Abstract
Non-physiological activation of the mineralocorticoid receptor (MR), e.g. by aldosterone under conditions of high salt intake, contributes to the pathogenesis of cardiovascular diseases, although beneficial effects of aldosterone also have been described. The epidermal growth factor receptor (EGFR) contributes to cardiovascular alterations and mediates part of the MR effects. Recently, we showed that EGFR is required for physiological homeostasis and function of heart and arteries in adult animals. We hypothesize that moderate high aldosterone/NaCl, at normal blood pressure, affects the cardiovascular system depending on cardiovascular EGFR. Therefore we performed an experimental series in male and female animals each, using a recently established mouse model with EGFR knockout in vascular smooth muscle cells and cardiomyocytes and determined the effects of a mild-high aldosterone-to-NaCl constellation on a.o. marker gene expression, heart size, systolic blood pressure, impulse conduction and heart rate. Our data show that (i) cardiac tissue of male but not of female mice is sensitive to mild aldosterone/NaCl treatment, (ii) EGFR knockout induces stronger cardiac disturbances in male as compared to female animals and (iii) mild aldosterone/NaCl treatment requires the EGFR in order to disturb cardiac tissue homeostasis whereas beneficial effects of aldosterone seem to be independent of EGFR.
Similar content being viewed by others
Introduction
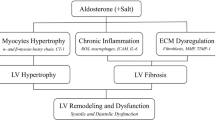
The mineralocorticoid receptor (MR) with its endogenous ligand aldosterone is one of the main effectors in the renin-angiotensin-aldosterone-system and has a pivotal role in water-electrolyte homeostasis and regulation of blood pressure. Recently the MR has gained further interest after the importance of the MR for pathological changes in the cardiovascular system and the kidneys became apparent. In two pivotal clinical studies the beneficial effect of MR antagonists like spironolactone and eplerenone for patients with cardiovascular disease was proven; however, without understanding the underlying mechanisms1,2. Since then, MR activation has been shown to be involved in different pathophysiological effects in the reno-cardiovascular system including endothelial dysfunction, inflammation, hypertrophy and fibrosis in both clinical studies and animal experiments1,3,4,5. The trigger that causes the MR to turn from a receptor regulating water-electrolyte homeostasis and not causing any harm into a receptor mediating pathological effects in the cardiovascular system is still an enigma. One way to achieve this is by having inappropriately high aldosterone levels in relation to a normal salt status in an individual. Although such a scenario is likely in case of hyperaldosteronism caused by adrenal adenoma or hyperplasia, this does not seem to apply for the majority of patients, where aldosterone levels were unremarkable. In animal studies, it is striking that aldosterone application only leads to pathological changes in the presence of additional permissive factors like salt, aging or oxidative stress, in other words a parainflammatory micro-milieu6. Among the plethora of molecules involved in aldosterone effects7 the epidermal growth factor receptor (EGFR) has been attributed major importance especially for the pathological effects of activated MR in the cardiovascular system8,9.
Beside its affore-mentioned detrimental effects aldosterone has also been reported to exert beneficial effects in the cardiovascular system10, e.g. in a mouse model of type 2 diabetes cardiac hyperaldosteronism prevented inflammation, oxidative stress and capillary rarefaction in the heart. The mechanisms mediating these beneficial effects are not completely understood.
EGFR is a receptor tyrosine kinase, that can be activated by its ligands, e.g. EGF, TGF-α, HB-EGF, which derive from neighbouring or the same cell by shedding11,12. But it can also be transactivated by binding of vasoactive substances to their distinct receptor, like angiotensin II13,14, α1- and β-adrenergic agonists15,16 and aldosterone17,18,19 and thereby support their pathophysiological effects in the cardiovascular system13,20. Thus, EGFR - via transactivation - has the potential to mediate signalling of non-EGFR ligands and thereby serve as a heterologous transducer of cellular signalling. Pathophysiological effects of EGFR include parainflammatory dysregulation of tissue homeostasis leading for example to vascular dysfunction and fibrosis as well as electrical remodelling by altered ion channel activity or expression21. Many attempts to delete or overexpress the EGFR family members or their ligands have been made22, revealing the importance of EGFR for perinatal and postnatal development. Mice lacking the EGFR die at day 11.5 of gestation or survive until the postnatal day 20, depending on the genetic background23.
In order to investigate the physiological importance of EGFR in the cardiovascular system further we recently generated a mouse model with simultaneous deletion of the EGFR in cardiomyocytes (CM) and vascular smooth muscle cells (VSMC)24. Under baseline conditions analysis without gender differentiation revealed cardiac hypertrophy, probably as a result of enhanced growth-promoting ROS formation due to greater NOX4 activity. Cardiac output was increased but no signs of fibrosis or inflammation could be observed. Furthermore, a reduced total peripheral vascular resistance and mean blood pressure due to dilated vascular phenotype with minor fibrosis and inflammation in knockout animals was detected. Thus, EGFR besides being a possible heterologous transducer of adverse cardiovascular stimuli it is also required for healthy basal cardiovascular tissue homeostasis24.
In the present study we extend our investigations regarding the cardiovascular importance of EGFR in two dimensions. First, we tested whether the loss of EGFR in CM and VSMCs24 leads to gender-related effects, comparing male and female knockout mice with their wildtype littermates. Second, we tested whether pathological cardiovascular effects of MR-activation by aldosterone depend on EGFR in CM and VSMC, again using the gender-specific approach. Because a growing body of evidence indicates that it is not the aldosterone concentration per se that induces pathological, blood pressure-independent, alterations but an inappropriately high aldosterone concentration in relation to the NaCl status or intake - even at aldosterone concentrations within the “normal” range25,26 -, we establish an experimental setup in which the intake of NaCl is increased and the aldosterone levels are inadequately high but in the physiological range. Furthermore, in our model no iatrogenic reduction of renal function was induced and blood pressure remained in the physiological range. This constellation represents most probably the situation of a large proportion of patients benefiting from MR blocking therapy. Using this model we show that (i) male are more susceptible to inappropriate high aldosterone, (ii) loss of EGFR leads to larger detrimental cardiac effects in male animals and (iii) mild aldosterone/NaCl treatment requires the EGFR in order to disturb cardiac tissue homeostasis whereas beneficial effects of aldosterone seem to be independent of EGFR.
