Abstract
The role of Body Mass Index (BMI) for Breast Cancer (BC) remains to be great interest for a long time. However, the precise effect of nonlinear dose-response for BMI and BC risk is still unclear. We conducted a dose-response meta-analysis to quantitatively assess the effect of BMI on BC risk. Twelve prospective studies with 4,699 cases identified among 426,199 participants and 25 studies of 22,809 cases identified among 1,155,110 participants in premenopausal and postmenopausal groups, respectively, were included in this meta-analysis. Significant non-linear dose-response (P < 0.001) association was identified between BMI and BC risk in postmenopausal women. Individuals with BMI of 25, 30 and 35 kg/m2 yielded relative risks (RRs) of 1.02 [95% confidence interval (CI): 0.98–1.06], 1.12 (95% CI: 1.01–1.24) and 1.26 (95% CI: 1.07–1.50), respectively, when compared to the mean level of the normal BMI range. However, inverse result though not significant was observed in premenopausal women. In conclusion, the results of this meta-analysis highlighted that obesity contributed to increased BC risk in a nonlinear dose-response manner in postmenopausal women and it is important to realize that body weight control may be a crucial process to reduce BC susceptibility.
Similar content being viewed by others
Introduction
Due to the high prevalence, obesity has been one of major public health burdens in the world. It is estimated that 10–20% men and 15–25% women in Europe are obese1, while 32.2% adult men and 35.5% adult women in United States2. In China, as a developing country with a large population, the total number of overweight and obese people is already close to one quarter of all population3. Epidemiological studies indicated that obesity may contribute to the increased incidence of various human cancers4, among which breast cancer (BC) is the most common and leading cause of cancer death among female in the world5. With increasing trend of obesity epidemic, much more attention should be paid on this public health problem and new intervention approaches should be proposed.
Current several epidemiological evidences suggested that higher body mass index (BMI) was positively associated with increased BC risk in postmenopausal women6,7,8 but inversely reduced BC risk in premenopausal women9,10. However, even restricted in same menopausal status population, conflicting results were obtained among different studies6,11. Though obesity was considered as a risk factor for BC by the International Agency for Research on Cancer (IRAC)12, meta-analysis is still the best way to integrate the all available data to better interpret the relationship pattern of obesity and BC risk. A meta-analysis conducted by Ursin G, et al13 identified that high BMI was inversely associated with BC risk in premenopausal women. In addition, another meta-analysis conducted by Suzuki, et al14 suggested that the relationship between body weight and risk of BC may vary based on the menopausal status or estrogen receptor (ER) and progesterone receptor (PR) status. However, to the best of our knowledge, those previous reported meta-analyses just considered the linear relationship between BMI and BC risk or just compared the highest BMI versus the reference category to evaluate the association of BMI and BC risk which did not take into account of the inconstant change of the BC risk per unit change of the BMI. Therefore, we conducted this nonlinear dose-response meta-analysis of prospective studies to quantitatively and precisely evaluate the relevance of BMI and BC risk in different menopausal status of participants.
Results
Studies characteristics
A total of 12 articles9,10,11,15,16,17,18,19,20,21,22,23 containing 12 studies of 4,699 cases identified among 426,199 women in premenopausal group and 20 articles6,8,10,11,15,16,17,19,20,21,23,24,25,26,27,28,29,30,31,32 containing 25 studies of 22,809 cases identified among 1,155,110 women in postmenopausal group were finally included in the meta-analysis. The duration of follow-up ranged from 2.14 to 24.1 years. Among these studies (Table 1), 24 studies were conducted in white population, 5 in Asian, 2 in black and 6 in mixed population. All studies were published in English except one published in Chinese.
Overall dose-response association between BMI and BC risk
The random-effects model was applied due to significant heterogeneity (P <0.001) was observed between studies in postmenopausal group. We found a significant nonlinear dose-response association (P <0.001, Figure 1) between BMI and BC risk with an increasing trend of RRs with per 1 kg/m2 increase in BMI. When compared with reference (mean level of the normal BMI range, BMI = 21.75), the pooled RRs for BC risk were 1.02 (95% CI: 0.98–1.06) for BMI at 25 kg/m2, 1.12 (95% CI: 1.01–1.24) for BMI at 30 kg/m2 and 1.26 (95% CI: 1.07–1.50) for BMI at 35 kg/m2 (Table S1). The Egger's regression test showed no evidence of publication bias with the P value of 0.236 (Figure S2).
