Abstract
In Drosophila, the male sex pheromone cis-vaccenyl acetate (cVA) elicits aggregation and courtship, through the odorant receptor Or67d. Long-lasting exposure to cVA suppresses male courtship, via a second channel, Or65a. In females, the role of Or65a has not been studied. We show that, shortly after mating, Drosophila females are no longer attracted to cVA and that activation of olfactory sensory neurons (OSNs) expressing Or65a generates this behavioral switch: when silencing Or65a, mated females remain responsive to cVA. Neurons expressing Or67d converge into the DA1 glomerulus in the antennal lobe, where they synapse onto projection neurons (PNs), that connect to higher neural circuits generating the attraction response to cVA. Functional imaging of these PNs shows that the DA1 glomerulus is inhibited by simultaneous activation of Or65a OSNs, which leads to a suppression of the attraction response to cVA. The behavioral role of postmating cVA exposure is substantiated by the observation that matings with starved males, which produce less cVA, do not alter the female response. Moreover, exposure to synthetic cVA abolishes attraction and decreases sexual receptivity in unmated females. Taken together, Or65a mediates an aversive effect of cVA and may accordingly regulate remating, through concurrent behavioral modulation in males and females.
Similar content being viewed by others
Introduction
Polyandry, females mating multiply with different males, leads to a gender conflict over optimum mating rates and remating intervals. Polyandry is widespread in Drosophila and other insects. Females mate more than once, since a single mating does not yield sufficient sperm to match their egg production capacity, whereas high mating rates decrease female fitness and lifetime. This gives rise to a sexual conflict, which mediates pre- and postcopulatory selection on female traits that influence optimum mating rates and remating intervals1,2,3,4.
After mating, insect females undergo vital behavioral changes, regarding receptivity to further mating, feeding and egg laying5,6,7. In the fruit fly D. melanogaster, a single component of the seminal fluid, dubbed sex peptide (SP), has been shown to trigger the post-mating switch in female reproductive behavior8,9,10,11,12,13. After mating for the first time, SP increases female egg production, while multiple receipt of SP during consecutive matings reduces female lifetime and fecundity. On the other hand, SP transfer increases male fitness, since it delays remating in females and thus reduces male sperm competition2,8,14,15,16.
Drosophila females become unreceptive immediately after mating. Two, partly overlapping components contribute to postmating physiological and behavioral changes, including the inhibition of remating: a short-lasting copulation effect and a long-lasting sperm effect, which is generated by seminal proteins including SP. The prolonged effect of insemination on female receptivity and egg production lasts several days, but becomes evident only several hours after mating. In contrast, the copulation effect persists only for about one day14,17,18,19. What triggers this immediate suppression of receptivity after mating is not known, but the male-produced sex pheromone cis-vaccenyl acetate (cVA) is a candidate stimulus, since it is transferred to the female via the ejaculate together with sperm20.
cVA is a key compound regulating Drosophila social and sexual behaviors. It acts as an aggregation pheromone to attract males and females to feeding and mating sites21 and elicits sex-specific courtship behaviors at close range22,23,24. On the fly antenna, cVA is detected by two odorant receptors, Or65a and Or67d, which are expressed in different olfactory sensory neurons (OSNs)22,25,26. First- and second order olfactory neurons show identical pheromone responses in both sexes. Differences in male and female courtship behavior arise from dimorphic third-order circuits in the central brain27, where neurons determined by the fruitless (fru) gene differentially couple sensory input to motor output in males and females27,28,29,30 and where doublesex (dsx) neurons, which are responsive to cVA, regulate receptivity of unmated females to male courtship31.
In males, cVA regulates courtship and aggression in a temporally differential manner: acutely, perception via Or67d elicits aggression and prevents courtship with other males22,23; chronically, perception via Or65a leads to a generalized suppression of aggression and courtship20,32. Or65a is not part of the fru circuit28, yet it achieves a sex-specific effect on male courtship and aggression through lateral interaction with the Or67d channel32. This raises the question which behavioral consequences cVA mediates in females through Or65a. Since cVA increases female receptivity prior to, but not after mating, we investigated whether mating modulates the female response to cVA at the behavioral and neurophysiological level.
Results
Males inhibit female attraction to cVA after mating
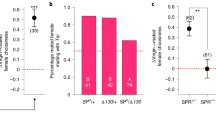
cVA attraction was tested in a Y-tube olfactometer, where cVA was released in one arm at a constant rate. Test females were virgin, mated with fed males, or mated with males that had been starved during 3 d. Virgin females were attracted to cVA, but copulation with fed males abolished female attraction. In contrast, females that had been mated with starved males continued to be attracted to cVA (Figure 1).