Results
Plasma aldosterone concentrations
Our aim was to reproduce a situation with inappropriately high plasma aldosterone relative to salt intake with absolute aldosterone plasma concentrations in the normal range, because it is known that this is most probably of high clinical prevalence. In order to obtain this condition animals received 1% NaCl in the drinking water - increasing daily NaCl intake from ~10 mg to ~60 mg per animal - and low dose aldosterone infusion from subcutaneous pellets. Analysis of plasma aldosterone concentrations in preparation of this study showed that the hormone levels were not different between the groups (WT: 341.6 ± 54.4 pM, KO: 342.1 ± 79.1 pM, aldosterone/NaCl (WT and KO): 262.0 ± 52.6 pM, N = 7–11 animals/group). Thus, in our experimental system aldosterone levels are indeed inappropriately high although still in a physiological range.
Survival
We have demonstrated before that mortality of the KO animals is higher as compared to WT animals aged > 6 month24. Yet, over the relative short 28d period of aldo/NaCl treatment in the present study, there was no significant difference in mortality for the two genotypes under control conditions (Fig. 1A and B). Aldosterone/NaCl-treatment enhanced mortality in male knockout animals (Fig. 1C) but not in the other groups (Fig. 1D). Treatment resulted in an increase in knockout male mortality by 45.5% over 28d, whereas mortality in knockout female animals was virtually not affected.
Kaplan-Meier-Plots on survival of wildtype and knockout animals depending on gender and treatment.
Mantel-Cox-Test for survival rate was performed revealing that male EGFR-knockout animals had a higher probability to die during the 28 days of aldosterone/NaCl treatment than male animals under control conditions or female EGFR-knockout animals upon aldosterone/NaCl treatment. a) survival of male wildtype and knockout animals during 28 days of control treatment (N (WT) = 9, N(KO) = 8), b) survival of female wildtype and knockout animals during 28 days of control treatment (N (WT) = 9, N(KO) = 7), c) survival of male wildtype and knockout animals during 28 days of aldosterone/NaCl treatment (N (WT) = 10, N(KO) = 9) and d) survival of female wildtype and knockout animals during 28 days of aldosterone/NaCl treatment (N (WT) = 9, N(KO) = 11).
As aldosterone can induce the expression of EGFR we analyzed EGFR mRNA expression via realtime qRT PCR. Inadequately high aldosterone/salt treatment indeed increased the expression of EGFR in the heart of male wildtype (196% of control, 95%CI: 140–252%, n = 9–11 animals/group) but not of female wildtype or the knockout animals. The expression of the other ErbB-family members, namely ErbB2-4, was not affected.
Organ weight
To rule out growth defects caused by the knockout of EGFR and as heart failure can lead to lung congestion organ weights were evaluated. There was no difference in body (Fig 2A and 2D) and lung weight (Fig 2C and 2F) for any of the four groups of both genders. As predicted from a previous study, heart weight was enhanced in knockout animals of the control groups (Fig 2B and 2E). In extension to previous studies, the data presented here show that the degree of cardiac hypertrophy is significantly higher in male knockout animals (306 ± 22% of WT for male KO animals; 218 ± 23% of WT for female KO animals, N = 8–10). In order to gain more insight into the time course of hypertrophy development we investigated heart size of newborn animals of either gender (N = 5 for each group). Heart weight per body weight was 5.73 ± 0.18 mg/g for male and 5.64 ± 0.16 mg/g for female wildtype animals. The values for knockout animals were 7.98 ± 0.26 mg/g (male) and 8.04 ± 0.52 mg/g (female). Thus, there is a similar (~35%) cardiac hypertrophy at birth in male and female knockout animals. After birth hypertrophy aggravates, yet to a greater extend in male animals as compared to females.
Effect of genotype and treatment on organ weights in male and female animals.
Wildtype and knockout animals were either untreated or treated with aldosterone/NaCl for 28 days. Afterwards the animals were sacrificed and the body weight (a, d), heart weight/tibia length (b, e) and lung weight/tibia length (c, f) were determined with respect to gender. Data are given as mean ± SEM, * p<0.05 compared to respective wildtype, # p<0.05 compared to respective control, N = 9–12 animals/group.
Treatment with aldosterone/NaCl did not affect heart weight with statistical significance in any of the groups, however hypertrophy in male KO animals was reduced by ~25% with p = 0.09 (Fig. 2B).
The slight increase in lung weight in male knockout animals results most probably from the strong cardiac hypertrophy with enhanced enddiastolic volume24.
Tissue analysis
To analyze gross morphological changes underlying the observed heart hypertrophy cardiomyocyte diameter, fibrosis (SR staining) and apoptosis (caspase-3 activity) were determined. Cardiomyocyte diameter was enhanced in knockout animals of both gender (Fig. 3 A and 3D). Aldosterone/NaCl treatment exerted not significant effects in wildtype animals but abolished cell hypertrophy in EGFR knockout, irrespective of gender, indicating a beneficial effect of the treatment.
Effect of genotype and treatment on cardiomyocyte diameter, caspase-3 activity and fibrosis depending on gender.
Wildtype and knockout animals were either untreated or treated with aldosterone/NaCl for 28 days. Afterwards the animals were sacrificed and the hearts were either partially embedded with paraffin or snap frozen for further analysis. Cardiomyocyte diameter (a, d) was determined in haematoxylin/eosin stained slices. At least 100 cardiomyocytes were analysed per mouse. Cardiac fibrosis was determined in picro sirius red stained, paraffin embedded heart sections as % red stained area per field in male (b) and female (e) animals. Cardiac caspase-3 activity was determined from snap frozen cardiac homogenates in male (c) and female (f) animals. Data are given as mean ± SEM, * p<0.05 compared to respective wildtype, # p<0.05 compared to respective control. N = 9–12 animals/group.