In premenopausal women, the random model was applied as significant heterogeneity (P = 0.061) was identified. And we did not find evidence of non-linear relationship of BMI and premenopausal BC risk (P = 0.608). Additionally, no significant association was found between BMI and BC risk in premenopausal women when we modeled the linear relation of BMI and BC risk (RR = 0.989, 95% CI = 0.977–1.002 for every 5 kg/m2 increase in BMI).
Stratified analyses in postmenopausal group
We performed stratified analyses according to ethnicity and follow-up year to explore the possible source of between-study heterogeneity in postmenopausal women. When stratified by ethnicity, 3 studies and 20 studies were included in Asian and European subgroups, respectively. The between-study heterogeneity was still existed in both groups (P <0.001 for Asian and P = 0.027 for European group). Furthermore, significant nonlinear dose-response relationship of BMI and BC risk was observed in European (P for nonlinear < 0.001, Figure S3). However, in Asian subgroup, no significant nonlinear association of BMI and BC was presented. The stratified analysis was not performed in Black subgroup due to only one study was included in this meta-analysis.
When stratified by follow-up year, 15 studies and 17 studies were included in <10 subgroup and ≧−10 subgroup, respectively. The between-study heterogeneities were still remained in both subgroups (P = 0.020 for <10 subgroup and P = 0.001 for ≧−10 subgroup). Significant nonlinear dose-response association was identified in <10 subgroup but was not find in ≧−10 subgroup (data not shown).
Sensitivity analysis
Similar results were presented in postmenopausal and premenopausal women before and after elimination of each study in the meta-analyses. These indicated that our results were stable.
Discussion
The meta-analysis of 25 prospective studies with 22,809 cases identified among 1,155,110 participants indicated a significant non-linear dose-response (P <0.001) association between BMI and BC risk in postmenopausal females. However, in the meta-analysis of 12 studies with 4,699 cases identified among 426,199 premenopausal women, we found inverse association of increasing BMI on BC development but with no significance. In postmenopausal women, the association was significantly identified among the subjects with a BMI more than 29. It suggested that the risk to develop BC was increased in women with obesity when compared to women in normal.
Although the mechanisms of the heterogeneous association of BMI and BC risk in different menopausal status women are still poorly understood, it is believed that the endogenous estrogen may be involved in the diverse attribution of BMI on BC risk in different menopausal status women33. In premenopausal women, it was reported that there were more frequent anovulatory cycles in obese34 which possibly protect against BC risk; and the clearance of free estrogen in liver was faster in obese than in lean women35. All of these may finally lead to lower levels of both progesterone and estrogen. In contrast, among postmenopausal women, the excess of adipose tissue may elevate the production of endogenous estrogen through the increased activity of enzymes aromatase and 17β-hydroxysteroid dehydrogenase (17β-HSD). In parallel, the decrease of sex-hormone-binding globulin (SHBG) caused by obesity along with the effect of increased formation of oestrone and testosterone may finally promote cellular proliferation and inhibit apoptosis in breast33. No significant association between BMI and BC risk in premenopausal women in our study was consistent with Cheraghi E et al.36 identified. However, it was different from the studies conducted by Renehan, et al.4 and Amadou A et al.37 reported that BMI was significantly associated with decreased BC risk in premenopausal women. These may be due to different methods applied in different meta-analyses. Those previous meta-analyses considered the linear association of BMI and BC risk and did not take into account the inconstant change of BC risk per unit change of BMI. In our study, we first tested the nonlinearity of the association between BMI and BC risk and then identified that BMI was associated with BC risk in a manner of non-linear dose-response in postmenopausal women. For all we know, this was the first report about the non-linear association of BMI and BC risk in postmenopausal women.
Subgroup analyses stratified by ethnicity and follow-up year were performed to explore the possible reasons of heterogeneity in postmenopausal group. However, the between-study heterogeneity was still remained when stratified by ethnicity or follow-up year. Significant non-linear dose-response association of BMI and BC risk was identified in White women. But in Asian subgroup, no significant association was observed. This may due to only three studies were included. The heterogeneous association of BMI and BC risk in different ethnicity may partly due to different genetic background. In addition, the difference in fat distribution, life styles and other BC risk factors in different ethnicities may also modify the association of BMI and BC risk. As for the follow-up, significant non-linear dose-response association between BC risk and BMI was identified in <10 years subgroup. However, no significant association was observed in ≧−10 years subgroup. This may be possibly owing to more complex confounders introduced into the longer follow-up studies.
