Abstract
Interleukin 17(A) (IL-17) is a potent pro-inflammatory cytokine that acts as a central regulator of inflammatory response within the brain, but its physiological roles under non-inflammatory conditions remain elusive. Here we report that endogenous IL-17 ablates neurogenesis in the adult dentate gyrus (DG) of hippocampus. Genetic deletion of IL-17 increased the number of adult-born neurons in the DG. Further, we found that IL-17 deletion altered cytokine network, facilitated basal excitatory synaptic transmission, enhanced intrinsic neuronal excitability and increased expression of proneuronal genes in neuronal progenitor cells (NPCs). Our findings suggest a profound role of IL-17 in the negative regulation of adult hippocampal neurogenesis under physiology conditions.
Similar content being viewed by others
Introduction
Neurogenesis in the subgranular zone (SGZ) of dentate gyrus is a major mechanism that occurs in the adult hippocampus to maintain its function and plasticity in response to extrinsic and intrinsic changes1,2,3. Abnormal neurogenesis in SGZ is likely to exacerbate major neurological disorders such as Alzheimer's disease, depression and drug addiction1,4,5,6. An understanding of how adult-born neurons develop and integrate into preformed neural networks may suggest targets for therapeutic intervention and assist in developing stem cell therapies for repairing damaged neuronal tissue7,8.
Environmental factors such as inflammatory mediators can influence neurogenesis9,10. Among these inflammatory mediators, interleukin 17 (A) (IL-17) has been found to play a pivotal role in the pathogenesis of numerous inflammatory diseases in the central nervous system (CNS), such as multiple sclerosis and stroke11,12. IL-17 acts synergistically with tumor necrosis factor (TNF) and IL-1 to shape CNS inflammation. Besides Th17 cells, a variety of cell types can produce IL-17 including γδ T cells, natural killer (NK) cells, NKT cells and lymphoid tissue inducer cells. In addition to immune cells, glial cells in the CNS also express IL-17 under physiology conditions13,14,15. IL-17 functions through a distinct ligand-receptor signaling system, IL-17 receptor (IL-17R), which is widely expressed and binds IL-17 with high affinity16. IL-17R deficiency results in reduced chemokine levels17 and its signaling has been implicated in both innate and adaptive immunity18. Recently, the expression of IL-17R has been detected within the CNS and upregulated under inflammatory conditions19,20. However, little is known about the effects of IL-17 on neurogenesis under non-inflammatory conditions.
In this study, we examined the role of endogenous IL-17 in hippocampal neurogenesis under physiological condition. Our results demonstrate that IL-17 is a negative regulator of adult hippocampal neurogenesis in the DG of hippocampus. Using IL-17 KO mice, we provided evidence that the absence of IL-17 significantly improved neurogenesis in the DG, enhanced synaptic function, reduced expression of inflammatory cytokines and increased expression of proneuronal genes in NPCs.
Methods
Animals
All mice were C57BL/6 background littermates and used at 2–3 months of age. IL-17 (A) KO mice were provided by Y. Iwakura (University of Tokyo, Tokyo, Japan). All experiments were performed in accordance with approved guidelines set by the Barrow Neurological Institute Ethical Committee.
Real-time RT-PCR
Total RNA was extracted from brain tissues or cultured progenitors with TRIzol (Invitrogen, CA). First-strand cDNA of each sample was synthesized using a reverse transcription kit (Invitrogen, CA). RT-PCR was performed as we previously described21, using an ABI Prism 7900-HT sequence system (Applied Biosystems, CA) with the QuantiTect SYBR Green PCR kit (QIAGEN, CA), in accordance with the manufacturer's instructions. The GAPDH gene was amplified and served as an endogenous control. All primer sequences used in this study were listed in supplemental Table 1. 1 μl of first-strand cDNA product was amplified with platinum Taq polymerase (Invitrogen, CA) and gene-specific primer pairs. Each sample was assayed in triplicate and experiments were repeated twice. The relative amounts of mRNA were calculated by plotting the Ct (cycle number). The mean relative expression was determined by the 2−ΔΔCt comparative method and expressed by relative expression levels against house-keeping gene (i.e. GAPDH).
Cell cultures
The method for cultures of neurons from adult DG of mouse hippocampus is the standard procedure as we previously described21,22. Briefly, animals were sacrificed and the DG of hippocampus was dissected under a stereological microscope. Tissue was minced with scissors in ice-cold Neurobasal medium (Invitrogen, Carlsbad, CA) and then digested with Papain (20 unit/mg, Worthington, NJ) at 30°C for 20 min in tubes shaken at 120 rpm in a water bath shaker. After enzyme digestion, the reaction was ceased by adding inactivated fetal bovine serum into the medium. Then, digested tissue was filtered and transferred into 15 ml tubes. After trituration, tissue was centrifuged at 1500 rpm for 3 min to form pellets containing dissociated cells and the supernatant was removed and replaced with Neurobasal medium, which was used to resuspend the pellets. This process was repeated three times. After the final centrifugation, the supernatant was replaced with Neurobasal medium supplemented with 0.5% (w/v) L-glutamine and 2% B27 serum-free supplement. Cells were re-suspended and counted based on Trypan blue exclusion and plated at a density of 4.5–5.0 × 104 cells per cm2 in 35 mm Falcon culture dishes (BD Biosciences, San Jose, California). Cells were kept within the 37°C/5% CO2 incubator for future use.