The fractions of fibrotic tissue were identical in between the different groups of both genders (Fig. 3B and 3E), which is not surprising, because histomorphologically detectable changes during mild pathological stimulation occur only after prolonged exposure.
In contrast, cardiac caspase-3 activity (Fig. 3C and 3F) was reduced in knockout animals of both genders as compared to sex-matched wildtype animals. Furthermore, aldosterone/NaCl reduced caspase-3-activity in male wildtype but not in female wildtype animals as shown in figure 3, again indicating a certain protective effect of treatment. As aldosterone/NaCl-treatment did not enhance the effect of EGFR-KO we conclude that the effect of aldo/NaCl requires the EGFR.
Blood pressure, heart rate, electrocardiography
Aldosterone influences blood pressure via the regulation of salt/water homeostasis, therefore systolic blood pressure via tail-cuff was determined. As expected in a situation of moderate high aldosterone/NaCl with intact renal function, neither systolic blood pressure nor heart rate were altered significantly (male: Fig. 4A and 4B, female: Fig. 4C and 4D) in any of the groups. These data confirm that we are investigating blood pressure independent effects.
Effect of genotype and treatment on systolic blood pressure and heart rate depending on gender.
Systolic blood pressure (a, c) and heart rate (b,d) was measured in 5–7month-old animals for 5 consecutive days (0 d) using external tail pulse detection. After this time period aldosterone releasing pellets were implanted subcutaneously in the back of the animals. After a three day recovery period blood pressure and heart rate were measured twice weekly for additional 28 days. Data are given as mean ± SEM, * p<0.05 compared to respective wildtype, # p<0.05 compared to respective control. N = 9–12 animals/group.
Electrocardiography was performed to analyze the electrical conduction in the heart. Electrical conduction (P-duration, PR-, QRS and QTc-interval) did not differ between the female groups (Fig. 5E–H), by contrast to the male groups (Fig. 5A–D), where EGFR-KO caused prolonged P-duration and QRS- as well as QTc-intervals (and a PR-prolongation in tendency) that were reversed by aldosterone/NaCl-treatment. The observed prolongation mirrors at least in part the cardiac hypertrophy.
Effect of genotype and treatment on electrocardiography parameters distinguished by gender.
After 28days with or without aldo/NaCl treatment animals were anaesthetized with isoflurane and electrocardiography recordings were obtained for at least ten minutes employing an Einthoven I limb lead recording. PR interval (a, e) was measured from the beginning of the P wave to the start of the chamber complex, P duration (b, f) was defined as the time from start to end of the P wave, QRS interval (c, g) was measured from start to end of the chamber excitation and the QTc interval (d,h; time from start of the chamber excitation to the end of the chamber repolarization, heartrate corrected according to Bazette). Data are given as mean ± SEM, * p<0.05 compared to respective wildtype, # p<0.05 compared to respective control. N = 3-5 animals/group.
Of note, aldosterone/NaCl-treatment induced a PR-interval prolongation in male wildtype but not in male knockout or female animals (Fig. 5A and 5E). PR-interval prolongation corresponds to enhanced PQ-intervals in patients with hyperaldosteronism27 and in mice with cardiac MR overexpression4, substantiating the validity of our model.
The parameters for heart rate variability were not different in the female groups (Fig. 6B). In contrast, all three parameters were significantly enhanced by aldosterone/NaCl in male EGFR knockout animals (Fig. 6A, C and D), leading to an increased heart rate variability.
Effect of genotype and treatment on heart rate variability distinguished by gender.
After 28 days with or without aldo/NaCl treatment animals were anaesthetized with isoflurane and electrocardiography recordings were obtained for at least ten minutes employing an Einthoven I limb lead recording, from these recordings heart rate variability was determined. SDNN (a, b), as standard deviation of RR interval of normal-to-normal intervals was analysed first. As no difference could be obtained in female animals, SD delta NN (standard deviation of averages of normal R-R intervals, c) and RMSSD (Square root of the mean of the sum of the squares of differences between adjacent NN intervals, d) was only analysed in male animals. Data are given as mean ± SEM, * p<0.05 compared to respective wildtype, # p<0.05 compared to respective control. N = 3–5 animals/group.
mRNA expression
In the heart of male knockout animals (Fig. 7) an increase in the expression of marker genes for hypertrophy (Nppa and Nppb), fibrosis (Col1a1, Fn1 and Serpine1) and ROS balance (Nos1 and Nox4) was observed under control conditions compared to wildtype. In contrast, expression of Ccl2, Tnf or Tgfb1 was not altered, arguing against a proinflammatory phenotype.
Alteration of marker gene expression in hearts of male animals regarding genotype and treatment.
After 28 days with or without aldo/NaCl treatment animals were sacrificed, the hearts dissected and snap frozen in liquid nitrogen. Gene expression was analysed according to the -Δ/Δct- method normalizing the individual mRNA amount to 18S and the mean mRNA amount in the untreated wildtype. In each graph the Δ/Δct amount is given on the left y-axis while the corresponding fold change in expression is given on the right y-axis. Data are given as mean ± SEM, * p<0.05 compared to respective wildtype, # p<0.05 compared to respective control. N = 6–11 animals/group.
Aldosterone/NaCl treatment increased the expression of marker genes for fibrosis (Col1a1, Col3a1 and Serpine1) and ROS balance (Nos1) in hearts of wildtype males (Fig. 7).
In the heart of male knockout animals (Fig. 7) aldosterone/NaCl treatment prevented the increase in Nppb expression, indicating again an EGFR-independent protective effect. On the other side, the expression of none of the genes affected in male wildtype animals was altered in male knockouts upon aldosterone/NaCl treatment, suggesting that EGFR is required for at least some of the detrimental effects of aldosterone/NaCl.
In the aorta of male animals (Supplementary figure S1), with the exception of Ccl2, no changes in mRNA expression were observed in wildtype or knockout animals with or without treatment.
In the hearts (Fig. 8) of female knockout animals the expression of Nppa, Nppb and Nos1 was enhanced compared to wildtype females. The expression of all other mRNAs investigated was not altered.
Alteration of marker gene expression in hearts of female animals regarding genotype and treatment.