Effect of mating on cVA attraction.
Attraction of D. melanogaster females to cVA released at 15 ng/min in one arm of a y-tube olfactometer. Females were tested before mating and 1 h after mating with fed males and starved males (n = 40, 27 and 20 respectively). The attraction index shows the time spent in the cVA arm of the olfactometer minus the time spent in the control arm divided by the total amount of time spent in both arms. Positive values indicate attraction to cVA whereas negative values show repellency. Asterisks indicate significant attraction (mean ± SEM, Wilcoxon test, ** p < 0.01). Photo by S. Lebreton.
Sex peptide does not modulate cVA attraction
Diverse mating-induced behavioral changes in Drosophila females, including reduced receptivity and increased oviposition, involve seminal fluid proteins8,19,33. Starvation has been shown to affect sperm-mediated traits34 and may hence decrease copulation duration and sperm transfer, or even deteriorate sperm quality. However, we found no difference in copulation duration and reproductive success of starved and fed males (Figure 2A).
Mating performance of fed and starved males and effect of SP on cVA attraction.
(A) Copulation duration and offspring production of fed (n = 19) and starved males (n = 18). No difference was observed between fed and starved males (Mann-Whitney test). (B) Effect of SP on cVA attraction in mated females. Females were mated to virgin males lacking SP (SP0) or control males producing SP (SP+)(n = 27 for each). No effect of SP was observed. In both case females were not significantly attracted to cVA. Data are mean ± SEM.
We then tested the hypothesis that sex peptide (SP) is involved in the termination of post-mating attraction to cVA. SP knockout males2,8 were used to this purpose. However, SP did not account for changes in postmating cVA attraction in females, since cVA attraction was down-regulated in females mated with SP-deficient males as well as in females mated with control males producing SP (Figure 2B).
cVA exposure during mating abolishes postmating cVA attraction
We next asked whether cVA itself is the signal that shuts down postmating female attraction to cVA. During mating, males transfer cVA to females21,35,36,37 and prolonged stimulation with cVA may ensue in behavioral modulation.
If olfactory exposure to cVA during mating causes the reduced female response, it would follow that starved males do not produce sufficient amounts of cVA to produce a female behavioral modulation. We employed chemical analysis to quantify cVA transfer during mating, showing (Figure 3A) that fed males transferred almost threefold more cVA to females during copulation than starved males. More cVA was extracted from females during 24 h than during 5 min (Figure 3A), which confirms that cVA is not only transferred onto the female cuticle, but also via the ejaculate into the genital tract20,38.
Effect of cVA exposure, during mating or to synthetic cVA, on subsequent cVA attraction and female receptivity.
(A) Amount of cVA extracted from females after mating with fed (n = 8) and starved males (n = 6). Single females were dropped in hexane, immediately after mating and extracted during 5 min or 24 h. Amounts found on females mated with fed and starved males were analyzed with a Mann-Whitney test (** p < 0.01). (B) Virgin females were exposed to 0.3 or 0.6 μg of synthetic cVA during 1 h and then tested for cVA attraction. Asterisks above bars indicate significant attraction (Wilcoxon test, *** p < 0.001; data are mean ± SEM). (C) Receptivity of unmated females. Females were exposed to 0 (hexane), 0.3, 0.6 or 1.2 μg of cVA (n = 44, 40, 43 and 41, respectively) during 1 h, before they came in contact with a random virgin fed male during 1 h. Data were analyzed using a Mixed-effect model followed by a multiple comparison test (for details see SI Material and Methods , *** p < 0.001). Photos by S. Lebreton.
cVA on the female cuticle is known to be detected by other males and to suppress further courtship20,24,39. Obviously, cVA is detected also by the female fly itself, since females carry odorant receptors (Ors) selectively tuned to cVA26, while the behavioral consequences have not been investigated. Since fed males transfer more cVA during mating than starved males, it is conceivable that females are exposed to a higher amount of cVA during copulation with fed males.
We therefore tested the effect of exposure to synthetic cVA on subsequent attraction of virgin females to cVA. Pre-exposure to a filter paper loaded with 300 ng cVA, corresponding to the amount of cVA transferred by starved males, did not have an effect on unmated females; whereas exposure to 600 ng, corresponding to the amount of cVA transferred by fed males, abolished attraction of unmated females to cVA (Figures 3A, B). The attraction response of virgin females, following olfactory exposure to a low and high dose of synthetic cVA, matched the behavior observed in females mated with starved and fed males, respectively (Figure 1).