Primary cultures of mouse astrocytes were prepared from the DG of C57BL/6 newly born mice (postnatal 1-3days) as previously described23. Briefly, the DG was dissected under sterile conditions and the tissues were dissociated in 0.25% trypsin (Invitrogen, CA) for 10 minutes at 37°C. The cell suspension was separated by centrifugation at 1500 rpm for 5 minutes and the cells were transferred to poly-D-lysine pre-coated cell culture flasks in DMEM containing 10% FCS, 100 U/ml penicillin and 100 μg/ml streptomycin. The cultures were maintained at 37°C in a humidified atmosphere of 5% CO2 and 95% air. Before the experiments, the purity of astrocytes in cultures was >95% determined by an astrocyte marker, GFAP (Santa Cruz, CA). Cells were used for ELISA assay after 10-14days in culture.
To prepare primary microglial cells as previously described24, after reaching a confluent monolayer of glial cells (10–14 days), microglia were separated from astrocytes by shaking off for 5 h at 100 rpm and re-plated on 24-well plates. After plating the microglia-enriched population for 48 h, cells were used for ELISA assay. The purity was >98% as determined by a microglia marker, Iba-1 (Wako, NY).
NPC cultures
NPC cultures derived from adult mice hippocampus were performed as previously described25. Briefly, mice were deeply anesthetized using halothane and decapitated and brains were removed. Hippocampi were dissected, pooled and dissociated in DMEM (Invitrogen, CA) containing 2.5 U/ml papain, 1 U/ml dispase II and 250 U/ml DNase 1 (Worthington Biochemical, NJ) for 45 min at +37°C. Dissociated cells were purified on two Percoll (Sigma, MI) gradients, 35 and 65%. The resulting cells were plated at 1 × 104 cells per well in uncoated 12-well culture plates in DMEM/F-12, supplemented with 15 mm HEPES, 2 mm l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 0.25 μg/ml Amphotericin B (Calbiochem, CA), N2 supplement (Invitrogen, CA) and 20 ng/ml basic FGF (Sigma, MI).
ELISA
Measurement of IL-17 levels in mouse DG homogenates and cultured cell lysates was performed with an ELISA kit specific to murine IL-17 (A). Procedures were carried out according to the manufacturer's instructions (Invitrogen, CA). Results are expressed in pg/mg protein for samples from DG homogenates or for samples from cultured cell lysates. Data were from three individual experiments performed in triplicate. A Multi-Analyte ELISArray kit (SABioscience) was used to measure inflammatory cytokines in homogenates of WT and IL-17 KO DG tissues, according to the manufacturer's instructions, as we previously described26,27.
Flow cytometry
Single cell suspensions (0.5 × 106 cells) were prepared from cultured neuron, astrocytes, microglia and NPCs and stained with fluorochrome-conjugated antibodies27,28. GFAP, CD11b, NeuN and IL-17 antibodies were purchased from eBioscience and Santa Cruz. Antibodies were directly labeled with one of the following fluorescent tags: FITC, PE, APC, or PerCP. Flow cytometric data were collected on a FACSAria flow cytometer and analyzed with Diva software.
BrdU labeling
BrdU was injected intraperitoneally at 10 mg/ml in sterile 0.9% NaCl to yield a single dose of 50 μg/g body weight on each of 4 consecutive days. Mice were singly housed for 2 or 4 weeks after the initial injection until tissue preparation. After perfusion, all steps were performed blind to genotype.
Tissue Preparation and immunohistochemistry
Mice were anesthetized by intraperitoneal injection of 10 mg/ml ketamine and 1 mg/ml xylazine in sterile 0.9% NaCl at a volume of 0.01 ml/g body weight. Toe pinch was used to determine effectiveness of anesthesia. Animals were transcardially perfused with 4% paraformaldehyde in phosphate-buffered saline (PBS). Whole brain was removed and post-fixed in 4% PFA in PBS overnight, then equilibrated in 30% sucrose. Next, the tissue was frozen and coronal slices (40 μm) were cut using a cryostat microtome and processed for BrdU immunohistochemistry.
For BrdU and neuron-specific nuclear protein (NeuN) double-labeled immunofluorescence, images were captured and quantitated by observers who were blind to genotype. Free-floating sections were denatured in 2N HCl for 30 min at 37°C, rinsed in KPBS (to neutralize pH), preincubated with 2% goat and donkey serum in 0.3% Triton X-100 for 1 h and incubated overnight with rat anti-BrdU antibody (1:100; Santa Cruz, CA) and mouse anti-NeuN antibody (1:100; Millipore, MA) or rabbit anti-DCX (1:100; Sigma, St. Louis, MO) or rabbit anti-GFAP (1:100; Millipore, MA) at +4°C. Sections were then rinsed and incubated in the dark for 2 h with Alexa Fluor 568 goat anti-rat IgG antibody (1:1000; Invitrogen, CA) and Alexa Fluor 488 donkey anti-mouse IgG antibody (1:1000; Invitrogen, CA). Sections were washed with PBS 3 times for 10 minutes. Sections were then mounted on glass slides and coverslipped with glycerol-based mounting medium (for fluorescence microscopy). After immunostaining, counts were made of BrdU-positive cells in the first third of the granule cell layer in every fourth section through the entire hippocampus. Adult-born neurons are mostly confined to the first third of the granule cell layer29. Imaging was performed within 48 hours of immunostaining using a Leica TCS STED CW confocal microscope. For BrdU+ cell counts in brain sections, images were captured mainly from germinal regions but other areas were also screened to ensure a complete analysis.