After 28 days with or without aldo/NaCl treatment animals were sacrificed, the hearts dissected and snap frozen in liquid nitrogen. Gene expression was analysed according to the -Δ/Δct- method normalizing the individual mRNA amount to 18S and the mean mRNA amount in the untreated wildtype. In each graph the Δ/Δct amount is given on the left y-axis while the corresponding fold change in expression is given on the right y-axis. Data are given as mean ± SEM, * p<0.05 compared to respective wildtype, # p<0.05 compared to respective control. N = 6–12 animals/group.
Aldosterone/NaCl-treatment exerted no significant effect on mRNA expression in hearts of female wildtype and female knockout animals (Fig. 8).
In the aortae (Supplementary figure S2) of female animals no major change in mRNA expression was observed for any of the groups.
Discussion
In the present study we investigate the cardiovascular impact of the clinical highly relevant constellation of normal plasma aldosterone levels despite enhanced NaCl intake, independent of blood pressure. In this constellation, achieved by feeding a 1% NaCl drinking solution (leading to an additional NaCl intake of >50 mg per day, which corresponds to a total NaCl intake of ~600% of control animals) and clamping plasma aldosterone to physiological values by continuous infusion with subcutaneous pellets, aldosterone is inappropriately high, because normally enhanced NaCl intake suppresses aldosterone secretion. The model refrains from partial kidney removal and is not accompanied by blood pressure elevations within the observation period of 28 days as expected, because it had been demonstrated that in mice28,29 and rats30 without nephrectomy even the treatment with higher salt concentrations does not result in an increase in blood pressure31. Thus, the model allows the investigations of effects elicited by inappropriately high aldosterone-to-NaCl concentrations, known to lead to inappropriate MR activation32. Furthermore, the dimension of increase in NaCl intake in the treated groups represents a known risk factor for cardiovascular morbidity and mortality in humans33.
Effects of Aldosterone/NaCl in wildtype animals
According to our data a moderate inappropriate high aldosterone/NaCl constellation induces a mild profibrotic cardiac phenotype with slowed atrio-ventricular conduction in hearts of male animals. In extension to previous studies, that reported a higher susceptibility of male animals under conditions of extensively increased aldosterone/NaCl in combination with reduced kidney function34,35,36, we now show that female animals are apparently not affected at all by mildly elevated aldosterone/NaCl, at least during the experimental period used in this study. Thus, we report, to our knowledge for the first time, that in the absence of changes in blood pressure and at preserved kidney function male but not female animals are affected by aldosterone/NaCl. Under our experimental conditions and with respect to the parameters investigated, the effects were restricted to the heart without significant alterations in the aorta. Of course we cannot exclude that treatment over a period exceeding 28 days would also affect the vasculature.
The prolonged PR interval is in line with the observations that (i) in uninephrectomized rats receiving aldosterone intraatrial and atrioventricular conduction are slower compared to untreated animals37, (ii) mice with cardiac MR overexpression4 also show extended PR intervals and (iii) human patients with hyperaldosteronism have longer PQ-intervals as compared to patients with essential hypertension27. It was hypothesized, that hyperaldosteronism might lead to site-specific conduction delay due to atrial anisotropy and thereby stabilize or promote atrial arrhythmias. There is additional evidence from humans, that the mineralocorticoid receptor might be involved in the onset of atrial fibrillation38,39. The pro-arrhythmogenic effect of aldosterone, suggested by several studies, most probably results from the cellular consequences of cardiac mineralocorticoid receptor activation, like disturbed control of cellular calcium homeostasis, action potential lengthening, alteration of calcium and potassium conductance and sarcoplasmic reticulum diastolic leaks40. In order to refine the analysis of electrical heart activity telemetrical studies will be performed in the future.
Effect of EGFR KO
In the last years our group, among others, demonstrated that the activated mineralocorticoid receptor is able to increase EGFR expression and can transactivate the EGFR8,17,18. Therefore we aimed to analyse the contribution of the EGFR to the aldo/NaCl induced cardiovascular alterations.
The ErbB-receptor family and its role in the heart gained interest as the anti-cancer drug herceptin (ErbB2-receptor antagonist) induced a dilative cardiomyopathy in a subset of patients14. Cardiac phenotypes in transgenic mice lacking HB-EGF41,42, or with a mutant EGFR43, developing cardiomyopathy, cardiac hypertrophy and premature death have been reported, indicating that beside ErbB2, EGFR is also important. Recently, we introduced the genetic mouse model with EGFR deletion in vascular smooth muscle cells and cardiomyocytes which now allows investigating the role of EGFR under pathophysiological conditions more specifically than using pharmacological inhibition24. In light of the above mentioned gender differences in wildtype animals we first had to perform a gender analysis with respect to VSMC- and CM-EGFR knockout. Our data show, to our knowledge for the first time, that the importance of cardiac EGFR is more pronounced in male as compared to female animals. Adult female animals are much less affected by EGFR-KO with respect to cardiac hypertrophy, cardiac and aortic parainflammation as well as electrical remodelling, indicating that the EGFR is of greater importance for tissue homeostasis in adult males. By contrast, cardiac hypertrophy in newborn animals was significantly smaller as compared to adult animals and there was no gender difference. These data indicate that more than one mechanism contributes to cardiac pathophysiology. Prenatally, there is a gender-independent mechanism, indicating that not the sex of the cardiomyocyte per se is responsible for part of the hypertrophy. Postnatal, hypertrophy aggravates, yet now with a significant gender difference, indicating that sex hormones may play a role, with androgens acting prohypertrophic. According to our data, it is conceivable that EGFR deletion leads to cardiac hypertrophy driven by at least two mechanisms, one gender-independent the other gender-dependent. The underlying molecular mechanisms of these effects will be the subject of future studies.
