passively cool buildings. Adv. Mater. 24, 5352–5356 (2012).
Li, D. H. W., Lam, J. C., Lau, C. C. S. & Huan, T. W. Lighting and energy performance of solar film coating in air-conditioned cellular offices. Renew. Energy 29, 921–937 (2004).
Baetens, R., Jelle, B. P. & Gustavsen, A. Properties, requirements and possibilities of smart windows for dynamic daylight and solar energy control in buildings: a state-of-the-art review. Sol. Energy Mater. Sol. Cells 94, 87–105 (2010).
Deb, S. K. Opportunities and challenges in science and technology of WO3 for electrochromic and related applications. Sol. Energy Mater. Sol. Cells 92, 245–258 (2008).
Lampert, C. M. Electrochromic materials and devices for energy efficient windows. Sol. Energy Mater. 11, 1–27 (1984).
Ko, H. C., Kang, M., Moon, B. & Lee, H. Enhancement of electrochromic contrast of poly(3,4-ethylenedioxythiophene) by incorporating a pendant viologen. Adv. Mater. 16, 1712–1716 (2004).
Nagai, J., McMeeking, G. D. & Saitoh, Y. Durability of electrochromic glazing. Sol. Energy Mater. Sol. Cells 56, 309–319 (1999).
Gotoh, T. & Murai, H. Preparation and characteristics of new reverse mode film of polymer dispersed liquid crystal type. Appl. Phys. Lett. 60, 392–394 (1992).
Cupelli, D. et al. Electrically switchable chromogenic materials for external glazing. Sol. Energy Mater. Sol. Cells 93, 329–333 (2009).
Seeboth, A., Ruhmann, R. & Mühling, O. Thermotropic and thermochromic polymer based materials for adaptive solar control. Materials 3, 5143–5168 (2010).
Watanabe, H. Intelligent window using a hydrogel layer for energy efficiency. Sol. Energy Mater. Sol. Cells 54, 203–211 (1998).
Chahroudi, D. Solar control system. U. S. Patent #4307942 (1981).
Zrínyi, M. et al. Smart gel-glass based on the responsive properties of polymer gels. Polym. Adv. Technol. 12, 501–505 (2001).
Schneider, J. & Seeboth, A. Natural thermotropic materials for solar switching glazing. Materialwiss. Werkstofftech. 32, 231–237 (2001).
Seeboth, A. & Holzbauer, H.-R. The optical behavior of lyotropic liquid crystalline polymer gel networks: dependence on temperature. Adv. Mater. 8, 408–411 (1996).
Kleinke, A. Temperature controlled radiation transmission material. U.S. Patent #6379769 (2002).
Raicu, A. et al. Façade systems with variable solar control using thermotropic polymer blends. Sol. Energy 72, 31–42 (2002).
Park, M. J. & Char, K. Two gel states of a PEO-PPO-PEO triblock copolymer formed by different mechanisms. Macromol. Rapid Commun. 23, 688–692 (2002).
Gong, X., Li, J., Chen, S. & Wen, W. Copolymer solution-based “smart window”. Appl. Phys. Lett. 95, 251907 (2009).
Fischer, Th., Lange, R. & Seeboth, A. Hybrid solar and electrically controlled transmission of light filters. Sol. Energy Mater. Sol. Cells 64, 321–331 (2000).
Gyenes, T., Szilágyi, A., Lohonyai, T. & Zrínyi, M. Electrically adjustable thermotropic windows based on polymer gels. Polym. Adv. Technol. 14, 757–762 (2003).
Kim, D., Lee, H. S. & Yoon, J. Remote control of volume phase transition of hydrogels containing graphene oxide by visible light irradiation. RSC Adv. 4, 25379–25383 (2014).
Schild, H. G. Poly(N-isopropylacrylamide): experiment, theory and application. Prog. Polym. Sci. 17, 163–249 (1992).
Abdelsayed, V. et al. Photothermal deoxygenation of graphite oxide with laser excitation in solution and graphene-aided increase in water temperature. J. Phys. Chem. Lett. 1, 2804–2809 (2010).
Cai, W. et al. Synthesis and solid-state NMR structural characterization of 13C-labeled graphite oxide. Science 321, 1815–1817 (2008).
Fischer, Th., Holzbauer, H.-R. & Seeboth, A. Influence of inorganic salts on optical transmission behaviour of thermotropic hydrogels. Materialwiss. Werkstofftech. 30, 473–477 (1999).
Galera-Gómez, P. A. & Gu, T. Cloud point of mixtures of polypropylene glycol and triton X-100 in aqueous solutions. Langmuir 12, 2602–2604 (1996).
Acknowledgements
This research was supported by the Ministry of Education (MOE), Ministry of Science, ICT and Future Planning (MSIP) and the National Research Foundation of Korea (NRF) through the Human Resource Training Project for Regional Innovation (2012H1B8A2025809) and Basic Research fund (2011-0015029).
Author information
Authors and Affiliations
Contributions
J.Y. planed and supervised the project. D.K. carried out bulk of experiments and analyzed data. E.S. analyzed data. H.S.L. designed photomask experiment. All authors contributed to discussion of the results. J.Y. primarily wrote the paper with input and comments from D.K., E.L. and H.S.L.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Kim, D., Lee, E., Lee, H. et al. Energy Efficient Glazing for Adaptive Solar Control Fabricated with Photothermotropic Hydrogels Containing Graphene Oxide. Sci Rep 5, 7646 (2015). https://doi.org/10.1038/srep07646
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep07646
This article is cited by
-
Highly bendable bilayer-type photo-actuators comprising of reduced graphene oxide dispersed in hydrogels
Scientific Reports (2016)
-
Reduced Graphene Oxide-Based Silver Nanoparticle-Containing Composite Hydrogel as Highly Efficient Dye Catalysts for Wastewater Treatment
Scientific Reports (2015)
-
Photothermally driven fast responding photo-actuators fabricated with comb-type hydrogels and magnetite nanoparticles
Scientific Reports (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.