Colocalization of BrdU-positive cells with NeuN was validated using a confocal scanning light microscope (MRC1024UV; Bio-Rad, Hemel Hempstead, UK) with krypton/argon 488 and 568 excitation filter. A double-stained cell was defined as having the strongest intensity of both stainings within the same or directly neighboring 1-μm-thick optical section through the cell in a consecutive Z-series of at least four sections, with an overlap of the stainings in at least three sections. Stereological estimations of the total number of BrdU+ cells in the SGZ of the dorsal hippocampal formation were performed using the optical fractionator method25,30.
Hippocampal slice preparation
Hippocampal slices that were prepared as described previously31,32. After isoflurane anesthesia, brain tissue was quickly removed and bathed in cold (4°C) artificial cerebrospinal fluid (ACSF) containing (in mM): NaCl, 119; KCl, 2.5; NaHCO3, 26; MgSO2, 1.3; NaH2PO4, 1.0; CaCl2, 2.5; and glucose, 11. The ACSF was continuously bubbled with 95% O2 - 5% CO2 (carbogen). Several coronal sections (400 μm) containing the dorsal hippocampus were cut using a vibratome (Vibroslice 725 M, WPI, Sarasota, FL) and transferred to a holding chamber and incubated at room temperature (21 ± 1°C) for at least 60 min prior to recording.
Electrophysiology
Current-clamp recordings to measure neuronal excitability were performed with a modified protocol as previously described21,33,34. Electrophysiology recordings were performed by experimenters who were blind to genotype. All recordings were performed at 31 ± 1°C. Neuronal intrinsic excitability was measured as the number of spikes in response to a series of fixed 500 ms current injection steps (0, 0.03, 0.06, 0.09, 0.12, 0.15, 0.18 and 0.21 nA). Data were acquired by Axonpatch 200B amplifier at 2 kHz with pClamp 9.2 software (Molecular Devices, Sunnyvale, CA) and analyzed with clampfit 9.2 software (Molecular Devices, Sunnyvale, CA) and Mini Analysis software (Synaptosoft, Decatur, GA). For field potential recordings from slices of hippocampal DG, a stimulating electrode was placed in the angular bundle of the perforant path and a recording electrode in the hilus of the DG. A single slice was transferred to a liquid-air interface chamber (Fine Science Tools, Inc., Foster City, CA, USA) and suspended on a nylon net at the liquid-air interface in a bath of continuously dripping oxygenated ACSF (2–2.5 ml/min).
Drugs and Solutions
All drugs used in this study were purchased from Sigma (Sigma, St. Louis, MO). Mouse IL-17 was purchased from Cell Signaling (Cell Signaling, MA). For patch-clamp recording from hippocampal cultures, the standard external solution contained 140 mM NaCl, 3 mM KCl, 2 mM MgCl2, 2 mM CaCl2, 10 mM d-glucose, 10 mM HEPES, adjusted to pH 7.4 with Tris base. The internal solution for current-clamp recordings to measure intrinsic excitability contained 130 mM KMeSO4, 10 mM KCl, 10 mM HEPES/K-HEPES, 2 mM MgSO4, 0.5 mM EGTA and 3 mM ATP; the pH was adjusted to 7.3 with KOH. For AMPA receptor-mediated miniature EPSC (mEPSC) recordings, 0.3 µM TTX, 50 µM picrotoxin and 50 µM APV were added into external solution. For whole-cell recordings of ligand-gated ion channels, the internal solution contains the following: 140 mM potassium gluconate, 5 mM KCl, 10 mM HEPES, 0.2 mM EGTA, 2 mM MgCl2, 4 mM MgATP, 0.3 mM Na2GTP and 10 mM Na2-phosphocreatine (pH 7.3 with KOH). For field potential recordings, the internal solution was 2 M NaCl.
Statistical analysis
Comparisons were performed using two-tailed student t-test or one-way ANOVA as appropriate. Data are presented as means ± SEM and differences are considered significant at p < 0.05. All statistical analyses were conducted using Graphpad Prism 5 software.
Results
IL-17 and IL-17R are expressed in the DG of hippocampus
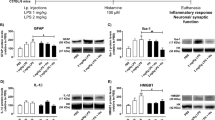
To evaluate the expression of IL-17 and its receptor (IL-17R) in the DG of hippocampus, we performed ELISA assay and found that IL-17 mRNA and protein are expressed in WT DG tissues (Figure 1A–B). To identify the source of IL-17 in WT DG tissues, we cultured astrocytes, microglia, neurons and NPCs. Thereafter, we used fluorescent-activated cell sorting (FACS) with intracellular staining to compare the expression of IL-17 among these cells. We found that astrocytes are the major source of IL-17 under physiological condition (Figure 1C–D). To assess the expression of IL-17R on NPCs, we examined the expression of IL-17R mRNA and protein via RT-PCR and FACS, respectively. We found that majority of NPCs expressed IL-17R (Figure F–G).
IL-17 and IL-17R are expressed in the DG of hippocampus.
(A). Real-time RT-PCR results show expression of IL-17 messenger relative to GAPDH in WT and IL-17 KO DG tissues. (B). Expression of IL-17R at protein level in WT and IL-17 KO DG tissues was confirmed using ELISA. (C–D). Results from FACS intracellular staining of cultured astrocytes, microglia, neurons and NPCs show astrocytes as a major source of IL-17. (E). IL-17R mRNA is expressed in cultured WT and IL-17 KO NPCs. (F–G). FACS analysis shows expression of IL-17R in cultured WT and IL-17 KO NPCs. Data were obtained from three individual experiments performed in triplicate. Means ± SEM; **p < 0.01.