Recently we showed, that cardiac EGFR deletion leads to an increase of NADPH-oxidase 4 (NOX4) expression and activity24 with enhanced ROS formation that presumably exerts the hypertrophic growth in adult animals. By contrast to adult animals, in the hearts of newborn animals we were not able to detect an enhanced NOX4-expression (84 ± 10% of wildtype, N = 5). Thus, prenatal gender-independent hypertrophy does not result from altered NADPH-oxidase expression. One mechanism for the postnatal gender-dependent hypertrophy, might be the gender-dependent increase in NOX4 expression (303 ± 57% of wildtype in male; 174 ± 33% of wildtype in female; figures 7 and 8), that can be explained by the stimulatory effect of androgens on NOX4 expression44. Additionally, the protective cardiovascular effects of oestrogens leading to greater non-specific resilience, e.g. to ROS, may also contribute. Furthermore, it is also conceivable that cardiac EGFR modulates cardiac androgen and oestrogen signalling – the receptors of both being able to interact with EGFR activity and/or expression – differently, resulting in sex hormone dependent cardiac phenotype after EGFR deletion. EGFR knockout could lead to a disinhibition especially of androgen-driven effects, by e.g. enhanced androgen receptor expression or insulin-like growth factor-1 signalling45,46.
From the clinical point of view our data suggest the need of more detailed investigations of side-effects during EGFR-targeted pharmacotherapy. In line with our findings, Barrick et al (2008)47 demonstrated that long-term treatment with EGFR-inhibitor (e.g. AG1478 or EKB-569) leads to a significant decrease in cardiovascular function. Taken, together with our data, these side-effects resulted, at least in part, from inhibition of cardiomyoctye EGFR.
The enhanced atrial and ventricular conduction times in male knockout mice are most probably not entirely attributable to enhanced heart size, because female knockout animals also presented with substantial cardiac hypertrophy, albeit not as extreme as male animals. The interaction of EGFR with cardiac ion channels (e.g. Kir3.1, Kv4.3) has been shown and therefore a lack of EGFR activity is expected to alter cardiac action potentials48,49. The enhanced conduction time combined with the increased heart size and the prolonged QTc-interval represent a high risk constellation for cardiac arrhythmias due to reentries, that may explain the previously reported increase in mortality in animals aged >6 month. In line with this conclusion are previous reports showing a contribution of EGFR to cardiac arrhythmias due to the modulation of Na+ and Ca2+-channel activity50. In future telemetry studies we will analyse also heart arrhythmia.
Effects of Aldosterone/NaCl in knockout animals
In male knockout animals aldosterone/NaCl treatment induced (i) a partial beneficial effect with respect to cardiac hypertrophy and altered intraatrial and intraventricular conduction induced by EGFR deletion, (ii) an increase in heart rate variability but (iii) no further increase of the profibrotic/parainflammatory phenotype compared to controls. According to these findings we propose that the detrimental effects aldosterone/NaCl exerts in the heart dependent, at least in part, on EGFR. On the contrary, the beneficial cardiac effects appear independent of EGFR. Recently, Akt-mediated cardioprotective effects of mineralocorticoid receptor activation have been described10. If these effects were EGFR-independent, they could explain also the beneficial effect observed in our study. Of course, this hypothesis needs further investigation in the future.
The development of heart fibrosis and hypertrophy induced by aldosterone has been described to depend on a high salt diet51,52, but the mechanisms involved are still under discussion53. Our data add another piece to the mechanistic puzzle of pathological MR action, namely the EGFR. At present we cannot quantify the relative importance of enhanced activation of preexisting EGFR and enhanced expression of EGFR as the underlying molecular mechanisms. The fact that cardiac EGFR-expression was enhanced in WT animals by aldosterone/NaCl suggests that this mechanism plays at least a partial role for the detrimental effects.
In conclusion, our data demonstrate that EGFR and moderately high aldosterone/NaCl seem to elicit Ying and Yang-like effects: While EGFR has a beneficial effect on physiological tissue homeostasis in hearts and aortas of male and female animals; it mediates at least some of the detrimental effects of high aldosterone-to-NaCl intake in male animals. On the other hand, high aldosterone-to-NaCl besides is detrimental effect also elicits beneficial effects in the heart, but presumably not mediated by the EGFR.
Methods
Ethics statement
All mouse experiments described in this manuscript were approved by the local government (Landesverwaltungsamt Sachsen-Anhalt, Germany, permit number: 203.k-42502-2-1039 MLU G) and were performed according to the guidelines of the American Physiological Society.
Generation of EGFRΔ/Δ VSMC mice
Generation of the mice and baseline phenotype were described before24,54. Mice were kept in the facilities of the University of Halle-Wittenberg at a 12 h/12 h light/dark cycle with a room temperature of 20 ± 1°C. EGFRΔ/ΔVSMC&CM mice were generated by mating C57BL/6 mice containing floxed EGFR alleles (EGFRflox/flox) to SM22-Cre mice. Genotyping was performed on tail biopsies by PCR as described before24. All animals were homozygous for the EGFR flox allele (EGFRflox/flox) and either heterozygous for the SM22-Cre allele (SM22-CRE+/-; EGFRΔ/ΔVSMC&CM = KO) or negative for the SM22-Cre allele (SM22-Cre-/-, WT).
Measurement of blood pressure
5-7month-old animals were trained 14 d before starting the blood pressure measurement for 5 consecutive days (0 d) using external tail pulse detection (ADI instruments, Spechbach, Germany) in awake animals. After this time period aldosterone releasing pellets (Innovative Research of America, Sarasota, USA) were implanted subcutaneously in the back of the animals under isoflurane anaesthesia (2%, 1 L/min O2). After a three day recovery period blood pressure and heart rate were measured twice weekly for additional 28 days. Release rate of aldosterone pellets was chosen to increase endogenous aldosterone levels to physiological plasma concentrations of mice even under high salt diet (see below).
Plasma aldosterone levels
The daily aldosterone synthesis rate of mice can be estimated from the known plasma half-life (t0,5 = 10–20 minutes), the approximate distribution volume (VD = 3–6 ml per animal) and the plasma concentration (CP = 100–200 ng/l) according to the formula synthesis rate = VD × CP × (ln2/t0,5) to range between 20 to 120 ng per day. In order to achieve a similar aldosterone application rate we used the above mentioned pellets that are supposed to release ~220 ng/d as determined by pre-tests in vitro. Our results show that by these means the animals maintained physiological plasma aldosterone concentrations despite consuming water containing 1% NaCl, known to reduce plasma aldosterone to ~1/3 of controls55.