In unmated females, cVA promotes sexual receptivity22. The abolished attraction response led thus to the question whether cVA pre-exposure also has an effect on female mating behavior. Exposure of unmated females to 600 ng and 1.200 ng synthetic cVA significantly reduced their receptivity to male courtship (Figure 3C), showing that exposure of females to cVA leads to a reduction of mating.
Mating reduces DA1 activity through activation of Or65a and suppresses cVA attraction
Olfactory input from Or67d-expressing olfactory sensory neurons (OSNs) is relayed, via the DA1 glomerulus in the AL, to a sexually dimorphic circuit in the lateral horn of the protocerebrum, which accounts for sex-specific behaviors to cVA27,40. In Drosophila males, acute stimulation of Or67d OSNs by cVA enhances aggression, whereas prolonged stimulation of neurons expressing Or65a, a receptor known to respond to large amount of cVA, down-regulates aggression and suppresses male courtship20,23,32. Recently, it has been shown that perception of cVA via Or65a OSNs activates a network of local interneurons (LNs) in the AL, which is thought to regulate the male behavioral response to cVA stimulus input via Or67d32. We therefore investigated the role of the Or65a and Or67d olfactory channels with respect to the cVA-induced response modulation in Drosophila females.
We first examined whether mating changes the sensitivity of Or67d OSNs in T1 sensilla on the female antenna, using single sensillum recordings. However, the response of Or67d OSNs to cVA did not change after mating (Figure 4A, B). Consequently, inhibition of cVA attraction cannot be attributed to a peripheral modulation at the OSN level.
Mating decreases DA1 activity and requires Or65a OSNs to suppress cVA attraction.
(A and B) Or67d OSN response to different amounts of cVA. (A) Spike trains in response to cVA (red bar shows stimulus duration) recorded from unmated females and (B) comparison between virgin and mated females (n = 10 for each). (C and D) Calcium imaging during cVA application in the DA1 glomerulus at the presynaptic (OSNs) and postsynaptic (PNs) level. (C) Representative false color-coded images showing the antennal lobe after stimulation with cVA in OSNs and PNs in unmated females. (D) Comparison of DA1 calcium activity in virgin (ORNs, n = 6; PNs, n = 9) and mated (ORNs, n = 7; PNs, n = 5) females elicited by cVA. Asterisks show significant differences between virgin and mated females (GLMM). (E) Effect of blocking Or65a OSN response on cVA attraction of mated females (n = 20 to 28). Tetanus toxin light chain (TeTxLC) was expressed in Or65a OSNs, using two independent lines (TeTxLC tnt1 and TeTxLC tnt2). As a negative control an inactive form of TeTxLC was expressed (TeTxLC (−)1 and TeTcLC (−)2). Asterisks above bars indicate significant attraction (Wilcoxon test, * p < 0.05, ** p < 0.01). Data are mean ± SEM. (F) Proposed mechanism underlying the suppression of cVA attraction in females after mating. During mating, females are exposed to high amounts of cVA, which activates Or65a neurons. Or65a OSNs decrease the activity of DA1 glomerulus, probably via LNs. Decreased DA1 activity results in an inhibition of cVA attraction. Photo by S. Lebreton.
Next, using transgenic flies expressing the calcium sensor GCaMP specifically in OSNs (Orco-Gal4 driver) or PNs (GH146-Gal4 driver), we recorded the activity of the DA1 glomerulus in response to cVA. At the presynaptic level (OSNs), the response to cVA was not affected (Figure 4C), whereas at the postsynaptic level (PNs), the response to cVA decreased significantly after mating (Figure 4D). This down-regulation of the DA1 output signal is consistent with the observed change in cVA attraction after mating (Figure 1). Moreover, processing of the cVA signal in the AL lends support to the idea that lateral interaction between Or67d and Or65a olfactory channels modulates the response to cVA not only in males32, but also in females.
In order to confirm that cVA exposure during mating suppresses further cVA attraction and that Or65a is involved, we targeted the expression of tetanus toxin light chain (TeTxLC tnt) to Or65a neurons, using two independent lines. TeTxLC blocks synaptic transmission by preventing neurotransmitter release and therefore suppresses any response from neurons in which it is expressed41. After mating, females expressing TeTxLC in Or65a OSNs remained to be attracted to cVA. This was not the case when an inactive form of TeTxLC (TeTxLC (−)) was expressed (Figure 4E), showing that input from Or65a OSNs is required to suppress cVA attraction after mating. Moreover, our results confirm that Or65a neurons are not needed for cVA attraction per se.