Despite the clear strength of the current study due to comprehensive analysis strategy, several limitations also should be acknowledged. Obesity could affect BC risk through impacting circulating endogenous estrogen levels. However, we did not assess the modifiable effect of ER and PR status or hormone replacement therapy on BC risk due to the insufficient data available in this meta-analysis. Additionally, the methods used in our study restricted the number of studies included. Furthermore, the definition of menopausal status varied between different cohorts may lead to misclassification bias. Moreover, it is considered that BMI may not be the valid indicator for some people to assess the adiposity. Such as for elderly, the waist-to-hip ratio or waist circumference may be more suitable to predict risk of disease38 since the shift of fat from peripheral to central abdominal sites with an accompanying increase in waist-to-hip ratio was discerned in older people39. In addition, the recent advance in genetic studies had identified many genes and variants were associated with BMI level or BC risk40,41,42 and whether there were interactions between genes and BMI should be further investigated.
In conclusion, the quantitative summary of the accumulated prospective evidence suggest that obesity may act as a risk factor for BC incidence in the manner of non-linear dose-response in postmenopausal women. Although the mechanism of obesity contributes to BC risk is still unclear; it is important to realize that body weight control should be considered as one of the most effective methods to reduce the BC susceptibility.
Methods
Selection of studies
A systematic literature research updated to 30 June 2014 was performed in the PubMed, ISI Web of Science with the language restriction in English and Chinese using the combination of the following on each term: “breast cancer” and “body mass index, overweight, or obesity” to identify eligible studies. In addition, all references listed in the retrieved articles and reviews were also scanned to further identify possible relevant publications.
Studies were eligible for inclusion in the meta-analysis if they satisfied the following criteria: (a) prospective studies evaluated the association between BMI and BC incidence; (b) study population was restricted in female humans; (c) the participants were categorized according to the menopausal status; (d) the exposure of interest was BMI with 3 or more quantitative categories; (e) the studies provide the RRs with 95% CIs and the number of cases and person - years for each BMI categories. When multiple studies had the same or overlapping study populations, only the studies contained the largest sample size or mostly completed were finally included.
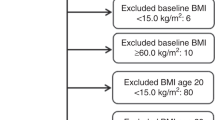
As shown in Figure S1, the systematic literature research identified 5073 records, of which 5007 articles were excluded after review the abstracts or roughly scanning the full texts. Among these articles, 15 articles reported overlapping samples and 5 articles together with another 15 articles lacked BMI level-specific RRs and cases or person-years of each BMI categories were further excluded. Moreover, 9 articles were removed due to the unclear menopausal status of the participants.
Data extraction
The following information was extracted from each of the eligible publications: the name of the first author, publication year, the country in which the study was conducted, the ethnicity of the major participants, years of follow-up and person-years, the number of total BC cases (BC patients identified in the cohort) and the sample size of cohort (sample size of the study), menopausal status (premenopausal-, postmenopausal-), the categories of BMI and RR with 95% CI for each category, the number of case and sample size of each BMI category, the covariates adjusted for in the multivariable analysis. In addition, the ER and PR status of cases which were expected to be extracted were not collected due to the unavailability of the information from each included study. We extracted the RRs with 95% CI that reflected the greatest degree of adjustment for potentially confounding variables. The adjusted RRs were included in the meta-analysis if the studies provided the crude RRs and adjusted RRs. Furthermore, if the results were reported for 2 or more multivariable models, we extracted the RRs that reflected the maximal adjustment for possible confounders. If data was reported separately by age category, study center or HRT use in one publication, they were considered as different studies8,23,25.
The midpoint of the upper and lower boundaries of each category was assigned as the mean BMI to each corresponding RRs of every study. If the upper boundary for the highest category (such as ≧−30) and the lower boundary for the lowest category (such as <18.5) were not provided in the articles, we assumed that the boundary had the same amplitude as the adjacent category43.