Blood was collected after 28 d of treatment via retroorbital puncture and anticoagulated with Na-citrate. After centrifugation (1.500 g, 10 min) plasma was removed from the reaction tube and transferred into a new 1.5 ml reaction tube. Immediately thereafter the plasma was snap frozen in liquid nitrogen. For plasma aldosterone estimation plasma was thawed and an ELISA was performed according to the manufacturer's protocol (IBL, Hamburg).
Electrocardiography
5–7 month old animals were anaesthetised with isoflurane 2% (1 L/min O2) and electrocardiography recordings were obtained for at least ten minutes employing AD Instruments LabChart equipment and an Einthoven I limb lead recording. The following parameters were analysed for standard ECG evaluation: PR interval (measured from the beginning of the P wave to the start of the chamber complex, a measure for the conduction time), P duration (time from start to end of the P wave, a measure for the dispersion of the excitation in the atria), QRS interval (time from start to end of the chamber excitation, a measure for the dispersion of the excitation in the chambers) and QTc interval (time from start of the chamber excitation to the end of the chamber repolarization, heart rate corrected according to Bazette, QTc = QT interval/√RR-interval). Heart rate variability was analysed in the above mentioned ECG tracings via the time-domain analysis according to Thireau et al (2008)56 with ΔNN = 6 ms. The following parameters were analysed: SDNN (standard deviation of RR interval of normal-to-normal intervals, a representation of overall heart rate variability), SD delta NN (standard deviation of averages of normal R-R intervals), RMSSD (Square root of the mean of the sum of the squares of differences between adjacent NN intervals).
Harvesting of organs and cardiomyocytes
Immediately after death, livers, kidneys, lungs, hearts and aortas were excised, carefully freed from adjacent tissue and partially weighed. Subsequently, the left ventricle was separated and also weighed. Furthermore, tibia length was measured for normalization of organ weights. Part of the kidneys and left ventricles were immediately snap frozen in liquid nitrogen for later RNA extraction while parts were stored in 3% paraformaldehyde solution for fixation. Paraformaldehyde fixated tissues were dehydrated by bathing in increasing concentrations of methanol or isopropanol, respectively. After embedding in paraffin, 3 µm sections were cut.
Microscopic analysis of hearts
The degree of interstitial fibrosis in hearts was determined by evaluation of Sirius Red stained area from at least 10 microscopic fields per animal, utilizing a point counting technique57. To analyse cardiomyocyte diameter haematoxylin/eosin stained crosssections of the left chamber were used. At least 100 cardiomyocytes were analysed per mouse.
Morphometric analysis was performed with a Keyence Biozero Fluorescence Microscope with 20× magnification. Images were analysed with the BZ image analysis software and ImageJ.
Caspase-3-activity
Caspase-3 activity was measured as described previously58. Snap frozen hearts were incubated with 100 µl cell lysis buffer (10 mmol/l TRIS, 100 mmol/l NaCl, 1 mmol/l EDTA, 0.01% Triton X-100, pH 7.5) for 10 min on ice and centrifuged at 16000 g for 10 min at 4°C. 60 µl of the supernatant was incubated with 65 µl reaction buffer (20 mmol/l PIPES, 4 mmol/l EDTA, 0.2% CHAPS, 10 mmol/l DTT, pH 7.4) containing 42 µmol/l DEVD-AFC (end-concentration) at 37°C and fluorescence of the cleaved product, 7-amino-4-trifluoromethylcoumarin (AFC), was measured at 400 nm excitation and 505 nm emission wavelength using a multiwell-multilabel counter (Infinite, Tecan, Berlin). Cleaved AFC was quantified by a calibration curve using known AFC concentrations. Protein content was determined with bicinchoninic acid assay (Interchim, Montluçon, France) using bovine serum albumin as standard.
Gene expression
Gene expression analysis was carried out as described before24,54. RNA was isolated using the RNeasy Mini Kit from Qiagen (Hilden, Germany) according to the manufacturer's instructions. Subsequently residual remaining DNA was digested with DNase I. Reverse transcription was performed with 1 μg RNA and random primers using qScript from Quanta Biosciences according to the manufacturers' protocol. Additionally each sample was analysed without reverse transcription. The signals obtained without RT were neglectable (<1%). Finally, realtime amplification was performed with the Stratagene Mx3005P using the Platinum SYBR Green kit (Invitrogen, Karlsruhe, Germany) according to the manufacturer's instructions. Primer sequences, names of the corresponding proteins, ref-sequences, as well as annealing temperature and fragment length are given in supplementary table S1. qPCR efficiency was >90%. All primers for genes of interest are intron-spanning and have been validated by RT-PCR and gel electrophoresis. Furthermore, the primers were validated by melting curve analysis. The relative expression of the two genes of interest was calculated using the 18S signal for normalization. Each sample was analysed as triplicate. All values are expressed as mean ± standard error of mean normalized to the wildtype control group.
Materials
Unless otherwise stated all materials were purchased from Sigma, Munich, Germany.
Statistical analysis
The data are presented as mean ± standard error of mean (SEM). Anova, followed by post hoc testing and Mantel-Cox (for survival rates) were applied. A p-value <0.05 was considered significant.
References
Pitt, B. et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N. Engl. J. Med. 341, 709–717 (1999).
Pitt, B. et al. The EPHESUS trial: eplerenone in patients with heart failure due to systolic dysfunction complicating acute myocardial infarction. Eplerenone Post-AMI Heart Failure Efficacy and Survival Study. Cardiovasc. Drugs Ther. 15, 79–87 (2001).
Nakano, S., Kobayashi, N., Yoshida, K., Ohno, T. & Matsuoka, H. Cardioprotective mechanisms of spironolactone associated with the angiotensin-converting enzyme/epidermal growth factor receptor/extracellular signal-regulated kinases, NAD(P)H oxidase/lectin-like oxidized low-density lipoprotein receptor-1 and Rho-kinase pathways in aldosterone/salt-induced hypertensive rats. Hypertens. Res. 28, 925–936 (2005).