Discussion
Our study brings new insights into how postmating behavior in Drosophila females is regulated by the male-produced sex pheromone cVA. We show, for the first time, that chronic exposure to cVA abolishes attraction of females to cVA and that it reduces receptivity to male courtship (Figures 1, 3).
The response to cVA is mediated via the same odorant receptors in both sexes: acute input via Or67d triggers direct responses, male-male aggression and attraction of females to males, respectively22,23; prolonged exposure via Or65a generates a response inhibition in males20,32, as well as in females (Figures 3, 4). A behavioral role of the Or65a channel in Drosophila females has not yet been described.
We show that exposure of females to cVA during mating activates Or65a OSNs and reduces the activity of the DA1 glomerulus receiving simultaneous input from Or67d OSNs (Figure 4C, D), which leads to a suppression of the female behavioral response to cVA following mating. Flies in which Or65a is silenced continue to be attracted to cVA even after mating (Figure 4E)
Or65a, which is not part of the fruitless circuit28, elicits nonetheless a gender-specific behavioral response. Or67d and Or65a OSNs converge in the adjacent DA1 and DL3 glomeruli, respectively42 and it has been suggested that the fine-tuning of male-to-male aggression response involves LNs that interconnect these two glomeruli32. We therefore propose that Or65a modulates the sex-specific Or67d olfactory channel27 through local circuits also in female flies (Figure 4F).
PN activity in DA1 is decreased in mated females, as shown by calcium imaging (Figure 4C, D). Most LNs in the AL are GABAergic and therefore inhibitory43 and GABAergic LNs are responsible for olfactory habituation44. It has been established that the activity of DA1 is modulated by activation of GABAB receptors, which are expressed in OSNs projecting to DA1 and the particularly high level of presynaptic inhibition in OSNs projecting to DA145 underlines the importance of gain control in pheromone detection and behavior.
The Drosophila SP has a long-term effect on female receptivity8,14,19, but a suppression of female receptivity during the first few hours following copulation cannot be attributed to SP17,18. Males transfer large amount of cVA to the females during mating, which consequently repels other males35,36,38. Here we show that the transfer of cVA elicits, in addition, the female short-term postmating response (Figure 3), well before SP induces long-term effects.
Polyandry in Drosophila drives pre- and postcopulatory sexual selection of female and male reproductive behaviour. Although is adaptive for females to mate more than once, multiple matings comprise a fitness cost14,16,46,47,48,49. However, females traits, including the sensory perception of male fecundity, are understudied in comparison with male traits, such as seminal fluid proteins49,50,51.
One element of Drosophila polyandry is that multiple matings diminish male fertility, probably due to sperm depletion43,52, which is congruent with the finding that females, after mating with recently mated males, show less pronounced postmating behavioral changes53,54. Shortly after mating with fed males, female receptivity decreases8 and abolished attraction to cVA prevents exposure to courting males at aggregation sites, from where cVA is being released in substantial amounts (Figures 1, 3)21. In contrast, matings with starved males, or exposure to smaller amounts of cVA, do not abolish attraction to cVA (Figures 1, 3).
Since high amounts of cVA are being transferred during first matings, it is conceivable that males transfer less cVA during subsequent matings, reflecting their reduced fecundity. The amount of cVA transferred may thus allow females to detect male mating status and to adjust sexual receptivity and remating rate accordingly.
Sexual selection is partitioned into male-male competition and female mate choice55. The Or65a olfactory channel, mediating the long-term effect of cVA exposure, is subject to sexual selection in Drosophila males and females. Or65a functions in both sexes to reinforce the effect of cVA on male competitors, through suppression of courtship in males20 and through reduced cVA attraction and receptivity in females (Figures 3, 4). In addition, cVA input through Or65a regulates remating in females as a function of the amount of cVA transferred by males, reflecting male quality (Figures 1, 3). The Or65a channel modulates accordingly polyandry in Drosophila.
Methods
Additional methods are provided in SI Material and Methods .