Statistical analysis
The dose-response meta-analysis was performed to evaluate a potential non-linear relationship between BMI levels and BC risk. We first applied restricted cubic splines with three knots in settled percentiles (10%, 50% and 90%) of the distribution to model the possible association44. Cubic splines are defined as piecewise-polynomial line segment which was used to present the nonlinearity association of response variable and covariate. And the boundaries of these segments are called knots. Then, the GLST command with the generalized least-squares regression, which required the cases, person-years and mean level of BMI in each category, as well as the BMI level-specific RRs with variance estimated for at least three quantitative categories of each article45 was used to carry out the dose-response meta-analysis. Testing the null hypothesis that the coefficient of the second spline was equivalent to zero was used to evaluate the nonlinearity association between BMI and BC risk46,47. Then, the procedure described by Orsini and Greenland was finally used to estimate the pooled RRs for specific exposure values (per 1 kg/m2 increase from BMI at 18 to 38 kg/m2) when compared to the reference (mean level of the normal BMI range)48. Since the normal BMI of 18.5 - 25 was recommended to maintain a healthy condition by IRAC12, we selected the mean level of normal BMI as the reference.
Heterogeneity among studies was assessed by the Q statistic test and was considered significant when P < 0.149. The fixed-effects model was applied when the heterogeneity was negligible; otherwise, the DerSimonian and Laird random-effects model50 was used in the meta-analysis. The meta-analysis was performed separately in different menopausal status patients. Besides, stratified analysis of ethnicity (Asian, white, or black) and years of follow-up (≧−10 or < 10) were separately conducted. Publication bias was evaluated by the Egger's regression test51. Additionally, sensitivity analysis was performed to assess the stable of results by removing one study each time. All statistical analyses were performed by Stata Software (version 10.0) and P values of two-sided less than 0.05 were considered statistically significant.
References
Carmichael, A. R. & Bates, T. Obesity and breast cancer: a review of the literature. Breast 13, 85–92 (2004).
Flegal, K. M., Carroll, M. D., Ogden, C. L. & Curtin, L. R. Prevalence and trends in obesity among US adults, 1999–2008. JAMA 303, 235–241 (2010).
Wu, Y. F. et al. The current prevalence status of body overweight and obesity in China: data from the China National Nutrition and Health Survey. Zhonghua Yu Fang Yi Xue Za Zhi 39, 316–320 (2005).
Renehan, A. G., Tyson, M., Egger, M., Heller, R. F. & Zwahlen, M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 371, 569–578 (2008).
Jemal, A. et al. Global cancer statistics. CA Cancer J Clin 61, 69–90 (2011).
Suzuki, R., Rylander-Rudqvist, T., Ye, W., Saji, S. & Wolk, A. Body weight and postmenopausal breast cancer risk defined by estrogen and progesterone receptor status among Swedish women: A prospective cohort study. Int J Cancer 119, 1683–1689 (2006).
Kawai, M. et al. Adiposity, adult weight change and breast cancer risk in postmenopausal Japanese women: the Miyagi Cohort Study. Brit J Cancer 103, 1443–1447 (2010).
Sweeney, C., Blair, C. K., Anderson, K. E., Lazovich, D. & Folsom, A. R. Risk factors for breast cancer in elderly women. Am J Epidemiol 160, 868–875 (2004).
Michels, K. B., Terry, K. L. & Willett, W. C. Longitudinal study on the role of body size in premenopausal breast cancer. Arch Intern Med 166, 2395–2402 (2006).
Tehard, B. & Clavel-Chapelon, F. Several anthropometric measurements and breast cancer risk: results of the E3N cohort study. Int J Obesity (Lond) 30, 156–163 (2006).
Kaaks, R. et al. Breast-cancer incidence in relation to height, weight and body-fat distribution in the Dutch "DOM" cohort. Int J Cancer 76, 647–651 (1998).
Bianchini, F., Kaaks, R. & Vainio, H. Weight control and physical activity in cancer prevention. Obes Rev 3, 5–8 (2002).
Ursin, G., Longnecker, M. P., Haile, R. W. & Greenland, S. A meta-analysis of body mass index and risk of premenopausal breast cancer. Epidemiology 6, 137–141 (1995).
Suzuki, R., Orsini, N., Saji, S., Key, T. J. & Wolk, A. Body weight and incidence of breast cancer defined by estrogen and progesterone receptor status--a meta-analysis. Int J Cancer 124, 698–712 (2009).