Genetic deletion of IL-17 promotes neurogenesis in the DG of hippocampus
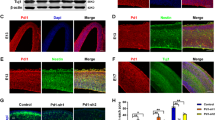
To determine whether endogenous IL-17 influences adult-born neuron proliferation and survival, we birthdated adult-born neurons in wild-type (WT) and IL-17 knockout (KO) mice by injecting BrdU and following their fate. Hippocampal slices were taken for BrdU immunostaining after 2 and 4 weeks to allow quantification before and after the critical period for activity-dependent adult-born neuron proliferation and survival. A significant increase in the number of BrdU-labeled cells was seen in the dentate gyrus of IL-17 KO mice as compared to age-matched WT mice at both 2 and 4 weeks (Figure 2A–B). The results indicate that IL-17 signaling appears to negatively regulate adult-born neurogenesis.
Genetic deficiency of IL-17 increases neurogenesis in the adult DG of hippocampus.
(A). Confocal images of the DG showing BrdU (red) and DAPI (blue) staining at 2 or 4 weeks after injection of BrdU to WT (top) and IL-17 KO (bottom) mice. (B). Quantification of BrdU-immunopositive cells at 2 weeks (left; 2 WPI) and 4 weeks (right; 4 WPI) post-BrdU injection (mean ± SEM; n = 4 mice per condition). Scale bar, 100 μm. (C). Confocal images of BrdU+DCX+ cells showing BrdU and DCX immunoreactivity as merged images at 2 weeks after 4 days of daily BrdU injections. (D). Number of new immature neurons (BrdU+/DCX+) in the dentate subgranular zone/granule cell layer at 2 weeks after 4 days of daily BrdU injections. (E). Confocal images of BrdU+NeuN+ cells showing BrdU and NeuN immunoreactivity as merged images at 4 weeks after 4 days of daily BrdU injections. (F). Number of new immature neurons (BrdU+/NeuN+) in the dentate subgranular zone/granule cell layer at 4 weeks after 4 days of daily BrdU injections. Means ± SEM; n = 6 for WT and IL-17 KO, respectively. **p < 0.01.
We then explored whether the absence of IL-17 can influence the formation of immature and mature neurons. IL-17 KO and WT mice were injected with BrdU once daily for 4 days and sacrificed 2 or 4 weeks. We found that the BrdU+ cells were distributed mainly in the SGZ/GCL. Genetic deletion of IL-17 caused a significant increase in the number of BrdU+ cells double labeled for both the specific marker of immature neurons (DCX) at 2 weeks after BrdU injections and mature neurons (NeuN) in the SGZ/GCL compared to WT animals at 4 weeks after BrdU injections (Figure 2C–F). These results suggest that deletion of IL-17 increases both adult-born immature and mature neurons in the DG of hippocampus.
Endogenous IL-17 regulates cytokine network in the DG of hippocampus
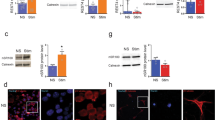
IL-17 participates in pathogenesis of neurological inflammatory diseases through an induction of inflammatory gene expression in target cells. Our finding that the deletion of IL-17 promoted neurogenesis suggests that endogenous IL-17 may influence cytokine environment in the DG. To test this possibility, we compared expression profile of key pro-inflammatory cytokines in the DG of hippocampus from IL-17 KO and WT mice. Using an ELISA array kit, we found that the deletion of IL-17 reduced the expression of TNF-α, IL-1β, IL-6 and IFN-γ, as compared to WT controls (Figure 3). These data suggest that IL-17 deletion may regulate the cytokine network in the DG, where progenitors reside.
Deficiency of IL-17 alters the expression of inflammatory cytokines in the adult DG of hippocampus.
ELISA results show that genetic deletion of IL-17 alters the expression of TNFα, IL-1, IL-4, IL-6, IL-10 and IFN-γ at protein level as compared to WT controls. Data were from four individual experiments performed in triplicate. Means ± SEM; *p < 0.05; **p < 0.01.
Deficiency of IL-17 alters synaptic functions in the DG of hippocampus
To investigate the neurophysiological consequences of IL-17 deletion in the DG of hippocampus, we examined baseline synaptic transmission and synaptic short- and long-term potentiation (LTP) in the DG of hippocampus in both IL-17 KO and WT mice. For field potential recordings from slices of hippocampal DG, the stimulating electrode was placed in the angular bundle of the perforant path and a recording electrode in the hilus of the DG. We conducted a systematic evaluation of changes in DG synaptic functions in IL-17 KO mice (Figure 4A). Although no changes in LTP was observed (Figure 4B), we found that the input - output (I/O) curve of field EPSP (fEPSP) slopes in response to different stimulation intensities were shifted to the left when compared with those of WT controls (Figure 4C), suggesting that basal synaptic transmission was significantly enhanced in the DG of IL-17 KO mice. We next examined synaptic short plasticity using paired-pulse stimulation protocol and found that the paired-pulse facilitation (PPF) was indistinguishable between IL-17 KO mice and WT controls at 50 ms inter-pulse intervals. However, PPF was significantly enhanced when the inter-pulse intervals were 100, 150 and 500 ms (Figure 4D). Therefore, short-lived presynaptic plasticity was increased in IL-17 KO mice.
Deficiency of IL-17 facilitates basal synaptic transmission in DG of hippocampal slices.
(A). For field potential recordings from slices of hippocampal DG, the stimulating electrode was placed in the angular bundle of the perforant path and a recording electrode in the hilus of the DG. (B). LTP recordings from the adult hippocampus DG shows no difference between WT and IL-17KO mice. (C). Input/output (I/O) curves relating the various input intensity (mV) to the slope of fEPSP in the DG region of adult IL-17 KO and WT mice. (D). Enhanced paired-pulse facilitation in the DG of hippocampal slices from IL-17 KO mice. Paired-pulse facilitation across different inter-stimulus intervals (50–500 ms) is increased in the DG of hippocampal slices from IL-17 KO mice. (n = 6 mice per group). Means ± SEM; **p < 0.01.