Fly stocks, crossings and rearing conditions
The Dalby strain of the fruit fly Drosophila melanogaster was used as a wild-type strain56. Optical imaging was performed using a transgenic fly line labeled in OSNs Orco-GAL4; END1-2,UAS-GC3.0; TM2/TM6B or labeled in PNs yω; GH146-GAL4, UAS-GCaMP-3.0/CyO; TM2/TM6B. The role of sex-peptide (SP) was assessed by using males lacking SP (SP0) and control males producing SP (SP+)2. SP0 males were obtained by crossing SP0/TM3,Sb,ry males to Δ130/TM3,Sb,ry females. Control males were produced by crossing SP0,SP+/TM3,Sb,ry males to Δ130/TM3,Sb,ry females, resulting in SP0,SP+/Δ130 (SP+) males. SP0/TM3,Sb,ry, SP0,SP+/TM3,Sb,ry and Δ130/TM3,Sb,ry lines were obtained from Claudia Fricke (University of East Anglia). To silence Or65a OSNs, we expressed a light chain of the tetanus toxin (TeTxLC) in these neurons using the UAS-GAL4 system. For that purpose, two UAS-TeTxLC tnt strains (Bloomington stock 28838 and 28997, respectively referred as 1 and 2 in this article) were crossed with a Or65a-GAL4 line (Bloomington stock 9994). Control experiments were done by expressing an inactive form of TeTxLC (UAS-TeTxLC (−)) using two independent strains (Bloomington stock 28840 and 28841, respectively referred as 1 and 2).
Flies were reared on a standard sugar-yeast-cornmeal medium diet at room temperature (19–22°C) and under a 10:14 h L:D photoperiod. For optical imaging experiments, flies were reared at 25°C. Newly emerged flies were anesthetized under CO2 and sexed under a microscope. Flies of the same sex were then kept together in 30 ml plastic tubes with fresh diet (fed flies) or with a humidified piece of cotton wool (starved males). All flies tested were 3 d old.
cVA attraction
Attraction to cVA was tested in a Y-tube olfactometer57. The olfactometer was composed of two branches (30 cm long glass tubes). Each branch was vertically connected to a 25-mL glass vial at its extremity. Both vials were filled with 8 ml of vinegar, producing a food odor background. cVA (1.5 ng/μL in hexane) was tested against hexane (control). These two solutions were released in each branch of the olfactometer from a glass capillary connected to a piezoelectric sprayer58 at a rate of 10 μL/min. An air-stream of 0.25 m/s was produced in each branch of the olfactometer.
Single females were introduced at the entrance of the Y-tube. The time spent in each branch was recorded. The tests lasted 5 min. An Attraction Index (AI) was calculated as follows: AI = (Time spent in the stimulus branch − Time spent in the control branch)/(Time spent in the stimulus branch + Time spent in the control branch). Inactive flies remaining at the entrance of the olfactometer were not taken into account.
Single sensillum recordings (SSR)
Extracellular recordings from OSNs were done using wild-type females, which were virgin or mated with fed wild-type males. A female fly was wedged into the narrow end of a 200-μL micropipette yellow tip with gentle pressure, leaving about half of the compound eye and antennae exposed. The right antenna was gently exposed and gently held between a double adhesive tape and a glass capillary, pressing on the second antennomere. This set up was mounted under a microscope (Nikon Eclipse E600FN) at ×750 magnification, in a stream of moist air. The insect was grounded through the eye using an electrode sharpened to about 1 μm. A second electrode was used to establish contact with the base of a T1 sensillum on the antenna. These sensilla could easily be identified by their characteristic spontaneous activity and single spike amplitude (OSN). The signals were pre-amplified 10× and fed into a computer via an IDAC 4 and recorded by Autospike software (Syntech, Hilversum, The Netherlands). Signals were recorded for 12 s, beginning 2 s prior to stimulation. Details about stimuli and odor delivery are provided in SI Material and Methods .
Optical imaging
Flies were dissected as previously described59. Briefly, flies were anesthetized on ice and mounted into a plastic stage whereby the head was fixed with Protemp II (3M ESPE). To prevent the antennae from getting in contact with saline, we bent the anterior part with a fine gold wire and placed a plate with a window on top that was sealed with two-component-silicone (KwikSil). Under saline (130 mM NaCl, 5 mM KCl, 2 mM MgCl2, 2 mM CaCl2, 36 mM saccharose, 5 mM HEPES, 1 M NaOH, pH 7.3) the vertex was opened between the eyes, the ocelli and the basis of the antennae. After removing the cuticle, fatty tissue and tracheal sacs, the antennal lobes was visible.
Imaging datasets were acquired using a CCD-camera (Pro-Imaging, Sensi-Cam) attached to an upright fluorescent microscope (OLYMPUS BX51WI), which was controlled via TILL visION (TILL Photonics). Excitation of the GCaMP-3.0 was provided via a Polychrome V (TILL Photonics). The stimulus was applied using a stimulus controller (Syntech Stimulus Controller CS-55; Kirchzarten, Germany) generating a continuous air flow of 1.0 L/min added with a stimulus flow of 0.5 L/min which was shifted between a blank and a stimulus pipette to prevent mechanical stimulation. cVA diluted in paraffin oil was applied on a circular filter paper (0.5, 5, 50 or 50 μg) and placed in Pasteur pipettes. These were then attached to the tubing of the CS-55. The imaging protocol lasted for 40 frames at 4 Hz with a stimulus duration of 2 s.