Ouvrard-Pascaud, A. et al. Conditional mineralocorticoid receptor expression in the heart leads to life-threatening arrhythmias. Circulation. 111, 3025–3033 (2005).
Pitt, B. Effect of aldosterone blockade in patients with systolic left ventricular dysfunction: implications of the RALES and EPHESUS studies. Mol. Cell Endocrinol. 217, 53–58 (2004).
Gekle, M. & Grossmann, C. Actions of aldosterone in the cardiovascular system: the good, the bad and the ugly? Pflugers Arch. 458, 231–246 (2009).
Grossmann, C. & Gekle, M. New aspects of rapid aldosterone signaling. Mol. Cell Endocrinol. 308, 53–62 (2009).
Grossmann, C. & Gekle, M. Interaction between mineralocorticoid receptor and epidermal growth factor receptor signaling. Mol. Cell Endocrinol. 350, 235–241 (2012).
Yavas, G., Elsurer, R., Yavas, C., Elsurer, C. & Ata, O. Does spironolactone ameliorate trastuzumab-induced cardiac toxicity? Med. Hypotheses. 81, 231–234 (2013).
Fazal, L. et al. Akt-mediated cardioprotective effects of aldosterone in type 2 diabetic mice. FASEB J. 28, 2430–2440 (2014).
Hackel, P. O., Zwick, E., Prenzel, N. & Ullrich, A. Epidermal growth factor receptors: critical mediators of multiple receptor pathways. Curr. Opin. Cell Biol. 11, 184–189 (1999).
Prenzel, N. et al. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 402, 884–888 (1999).
Kagiyama, S. et al. Angiotensin II-induced cardiac hypertrophy and hypertension are attenuated by epidermal growth factor receptor antisense. Circulation. 106, 909–912 (2002).
Smith, N. J., Chan, H. W., Osborne, J. E., Thomas, W. G. & Hannan, R. D. Hijacking epidermal growth factor receptors by angiotensin II: new possibilities for understanding and treating cardiac hypertrophy. Cell Mol. Life Sci. 61, 2695–2703 (2004).
Noma, T. et al. Beta-arrestin-mediated beta1-adrenergic receptor transactivation of the EGFR confers cardioprotection. J. Clin. Invest. 117, 2445–2458 (2007).
Ulu, N. et al. alpha1-Adrenoceptor-mediated contraction of rat aorta is partly mediated via transactivation of the epidermal growth factor receptor. Br. J. Pharmacol. 161, 1301–1310 (2010).
Grossmann, C. et al. Human mineralocorticoid receptor expression renders cells responsive for nongenotropic aldosterone actions. Mol. Endocrinol. 19, 1697–1710 (2005).
Grossmann, C. et al. Aldosterone-induced EGFR expression: interaction between the human mineralocorticoid receptor and the human EGFR promoter. Am. J. Physiol Endocrinol. Metab. 292, E1790–E1800 (2007).
Kagiyama, S. et al. Aldosterone-and-salt-induced cardiac fibrosis is independent from angiotensin II type 1a receptor signaling in mice. Hypertens. Res. 30, 979–989 (2007).
Asakura, M. et al. Cardiac hypertrophy is inhibited by antagonism of ADAM12 processing of HB-EGF: metalloproteinase inhibitors as a new therapy. Nat. Med. 8, 35–40 (2002).
Pullar, C. E. & Isseroff, R. R. The beta 2-adrenergic receptor activates pro-migratory and pro-proliferative pathways in dermal fibroblasts via divergent mechanisms. J. Cell Sci. 119, 592–602 (2006).
Sibilia, M. et al. The epidermal growth factor receptor: from development to tumorigenesis. Differentiation. 75, 770–787 (2007).
Sibilia, M. & Wagner, E. F. Strain-dependent epithelial defects in mice lacking the EGF receptor. Science. 269, 234–238 (1995).
Schreier, B. et al. Loss of epidermal growth factor receptor in vascular smooth muscle cells and cardiomyocytes causes arterial hypotension and cardiac hypertrophy. Hypertension. 61, 333–340 (2013).
Rossi, G. P. et al. Primary aldosteronism: cardiovascular, renal and metabolic implications. Trends Endocrin. Met. 19, 88–90 (2008).
Catena, C., Colussi, G. & Sechi, L. A. Aldosterone, organ damage and dietary salt. Clin. Exp. Pharmacol. Physiol. 40, 922–928 (2013).
Rossi, G. P. et al. Excess aldosterone is associated with alterations of myocardial texture in primary aldosteronism. Hypertension. 40, 23–27 (2002).
Pruthi, D. et al. Aldosterone promotes vascular remodeling by direct effects on smooth muscle cell mineralocorticoid receptors. Arterioscler. Thromb. Vasc. Biol. 34, 355–364 (2014).
McCurley, A. et al. Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors. Nat. Med. 18, 1429–1433 (2012).
Kittikulsuth, W., Looney, S. W. & Pollock, D. M. Endothelin ET(B) receptors contribute to sex differences in blood pressure elevation in angiotensin II hypertensive rats on a high-salt diet. Clin. Exp. Pharmacol. Physiol. 40, 362–370 (2013).
Kim, S. M. et al. Salt sensitivity of blood pressure in NKCC1-deficient mice. Am. J. Physiol. Renal Physiol. 295, F1230–F1238 (2008).
Shibata, H. & Itoh, H. Mineralocorticoid receptor-associated hypertension and its organ damage: clinical relevance for resistant hypertension. Am. J. Hypertens. 25, 514–523 (2012).
Alderman, M. H. & Cohen, H. W. Dietary sodium intake and cardiovascular mortality: controversy resolved? Am. J. Hypertens. 25, 727–734 (2012).
Crofton, J. T. & Share, L. Gonadal hormones modulate deoxycorticosterone-salt hypertension in male and female rats. Hypertension. 29, 494–499 (1997).
Bayorh, M. A., Socci, R. R., Eatman, D., Wang, M. & Thierry-Palmer, M. The role of gender in salt-induced hypertension. Clin. Exp. Hypertens. 23, 241–255 (2001).