Galanis, D. J., Kolonel, L. N., Lee, J. & Le Marchand, L. Anthropometric predictors of breast cancer incidence and survival in a multi-ethnic cohort of female residents of Hawaii, United States. Cancer Cause Control 9, 217–224 (1998).
Sonnenschein, E. et al. Body fat distribution and obesity in pre- and postmenopausal breast cancer. Int J Epidemiol 28, 1026–1031 (1999).
Manjer, J., Kaaks, R., Riboli, E. & Berglund, G. Risk of breast cancer in relation to anthropometry, blood pressure, blood lipids and glucose metabolism: a prospective study within the Malmo Preventive Project. Eur J Cancer Prev 10, 33–42 (2001).
Weiderpass, E. et al. A prospective study of body size in different periods of life and risk of premenopausal breast cancer. Cancer Epidemiol Biomarkers Prev 13, 1121–1127 (2004).
Wada, K. et al. Body mass index and breast cancer risk in Japan: a pooled analysis of eight population-based cohort studies. Ann Oncol 25, 519–524 (2014).
Li, H. L., Gao, Y. T., Li, Q. & Liu, D. K. Anthropometry and female breast cancer: a prospective cohort study in urban Shanghai. Zhonghua Liu Xing Bing Xue Za Zhi 27, 488–493 (2006).
Palmer, J. R., Adams-Campbell, L. L., Boggs, D. A., Wise, L. A. & Rosenberg, L. A prospective study of body size and breast cancer in black women. Cancer Epidemiol Biomarkers Prev 16, 1795–1802 (2007).
Manders, P. et al. Body weight and risk of breast cancer in BRCA1/2 mutation carriers. Breast Cancer Res Tr 126, 193–202 (2011).
Cecchini, R. S. et al. Body mass index and the risk for developing invasive breast cancer among high-risk women in NSABP P-1 and STAR breast cancer prevention trials. Cancer Prev Res (Phila) 5, 583–592 (2012).
van den Brandt, P. A., Dirx, M. J., Ronckers, C. M., van den Hoogen, P. & Goldbohm, R. A. Height, weight weight change and postmenopausal breast cancer risk: The Netherlands Cohort Study. Cancer Cause Control 8, 39–47 (1997).
Mellemkjaer, L. et al. Body composition and breast cancer in postmenopausal women: a Danish prospective cohort study. Obesity (Silver Spring) 14, 1854–1862 (2006).
Song, Y. M., Sung, J. & Ha, M. Obesity and risk of cancer in postmenopausal Korean women. J Clin Oncol 26, 3395–3402 (2008).
Kerlikowske, K. et al. Obesity, mammography use and accuracy and advanced breast cancer risk. J Natl Cancer Inst 100, 1724–1733 (2008).
Lacey, J. V., Jr et al. Breast cancer epidemiology according to recognized breast cancer risk factors in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial Cohort. BMC Cancer 9, 84 (2009).
Phipps, A. I. et al. Body size, physical activity and risk of triple-negative and estrogen receptor-positive breast cancer. Cancer Epidemiol Biomarkers Prev 20, 454–463 (2011).
Opdahl, S., Alsaker, M. D., Janszky, I., Romundstad, P. R. & Vatten, L. J. Joint effects of nulliparity and other breast cancer risk factors. Brit J Cancer 105, 731–736 (2011).
Canchola, A. J. et al. Body size and the risk of postmenopausal breast cancer subtypes in the California Teachers Study cohort. Cancer Cause Control 23, 473–485 (2012).
Gaudet, M. M. et al. Waist circumference, body mass index and postmenopausal breast cancer incidence in the Cancer Prevention Study-II Nutrition Cohort. Cancer Cause Control 25, 737–745 (2014).
Calle, E. E. & Kaaks, R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer 4, 579–591 (2004).
Sherman, B., Wallace, R., Bean, J. & Schlabaugh, L. Relationship of body weight to menarcheal and menopausal age: implications for breast cancer risk. J Clin Endocrinol Metab 52, 488–493 (1981).
Siiteri, P. K. et al. The serum transport of steroid hormones. Recent Prog Horm Res 38, 457–510 (1982).