Increased intrinsic excitability and excitatory synaptic activities on adult-born IL-17 KO neurons from the DG of hippocampus cultures
Next, we compared neuronal intrinsic excitability and glutamatergic inputs between these adult-born IL-17 KO neurons and WT controls by performing somatic whole-cell current-clamp recordings in cultured hippocampal DG neurons dissociated from mice injected with BrdU (i.p. injected with Brdu for 4 days before cell dissociation). After DIV for over 14 days, the cultured cells were recorded and biocytin was microinjected afterward. Then biocytin-injected cells were stained with streptavidin conjugated antibody and anti-Brdu antibody to confirm that the recorded cells were newly born from the DG of hippocampus following Brdu injections (Figure 5A). We examined the effects of IL-17 deletion on intrinsic membrane excitability in response to injection of a 90 pA current from cultured BrdU+ neurons. Results showed that IL-17 deletion increased neuronal excitability represented as an increase in spike numbers (Figure 5B). I/O relationships derived from plots of spike numbers occurring each 500 ms as a function of injected, different depolarizing currents were used to evaluate neuronal excitability33,34. IL-17 KO mice exhibited a significantly increase in neuronal excitability represented as a left shift of I-O relationship curve (in response to injected currents ranging from 0-180 pA) compared to WT controls (Figure 5C). These results suggest that IL-17 deletion enhances intrinsic excitability in the newly born neurons from DG of hippocampus.
Adult-born neurons lacking IL-17 exhibit increased intrinsic excitability and receive more excitatory synaptic activity.
(A). A representative cultured neuron from the adult DG of hippocampus (i.p. injected with Brdu for 4 days before cell dissociation) was recorded and microinjected with biocytin then immunostained with streptavidin conjugated antibody (green) and BrdU antibody (red). (B). Typical traces of action potential generation in response to 90 pA current injection (note the -60 mV holding potential and the time course and membrane potential calibration bars) show that deletion of IL-17 signaling causes increased neuronal excitability in BrdU+ primary cultured neuron from the adult DG of hippocampus. (C). Calculated input-output relationships for action potential spike activity (ordinate) as a function of currents injected (0-180 pA) in cultured BrdU+ cortical neurons prepared from the adult DG of hippocampus. Results show that deletion of IL-17 induces the increase of action potential numbers as compared to WT controls. (D–F). Deletion of IL-17 also increases the amplitude and frequency of mEPSCs recorded from cultured BrdU+ neurons from the DG of hippocampus as compared to those from WT controls. n = 8. Means ± SEM; *p < 0.05; **p < 0.01.
To test the impact of IL-17 deletion on synaptic excitatory glutamatergic activities, we examined the AMPA (2-amino-3-(5-methyl-3-oxo-1,2- oxazol-4-yl) propanoic acid) receptor-mediated miniature excitatory postsynaptic currents (mEPSCs) that occur spontaneously in cultured neurons in the presence of tetrodotoxin. We found a significant increase in both mEPSC amplitude (Figure 5D–E) and frequency (Figure 5D–F) in cultured IL-17KO BrdU+ neurons as compared to WT controls. Statistical analysis demonstrated that the mEPSC amplitude was increased in IL-17 KO neurons as compared to that of WT controls (Figure 5E). Meanwhile, mEPSC frequency was enhanced in IL-17KO neurons as well (Figure 6F). These results suggest that deletion of IL-17 results in increased mEPSC amplitude and frequency, which may be caused by increased post-synaptic AMPARs levels and the probability of presynaptic vesicular glutamate release. In the absence of IL-17, adult-born neurons receive more synaptic activity, offering a possible explanation for the increased neurogenesis in the DG of hippocampus.
Endogenous IL-17 regulates the expression of proneuronal and progial genes.
Real-time RT-PCR analysis of the messenger expression levels of promeuronal genes (NGN2, MASH1 and NeuroD1) and proglia genes (HES1 and ld2) in cultured NPCs from IL-17 KO and WT mice. The mRNA expression of NGN2, Mash1 and NeuroD1 were increased in IL-17 KO progenitor cells compared with WT controls. Proglial gene expressions of HES1 and ld2 were decreased in IL-17 KO progenitors as compared to WT controls. Data were obtained from four individual experiments performed in triplicate. Means ± SEM; *p < 0.05; **p < 0.01.
IL-17 regulates the expression of the proneuronal and proglial genes in adult-born DG neural stem cells
Since most of the neuronal progenitors are regulated by proneural genes, we asked whether the expressions of these proneural genes were changed in IL-17 KO progenitors. By using real-time RT-PCR techniques, we measured the messenger expression levels of neurogenin 2 (Ngn2), mammalian achaete-scute homolog 1 (Mash1) and neurogenic differentiation factor 1 (NeuroD1). IL-17 KO progenitors exhibited increased expression of Ngn2, Mash1 and NeuroD1 compared to WT controls after 3 days in differentiation medium (Figure 6). In addition, reduced gliagenic differentiation genes, i.e. HES1 and ld2 were observed in IL-17 KO progenitors as well (Figure 6). These findings suggest that the elevated messenger expression of neurogenic gene and reduced expression of gliagenic differentiation genes may be related to the increased differentiation toward neurons in IL-17-KO progenitors.