Ouchi, Y., Share, L., Crofton, J. T., Iitake, K. & Brooks, D. P. Sex difference in the development of deoxycorticosterone-salt hypertension in the rat. Hypertension. 9, 172–177 (1987).
Reil, J. C. et al. Aldosterone promotes atrial fibrillation. Eur. Heart J. 33, 2098–2108 (2012).
Swedberg, K. et al. Eplerenone and atrial fibrillation in mild systolic heart failure: results from the EMPHASIS-HF (Eplerenone in mild patients hospitalization and survival study in heart failure) Study. J. Am. Coll. Cardiol. 59, 1598–1603 (2012).
Mentz, R. J. et al. The past, present and future of renin-angiotensin aldosterone system inhibition. Int. J. Cardiol. 167, 1677–1687 (2013).
Gravez, B., Tarjus, A. & Jaisser, F. Mineralocorticoid receptor and cardiac arrhythmia. Clin. Exp. Pharmacol. Physiol. 40, 910–915 (2013).
Iwamoto, R. et al. Heparin-binding EGF-like growth factor and ErbB signaling is essential for heart function. Proc. Natl. Acad. Sci. U. S. A. 100, 3221–3226 (2003).
Iwamoto, R. & Mekada, E. ErbB and HB-EGF signaling in heart development and function. Cell Struct. Funct. 31, 1–14 (2006).
Chen, B. et al. Mice mutant for egfr and shp2 have defective cardiac semilunar valvulogenesis. Nat. Genet. 24, 296–299 (2000).
Chignalia, A. Z. et al. Testosterone induces vascular smooth muscle cell migration by NADPH oxidase and c-Src-dependent pathways. Hypertension. 59, 1263–1271 (2012).
Nahrendorf, M. et al. Effect of testosterone on post-myocardial infarction remodeling and function. Cardiovasc. Res. 57, 370–378 (2003).
Phillip, M., Maor, G., Assa, S., Silbergeld, A. & Segev, Y. Testosterone stimulates growth of tibial epiphyseal growth plate and insulin-like growth factor-1 receptor abundance in hypophysectomized and castrated rats. Endocrine. 16, 1–6 (2001).
Barrick, C. J., Yu, M., Chao, H. H. & Threadgill, D. W. Chronic pharmacologic inhibition of egfr leads to cardiac dysfunction in C57BL/6J mice. Toxicol. Appl. Pharmacol. 228, 315–325 (2008).
Kobrinsky, E., Mirshahi, T., Zhang, H., Jin, T. & Logothetis, D. E. Receptor-mediated hydrolysis of plasma membrane messenger pip2 leads to K+-current desensitization. Nat. Cell Biol. 2, 507–514 (2000).
Zhang, Y. H. et al. Modulation of human cardiac transient outward potassium current by egfr tyrosine kinase and src-family kinases. Cardiovasc. Res. 93, 424–433 (2012).
Feng, M. et al. Activation of epidermal growth factor receptor mediates reperfusion arrhythmias in anaesthetized rats. Cardiovasc. Res. 93, 60–68 (2012).
Robert, V. et al. Biological determinants of aldosterone-induced cardiac fibrosis in rats. Hypertension. 26, 971–978 (1995).
Robert, V. et al. Angiotensin AT(1) receptor subtype as a cardiac target of aldosterone - Role in aldosterone-salt-induced fibrosis. Hypertension. 33, 981–986 (1999).
Robert, V. et al. Increased cardiac type-i and type-iii collagen messenger-rnas in aldosterone-salt hypertension. Hypertension. 24, 30–36 (1994).
Schreier, B. et al. Consequences of epidermal growth factor receptor (erbB1) loss for vascular smooth muscle cells from mice with targeted deletion of erbB1. Arterioscler. Thromb. Vasc. Biol. 31, 1643–1653 (2011).
Huang, D. Y. B. K. Resistance of mice lacking the serum- and glucocorticoid-inducible kinase SGK1 against salt-sensitive hypertension induced by a high-fat diet. Am. J. Physiol. - Renal. 291, F1264–F1273 (2006).
Thireau, J., Zhang, B. L., Poisson, D. & Babuty, D. Heart rate variability in mice: a theoretical and practical guide. Exp. Physiol. 93, 83–94 (2008).
Rangan, G. K. & Tesch, G. H. Quantification of renal pathology by image analysis. Nephrology. 12, 553–558 (2007).
Schwerdt, G. et al. Long-term effects of ochratoxin A on fibrosis and cell death in human proximal tubule or fibroblast cells in primary culture. Toxicology. 232, 57–67 (2007).
Acknowledgements
This study was supported by the Deutsche Forschungsgemeinschaft (Grant GE 905/19-1).
Author information
Authors and Affiliations
Contributions
B.S. performed and designed most of the experiments, analysed and interpreted the data, designed the artwork, performed the statistical analysis and is the main author of the manuscript. Si. Ra. performed most of the experiments, S.W. performed the genotyping on the tail biopsies, St. Ru. took part in the tailcuff measurements and assisted in the organ harvesting, S.M. took part in the tailcuff measurements, Be. Sch. cut and stained the paraffin embedded organs, M.S. generated and provided the EGFR flox/flox mouse, Michael Gotthardt generated and provided the SM22-Cre mouse, S.K. and K.M. performed the experiments on the aldosterone release, C.G. took part in the experiment designing and the fund raising. M.G. wrote the manuscript, discussed the data and gave substantial input to the interpretation of the data, designed part of the experiments and is responsible for funding. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Schreier, B., Rabe, S., Winter, S. et al. Moderate inappropriately high aldosterone/NaCl constellation in mice: cardiovascular effects and the role of cardiovascular epidermal growth factor receptor. Sci Rep 4, 7430 (2014). https://doi.org/10.1038/srep07430
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep07430
This article is cited by
-
The mineralocorticoid receptor leads to increased expression of EGFR and T-type calcium channels that support HL-1 cell hypertrophy
Scientific Reports (2021)
-
miR-221 and -222 target CACNA1C and KCNJ5 leading to altered cardiac ion channel expression and current density
Cellular and Molecular Life Sciences (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.