Cheraghi, Z., Poorolajal, J., Hashem, T., Esmailnasab, N. & Doosti Irani, A. Effect of body mass index on breast cancer during premenopausal and postmenopausal periods: a meta-analysis. PLoS One 7, e51446 (2012).
Amadou, A. et al. Overweight, obesity and risk of premenopausal breast cancer according to ethnicity: a systematic review and dose-response meta-analysis. Obes Rev 14, 665–678 (2013).
Pischon, T. et al. Body size and risk of colon and rectal cancer in the European Prospective Investigation Into Cancer and Nutrition (EPIC). J Natl Cancer Inst 98, 920–931 (2006).
Borkan, G. A., Hults, D. E., Gerzof, S. G., Robbins, A. H. & Silbert, C. K. Age changes in body composition revealed by computed tomography. J Gerontol 38, 673–677 (1983).
Frayling, T. M. et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 316, 889–894 (2007).
Chen, W. et al. The SLC4A7 variant rs4973768 is associated with breast cancer risk: evidence from a case-control study and a meta-analysis. Breast Cancer Res Tr 136, 847–857 (2012).
Chen, W. et al. Risk of GWAS-identified genetic variants for breast cancer in a Chinese population: a multiple interaction analysis. Breast Cancer Res Tr 142, 637–644 (2013).
Discacciati, A., Orsini, N. & Wolk, A. Body mass index and incidence of localized and advanced prostate cancer--a dose-response meta-analysis of prospective studies. Ann Oncol 23, 1665–1671 (2012).
Harrell, F. E., Jr, Lee, K. L. & Pollock, B. G. Regression models in clinical studies: determining relationships between predictors and response. J Natl Cancer Inst 80, 1198–1202 (1988).
Orsini, N., Bellocco, R. & Greenland, S. Generalized least squares for trend estimation of summarized dose-response data. The stata journal 6, 40–57 (2006).
Larsson, S. C. & Orsini, N. Coffee consumption and risk of stroke: a dose-response meta-analysis of prospective studies. Am J Epidemiol 174, 993–1001 (2011).
Zhu, B., Zou, L., Qi, L., Zhong, R. & Miao, X. Allium Vegetables and Garlic Supplements Do Not Reduce Risk of Colorectal Cancer, Based on Meta-analysis of Prospective Studies. Clin Gastroenterol Hepatol. 10.1016/j.cgh.2014.03.019 (2014).
Orsini, N. & Greenland, S. A procedure to tabulate and plot results after flexible modeling of a quantitative covariate. The stata journal 11, 1–29 (2011).
Higgins, J. P. & Thompson, S. G. Quantifying heterogeneity in a meta-analysis. Stat Med 21, 1539–1558 (2002).
DerSimonian, R. & Laird, N. Meta-analysis in clinical trials. Control Clin Trials 7, 177–188 (1986).
Egger, M., Davey Smith, G., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 (1997).
Acknowledgements
This work was supported by National Natural Science Foundation of China (Grant No. 81202109) and Union Youth fund of Peking Union Hospital (Grant No. 333201361)
Author information
Authors and Affiliations
Contributions
Conceived and designed the study strategy: J.G.; Acquisition of data, statistical analysis and interpretation of data: W.C., J.Y.L., R.R.; Drafting or revision of the manuscript: W.C., L.L., X.P.X.; Reference collection and data management: X.Q.C., Y.S.; Prepared the tables and figures: W.C., C.L.; Study supervision: J.G., P.Y.; All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Xia, X., Chen, W., Li, J. et al. Body Mass Index and Risk of Breast Cancer: A Nonlinear Dose-Response Meta-Analysis of Prospective Studies. Sci Rep 4, 7480 (2014). https://doi.org/10.1038/srep07480
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep07480
This article is cited by
-
Dose-dependent relation between metformin and the risk of hormone receptor-positive, her2-negative breast cancer among postmenopausal women with type-2 diabetes
Breast Cancer Research and Treatment (2022)
-
The role of obesity and bariatric surgery-induced weight loss in breast cancer
Cancer and Metastasis Reviews (2022)
-
Associations between obesity, metabolic health, and the risk of breast cancer in East Asian women
British Journal of Cancer (2021)
-
Gender Differences in Obesity-Related Cancers
Current Obesity Reports (2021)
-
Effect of increased body mass index on risk of diagnosis or death from cancer
British Journal of Cancer (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.