Discussion
By using IL-17 KO mice, we provide the first evidence that IL-17 negatively regulates neurogenesis in the adult DG in vivo under non-inflammatory conditions. We observed enhanced intrinsic neuronal excitability, increased basal excitatory synaptic transmission and altered cytokine environment in the hippocampal DG of IL-17 KO mice. In addition, we also demonstrate that genetic deletion of IL-17 promotes the mRNA expression of pro-neuronal genes. Considering that there is no evidence of any developmental abnormalities in the IL-17 KO mice that would explain the observed alterations of hippocampal neurogenesis, our findings suggest a profound role of endogenous IL-17 in the negative regulation of hippocampal neurogenesis and its impact on the cytokine environment as well as basal neurotransmission.
Previous findings have revealed a pivotal role of IL-17 in the pathogenesis of CNS inflammatory diseases11,12, but the physiological function of IL-17 is much less studied. One recent report suggests suppressive effects of exogenous IL-17 on the proliferation of stem cells in vitro35. However, the role of endogenous IL-17 in the hippocampal neurogenesis remains unknown. By using IL-17 KO mice, we show that endogenous IL-17 negatively regulates neurogenesis in the DG. We found that progenitor cell proliferation in hippocampal DG was enhanced in IL-17 KO mice as compared to their WT counterparts. BrdU+ cells from the DG of IL-17 KO mice showed positive staining of DCX at 2 weeks post BrdU injection, supporting that the proliferating cells were NPCs36. Moreover, BrdU+ cells from the DG of IL-17 KO mice showed higher numbers of DCX+ staining 2 weeks post BrdU injection, reflecting the increased SGZ proliferation. The increased numbers of BrdU+ NeuN+ cells in the DG of IL-17 KO mice was seen 4 weeks post BrdU injection, which suggests increased newly born mature neurons in the SGZ. Both IL-17 and its receptors were found expressed in the DG tissue, IL-17R was also found expressed in the cultured progenitors, supporting that the effects of the ligand are directly mediated by IL-17R on these cells.
It's reported that IL-17 is produced by CNS glia cells and the IL 17R is expressed by various CNS cells13,14,15. One recent study also showed that IL-17 treatment can reduce NPC proliferation and differentiation by acting on IL-17R in vitro35. In the present study, we identified astrocytes as a major source of IL-17 in the DG tissues and IL-17R is expressed by NPCs. Our results show the anti-proneurogenic effects of proinflammatory cytokines, because loss of endogenous IL-17 potentiates neurogenesis, as evidenced by the experiments with IL-17 KO mice in vivo. Nevertheless, the role of IL-17 in neurogenesis during pathological conditions and in the cross-talk between glial cells and NPCs require further investigation, which could be a relevant molecular link during the development of neuroinflammation and brain repair. Additionally, whether IL-17 deletion can improve learning and memory behavior remains unclear, future investigations are warranted to reveal the potential impact of IL-17 on cognitive functions.
Neurogenesis is affected by their local cytokine environment. For example, several studies show that TNF-α, IL-1β and IL-6 inhibit neurogenesis by activating their receptors10,37,38,39,40,41. The net effects of IL-17 on neurogenesis are most likely dependent on its levels and on the expression of IL-17R in the progenitor cells. IL-17 binds to IL-17R with high affinity42,43. The altered CNS inflammatory environment may underlie this enhanced neurogenesis in the DG. On the other hand, the intracellular mechanisms mediating the actions of IL-17 through its receptor IL-17R on progenitor proliferation and survival in vivo are largely unknown. Previous findings demonstrated that activation of IL-17R induced cell apoptosis by activating caspase-3, caspase-9 and up-regulating the ratio of Bax/Bcl-244. We observed that both IL-17 and IL-17R were expressed in DG tissues as well as in cultured progenitors (Figure 2), although at a relatively low amount, which is similar to what has been reported previously in rats and humans13,45. Thus, the ligand acting on the IL-17R could originate from the CNS-resident cells including hippocampal progenitors themselves. In addition, we found that deletion of endogenous IL-17 alters the cytokine environment in the DG tissues, including several proinflammatory cytokines that may be detrimental for neurogenesis. This altered cytokine microenvironment may be involved in the enhanced neurogenesis caused by the deletion of IL-17 in the DG of hippocampus.
Our findings on the deletion of IL-17 promoting basal neurotransmission and intrinsic excitability do not conclude that increased neurogenesis in IL-17 KO mice results from the neurophysiological consequences of IL-17 deletion. Recent evidence suggests that neurogenesis can be directly coupled with neuronal excitation46,47 and the survival of NPC-derived immature neurons through the critical period depends on their excitatory inputs46,47,48. Meanwhile, several lines of studies also support that increased neurogenesis can modulate synaptic transmission and neuronal activities49,50,51. These studies have highlighted the link between neurogenesis and neuronal network activities. In this study, we found that deletion of IL-17 increased basal synaptic transmission, as well as the intrinsic excitability and glutamatergic inputs of newly born neurons. These results suggest that endogenous IL-17 can regulate neuronal network activities. Further investigations are warranted to reveal whether or not the increased neurogenesis seen in IL-17 KO mice results from these neurophysiological consequences of IL-17 deletion.
Our results show altered expression of proneural and glia genes in cultured NPCs from IL-17 KO mice. These findings are consistent with previous studies that inhibited expression of glia fate genes but increased proneural genes promotes neurogenesis36,46. We found that deletion of IL-17 down-regulates Hes1 and Id2 and up-regulates MASH1, NGN2 and NeuroD messengers in cultured NPCs, which may be related to the increased differentiation toward neurons seen in IL-17-KO progenitors.