Further analysis was carried out with custom-written programs in IDL 6.4 (ITT Visual Information Solutions). Beginning with a background (percentage of change from background), bleach and movement correction to minimize artifacts and continuing with identification of the observed glomeruli, a precise response kinetic (ΔF/F) for each glomerulus was calculated.
Chemical analysis
We analyzed the amount of cVA transferred to females during mating with fed and starved males. Just after mating, individual females were dropped into a 1.5-mL glass vial containing 100 μL hexane, to which 200 ng of heptadecenyl acetate was added as an internal standard, during 5 min, for a brief extraction of compounds present on the cuticle, or during 24 h. These extracts were then analyzed on a gas chromatograph coupled with a mass spectrometer (GC-MS). For details on chemical analysis, see SI Material and Methods .
Statistics
For details, see SI Material and Methods .
References
Arnqvist, G. & Nilsson, T. The evolution of polyandry: multiple mating and female fitness in insects. Anim. Behav. 60, 145–164 (2000).
Fricke, C., Wigby, S., Hobbs, R. & Chapman, T. The benefits of male ejaculate sex peptide transfer in Drosophila melanogaster. J. Evol. Biol. 22, 275–286 (2009).
Long, T. A. F., Pischedda, A., Nichols, R. V. & Rice, W. R. The timing of mating influences reproductive success in Drosophila melanogaster: implications for sexual conflict. J. Evol. Biol. 23, 1024–1032 (2010).
Kvarnemo, C. & Simmons, L. W. Polyandry as a mediator of sexual selection before and after mating. Phil. Trans. R. Soc. B 368, 20120042 (2013).
Ringo, J. Sexual receptivity in insects. Annu. Rev. Entomol. 41, 473–494 (1996).
Vargas, M. A., Luo, N. G., Yamaguchi, A. & Kapahi, P. A role for S6 kinase and serotonin in postmating dietary switch and balance of nutrients in D. melanogaster. Curr. Biol. 20, 1006–1011 (2010).
Saveer, A. M. et al. Floral to green: mating switches moth olfactory coding and preference. Proc. R. Soc. B 279, 2314–2322 (2012).
Liu, H. F. & Kubli, E. Sex-peptide is the molecular basis of the sperm effect in Drosophila melanogaster. Proc. Natl. Acad. Sc. USA 100, 9929–9933 (2003).
Yapici, N., Kim, Y.-J., Ribeiro, C. & Dickson, B. J. A receptor that mediates the post-mating switch in Drosophila reproductive behaviour. Nature 451, 33–38 (2008).
Hasemeyer, M., Yapici, N., Heberlein, U. & Dickson, B. J. Sensory neurons in the Drosophila genital tract regulate female reproductive behavior. Neuron 61, 511–518 (2009).
Yang, C. H. et al. Control of the postmating behavioral switch in Drosophila females by internal sensory neurons. Neuron 61, 519–526 (2009).
Rezaval, C. et al. Neural circuitry underlying Drosophila female postmating behavioral responses. Curr. Biol. 22, 1155–1165 (2012).
Haussmann, I. U., Hemani, Y., Wijesekera, T., Dauwalder, B. & Soller, M. Multiple pathways mediate the sex peptide-regulated switch in female Drosophila reproductive behaviours. Proc. R. Soc. B 280, 20131938 (2013).
Chapman, T. et al. The sex peptide of Drosophila melanogaster: female post-mating responses analyzed by using RNA interference. Proc. Natl. Acad. Sci. USA 100, 9923–9928 (2003).
Wigby, S. & Chapman, T. Sex peptide causes mating costs in female Drosophila melanogaster. Curr. Biol. 15, 316–321 (2005).
Kuijper, B., Stewart, A. D. & Rice, W. R. The cost of mating rises nonlinearly with copulation frequency in a laboratory population of Drosophila melanogaster. J. Evol. Biol. 19, 1795–1802 (2006).
Manning, A. Control of sexual receptivity in female Drosophila. Anim. Behav. 15, 239–250 (1967).
Scott, D. The timing of the sperm effect on female Drosophila melanogaster receptivity. Anim. Behav. 35, 142–149 (1987).
Ram, K. R. & Wolfner, M. F. A network of interactions among seminal proteins underlies the long-term postmating response in Drosophila. Proc. Ntl. Acad. Sc. USA 106, 15384–15389 (2009).
Ejima, A. et al. Generalization of courtship learning in Drosophila is mediated by cis-vaccenyl acetate. Curr. Biol. 17, 599–605 (2007).
Bartelt, R. J., Schaner, A. M. & Jackson, L. L. cis-Vaccenyl acetate as an aggregation pheromone in Drosophila melanogaster. J. Chem. Ecol. 11, 1747–1756 (1985).
Kurtovic, A., Widmer, A. & Dickson, B. J. A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature 446, 542–546 (2007).
Wang, L. & Anderson, D. J. Identification of an aggression-promoting pheromone and its receptor neurons in Drosophila. Nature 463, 227–231 (2010).
Keleman, K. et al. Dopamine neurons modulate pheromone responses in Drosophila courtship learning. Nature 489, 145–U210 (2012).
Ha, T. S. & Smith, D. P. A pheromone receptor mediates 11-cis-vaccenyl acetate-induced responses in Drosophila. J. Neurosc. 26, 8727–8733 (2006).
Van der Goes van Naters, W. & Carlson, J. R. Receptors and neurons for fly odors in Drosophila. Curr. Biol. 17, 606–612 (2007).
Kohl, J., Ostrovsky, A. D., Frechter, S. & Jefferis, G. S. X. E. A bidirectional circuit switch reroutes pheromone signals in male and female brains. Cell 155, 1610–1623 (2013).
Stockinger, P., Kvitsiani, D., Rotkopf, S., Tirian, L. & Dickson, B. J. Neural circuitry that governs Drosophila male courtship behavior. Cell 121, 795–807 (2005).
Ruta, V. et al. A dimorphic pheromone circuit in Drosophila from sensory input to descending output. Nature 468, 686–U106 (2010).
Dauwalder, B. The roles of fruitless and doublesex in the control of male courtship. Int. Rev. Neurobiol. 99, 87–105 (2011).
Zhou, C., Pan, Y. F., Robinett, C. C., Meissner, G. W. & Baker, B. S. Central brain neurons expressing doublesex regulate female receptivity in Drosophila. Neuron 83, 149–163 (2014).
Liu, W. W. et al. Social regulation of aggression by pheromonal activation of Or65a olfactory neurons in Drosophila. Nat. Neurosc. 14, 896–U119 (2011).
Wigby, S. et al. Seminal fluid protein allocation and male reproductive success. Curr. Biol. 19, 751–757 (2009).
Fricke, C., Bretman, A. & Chapman, T. Adult male nutrition and reproductive success in Drosophila melanogaster. Evolution 62, 3170–3177 (2008).
Yew, J. Y., Cody, R. B. & Kravitz, E. A. Cuticular hydrocarbon analysis of an awake behaving fly using direct analysis in real-time time-of-flight mass spectrometry. Proc. Natl. Acad. Sci. USA 105, 7135–7140 (2008).
Yew, J. Y. et al. A new male sex pheromone and novel cuticular cues for chemical communication in Drosophila. Curr. Biol. 19, 1245–1254 (2009).
Everaerts, C., Farine, J.-P., Cobb, M. & Ferveur, J.-F. Drosophila cuticular hydrocarbons revisited: mating status alters cuticular profiles. PloS One 5, e9607 (2010).
Butterworth, F. M. Lipids of Drosophila: a newly detected lipid in the male. Science 163, 1356–1357 (1969).
Zawistowski, S. & Richmond, R. C. Inhibition of courtship and mating of Drosophila melanogaster by the male-produced lipid, cis-vaccenyl acetate. J. Insect Physiol. 32, 189–192 (1986).
Cachero, S., Ostrovsky, A. D., Yu, J. Y., Dickson, B. J. & Jefferis, G. S. X. E. Sexual dimorphism in the fly brain. Curr. Biol. 20, 1589–1601 (2010).
Sweeney, S. T., Broadie, K., Keane, J., Niemann, H. & O'Kane, C. J. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron 14, 341–351 (1995).
Couto, A., Alenius, M. & Dickson, B. J. Molecular, anatomical and functional organization of the Drosophila olfactory system. Curr. Biol. 15, 1535–1547 (2005).
Olsen, S. R. & Wilson, R. I. Lateral presynaptic inhibition mediates gain control in an olfactory circuit. Nature 452, 956–960 (2008).