Our data shows that endogenous IL-17 can negatively regulate adult neurogenesis in the DG of hippocampus. However, caveats should be taken when extrapolate these findings to all adult neurogenesis, including neurogenesis in the subventricular zone. Future studies are needed to further clarify whether endogenous IL-17 can influence neurogenesis in other brain regions as well.
In summary, we have demonstrated the involvement of endogenous IL-17 in the negative regulation of adult hippocampal neurogenesis under non-inflammatory conditions. Given that circumstantial evidence indicates a link between hippocampal neurogenesis and cognitive function52, our findings suggest that the ablation of endogenous IL-17 may serve as a potential strategy to promote adult hippocampal neurogenesis.
References
Rola, R. et al. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol 188, 316–330 (2004).
Shors, T. J. et al. Neurogenesis in the adult is involved in the formation of trace memories. Nature 410, 372–376 (2001).
Snyder, J. S., Hong, N. S., McDonald, R. J. & Wojtowicz, J. M. A role for adult neurogenesis in spatial long-term memory. Neuroscience 130, 843–852 (2005).
Sahay, A. & Hen, R. Adult hippocampal neurogenesis in depression. Nat Neurosci 10, 1110–1115 (2007).
Verret, L., Trouche, S., Zerwas, M. & Rampon, C. Hippocampal neurogenesis during normal and pathological aging. Psychoneuroendocrinology 32 Suppl 1S26–30 (2007).
Noonan, M. A., Bulin, S. E., Fuller, D. C. & Eisch, A. J. Reduction of adult hippocampal neurogenesis confers vulnerability in an animal model of cocaine addiction. J Neurosci 30, 304–315 (2010).
Sohur, U. S., Emsley, J. G., Mitchell, B. D. & Macklis, J. D. Adult neurogenesis and cellular brain repair with neural progenitors, precursors and stem cells. Philos Trans R Soc Lond B Biol Sci 361, 1477–1497 (2006).
Okano, H. & Sawamoto, K. Neural stem cells: involvement in adult neurogenesis and CNS repair. Philos Trans R Soc Lond B Biol Sci 363, 2111–2122 (2008).
Aarum, J., Sandberg, K., Haeberlein, S. L. & Persson, M. A. Migration and differentiation of neural precursor cells can be directed by microglia. Proc Natl Acad Sci U S A 100, 15983–15988 (2003).
Monje, M. L., Toda, H. & Palmer, T. D. Inflammatory blockade restores adult hippocampal neurogenesis. Science 302, 1760–1765 (2003).
Jadidi-Niaragh, F. & Mirshafiey, A. Th17 cell, the new player of neuroinflammatory process in multiple sclerosis. Scand J Immunol 74, 1–13 (2011).
Shichita, T. et al. Pivotal role of cerebral interleukin-17-producing gammadeltaT cells in the delayed phase of ischemic brain injury. Nat Med 15, 946–950 (2009).
Li, G. Z. et al. Expression of interleukin-17 in ischemic brain tissue. Scand J Immunol 62, 481–486 (2005).
Tzartos, J. S. et al. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol 172, 146–155 (2008).
Kawanokuchi, J. et al. Production and functions of IL-17 in microglia. J Neuroimmunol 194, 54–61 (2008).
Moseley, T. A., Haudenschild, D. R., Rose, L. & Reddi, A. H. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev 14, 155–174 (2003).
Huang, W., Na, L., Fidel, P. L. & Schwarzenberger, P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis 190, 624–631 (2004).
Kolls, J. K. & Linden, A. Interleukin-17 family members and inflammation. Immunity 21, 467–476 (2004).
Kebir, H. et al. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med 13, 1173–1175 (2007).
Das Sarma, J. et al. Functional interleukin-17 receptor A is expressed in central nervous system glia and upregulated in experimental autoimmune encephalomyelitis. J Neuroinflammation 6, 14 (2009).
Liu, Q., Xie, X., Lukas, R. J., St John, P. A. & Wu, J. A novel nicotinic mechanism underlies beta-amyloid-induced neuronal hyperexcitation. J Neurosci 33, 7253–7263 (2013).
He, P., Liu, Q., Wu, J. & Shen, Y. Genetic deletion of TNF receptor suppresses excitatory synaptic transmission via reducing AMPA receptor synaptic localization in cortical neurons. FASEB J 26, 334–345 (2012).
Yang, Y. J., Zhang, S., Ding, J. H., Zhou, F. & Hu, G. Iptakalim protects against MPP+-induced degeneration of dopaminergic neurons in association with astrocyte activation. Int J Neuropsychopharmacol 12, 317–327 (2009).
Gao, H. M., Hong, J. S., Zhang, W. & Liu, B. Distinct role for microglia in rotenone-induced degeneration of dopaminergic neurons. J Neurosci 22, 782–790 (2002).
West, M. J., Slomianka, L. & Gundersen, H. J. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec 231, 482–497 (1991).
Gan, Y. et al. Ischemic neurons recruit natural killer cells that accelerate brain infarction. Proc Natl Acad Sci U S A 111, 2704–2709 (2014).
Hao, J. et al. Central nervous system (CNS)-resident natural killer cells suppress Th17 responses and CNS autoimmune pathology. J Exp Med 207, 1907–1921 (2010).
Pastrana, E., Cheng, L. C. & Doetsch, F. Simultaneous prospective purification of adult subventricular zone neural stem cells and their progeny. Proc Natl Acad Sci U S A 106, 6387–6392 (2009).
Zhao, C., Teng, E. M., Summers, R. G., Jr, Ming, G. L. & Gage, F. H. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci 26, 3–11 (2006).