Das, S. et al. Plasticity of local GABAergic interneurons drives olfactory habituation. Proc. Natl. Acad. Sci. USA 108, E646–E654 (2011).
Root, C. M. et al. A presynaptic gain control mechanism fine-tunes olfactory behavior. Neuron 59, 311–321 (2008).
Chapman, T., Liddle, L. F., Kalb, J. M., Wolfner, M. F. & Partridge, L. Cost of mating in Drosophila melanogaster females is mediated by male accessory gland products. Nature 373, 241–244 (1995).
Pitnick, S. & García-González, F. Harm to females increases with male body size in Drosophila melanogaster. Proc. R. Soc. B 269, 1821–1828 (2002).
Wigby, S., Chapman, T., Building, D. & Street, G. Sex peptide causes mating costs in female Drosophila melanogaster. Curr. Biol. 15, 316–321 (2005).
Wolfner, M. F. Battle and ballet: molecular interactions between the sexes in Drosophila. J. Hered. 100, 399–410 (2009).
Pischedda, A. & Rice, W. R. Partitioning sexual selection into its mating success and fertilization success components. Proc. Natl. Acad. Sci. USA 109, 2049–2053 (2012).
Kvarnemo, C. & Simmons, L. W. Polyandry as a mediator of sexual selection before and after mating. Phil. Trans. R. Soc. B 368, 20120042 (2013).
Markow, T. A., Quaid, M. & Kerr, S. Male mating experience and competitive courtship success in Drosophila melanogaster. Nature 276, 821–822 (1978).
Van Vianen, A. & Bijlsma, R. The adult component of selection in Drosophila melanogaster - some aspects of early-remating activity of females. Heredity 71, 269–276 (1993).
Avila, F. W., Sirot, L. K., LaFlamme, B. A., Rubinstein, C. D. & Wolfner, M. F. Insect seminal fluid proteins: identification and function. Annu. Rev. Entomol. 56, 21–40 (2011).
Hunt, J., Breuker, C. J., Sadowski, J. A. & Moore, A. J. Male-male competition, female mate choice and their interaction: determining total sexual selection. J. Evol. Biol. 22, 13–26 (2009).
Ruebenbauer, A., Schlyter, F., Hansson, B. S., Löfstedt, C. & Larsson, M. C. Genetic variability and robustness of host odor preference in Drosophila melanogaster. Curr. Biol. 18, 1438–1443 (2008).
Lebreton, S., Becher, P. G., Hansson, B. S. & Witzgall, P. Attraction of Drosophila melanogaster males to food-related and fly odours. J. Insect Physiol. 58, 125–129 (2012).
Becher, P. G., Bengtsson, M., Hansson, B. S. & Witzgall, P. Flying the fly: long-range flight behavior of Drosophila melanogaster to attractive odors. J. Chem. Ecol. 36, 599–607 (2010).
Strutz, A., Völler, T., Riemensperger, T., Fiala, A. & Sachse, S. Calcium imaging of neural activity in the olfactory system of Drosophila. Neuromethods/Genetically Encoded Functional Indicators, pp 43–70, ed. Martin, J.-R. (Humana Press, Totowa, NJ, 2012).
Acknowledgements
This work was funded by the Linnaeus grant “Insect Chemical Ecology, Ethology and Evolution” IC-E3 (Formas, SLU). We thank Claudia Fricke (University of East Anglia) for supplying fly lines.
Author information
Authors and Affiliations
Contributions
S.L. and P.W. wrote the main manuscript text and prepared the figures. V.G., S.S. and B.S.H. designed and conducted functional imaging tests, A.B.O. and R.I. did single cell recordings, P.G.B. contributed to behavioural studies. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Lebreton, S., Grabe, V., Omondi, A. et al. Love makes smell blind: mating suppresses pheromone attraction in Drosophila females via Or65a olfactory neurons. Sci Rep 4, 7119 (2014). https://doi.org/10.1038/srep07119
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep07119
This article is cited by
-
Aggregation pheromones have a non-linear effect on oviposition behavior in Drosophila melanogaster
Nature Communications (2023)
-
Molecular and neural mechanisms regulating sexual motivation of virgin female Drosophila
Cellular and Molecular Life Sciences (2021)
-
Olfactory processing in the lateral horn of Drosophila
Cell and Tissue Research (2021)
-
Flying Drosophila show sex-specific attraction to fly-labelled food
Scientific Reports (2019)
-
Factors Influencing Mating Behavior and Success in the Red Palm Weevil, Rhynchophorus ferrugineus Olivier (Coleoptera: Dryophthoridae)
Neotropical Entomology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.