Gundersen, H. J. & Jensen, E. B. The efficiency of systematic sampling in stereology and its prediction. J Microsc 147, 229–263 (1987).
Song, C. et al. Role of alpha7-nicotinic acetylcholine receptors in tetanic stimulation-induced gamma oscillations in rat hippocampal slices. Neuropharmacology 48, 869–880 (2005).
Kimura, R., MacTavish, D., Yang, J., Westaway, D. & Jhamandas, J. H. Beta amyloid-induced depression of hippocampal long-term potentiation is mediated through the amylin receptor. J Neurosci 32, 17401–17406 (2012).
Desai, N. S., Rutherford, L. C. & Turrigiano, G. G. Plasticity in the intrinsic excitability of cortical pyramidal neurons. Nat Neurosci 2, 515–520 (1999).
Karmarkar, U. R. & Buonomano, D. V. Different forms of homeostatic plasticity are engaged with distinct temporal profiles. Eur J Neurosci 23, 1575–1584 (2006).
Li, Z. et al. Inhibitory effect of IL-17 on neural stem cell proliferation and neural cell differentiation. BMC Immunol 14, 20 (2013).
He, P. & Shen, Y. Interruption of beta-catenin signaling reduces neurogenesis in Alzheimer's disease. J Neurosci 29, 6545–6557 (2009).
Ekdahl, C. T., Kokaia, Z. & Lindvall, O. Brain inflammation and adult neurogenesis: the dual role of microglia. Neuroscience 158, 1021–1029 (2009).
Carpentier, P. A. & Palmer, T. D. Immune influence on adult neural stem cell regulation and function. Neuron 64, 79–92 (2009).
Goshen, I. et al. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol Psychiatry 13, 717–728 (2008).
Koo, J. W. & Duman, R. S. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci U S A 105, 751–756 (2008).
Spulber, S., Oprica, M., Bartfai, T., Winblad, B. & Schultzberg, M. Blunted neurogenesis and gliosis due to transgenic overexpression of human soluble IL-1ra in the mouse. Eur J Neurosci 27, 549–558 (2008).
Kuestner, R. E. et al. Identification of the IL-17 receptor related molecule IL-17RC as the receptor for IL-17F. J Immunol 179, 5462–5473 (2007).
Gaffen, S. L. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol 9, 556–567 (2009).
Zhu, F. et al. IL-17 induces apoptosis of vascular endothelial cells: a potential mechanism for human acute coronary syndrome. Clin Immunol 141, 152–160 (2011).
Li, H. L. et al. IL-17 and IFN-gamma mRNA expression is increased in the brain and systemically after permanent middle cerebral artery occlusion in the rat. J Neuroimmunol 116, 5–14 (2001).
Deisseroth, K. et al. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron 42, 535–552 (2004).
Tashiro, A., Sandler, V. M., Toni, N., Zhao, C. & Gage, F. H. NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature 442, 929–933 (2006).
Tashiro, A., Makino, H. & Gage, F. H. Experience-specific functional modification of the dentate gyrus through adult neurogenesis: a critical period during an immature stage. J Neurosci 27, 3252–3259 (2007).
Ikrar, T. et al. Adult neurogenesis modifies excitability of the dentate gyrus. Front Neural Circuits 7, 204 (2013).
Jakubs, K. et al. Environment matters: synaptic properties of neurons born in the epileptic adult brain develop to reduce excitability. Neuron 52, 1047–1059 (2006).
Eisch, A. J. et al. Adult neurogenesis, mental health and mental illness: hope or hype? J Neurosci 28, 11785–11791 (2008).
Abrous, D. N., Koehl, M. & Le Moal, M. Adult neurogenesis: from precursors to network and physiology. Physiol Rev 85, 523–569 (2005).
Acknowledgements
This study was supported in part by National Basic Research Program of China Grant 2013CB966900, National Key-Project of Clinical Neurology, National Science Foundation of China Grant 81230028 and 81471535, Foundation of Tianjin Education Commission, National Institutes of Health Grants R01AI083294, American Heart Association Grant GRNT18970031 and Hanley family charitable trust.
Author information
Authors and Affiliations
Contributions
Q.L. designed and performed experiments and wrote the main manuscript text, W.X. designed and performed experiments and data analysis, P.H. performed cell biological experiments and data analysis, D.T. performed electrophysiological experiments and data analysis, J.X.Y. performed immunohistochemical staining and contributed to the Fig. 2, Y.G. performed some immunological experiments and contributed to the part of Fig. 1,3, F.D.S. designed experiments and revised MS, J.W. designed experiments, wrote part of MS and revised MS. All authors reviewed the manuscript
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplemental table 1
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Liu, Q., Xin, W., He, P. et al. Interleukin-17 inhibits Adult Hippocampal Neurogenesis. Sci Rep 4, 7554 (2014). https://doi.org/10.1038/srep07554
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep07554
This article is cited by
-
Evaluation of serum interleukin-17 A and interleukin-22 levels in pediatric patients with autism spectrum disorder: a pilot study
BMC Pediatrics (2024)
-
Th17 Cells and IL-17A in Ischemic Stroke
Molecular Neurobiology (2023)
-
The Dialogue Between Neuroinflammation and Adult Neurogenesis: Mechanisms Involved and Alterations in Neurological Diseases
Molecular Neurobiology (2023)
-
Interleukin-17A regulates ependymal cell proliferation and functional recovery after spinal cord injury in mice
Cell Death & Disease (2021)
-
Transplantation of mesenchymal stem cells causes long-term alleviation of schizophrenia-like behaviour coupled with increased neurogenesis
Molecular Psychiatry (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.