Abstract
Bio-fouling is a serious problem in many membrane-based separation processes for water and wastewater treatment. Current state of the art methods to overcome this are to modify the membranes with either hydrophilic additives or with an antibacterial compound. In this study, we propose and practise a novel concept to prevent bio-fouling by developing a killing and self-cleaning membrane surface incorporating antibacterial silver nanoparticles and highly hydrophilic negatively charged carboxylic and amine functional groups. The innovative surface chemistry helps to reduce the contact angle of the novel membrane by at least a 48% and increase the pure water flux by 39.4% compared to the control membrane. The flux drop for the novel membrane is also lower (16.3% of the initial flux) than the control membrane (55.3% of the initial flux) during the long term experiments with protein solution. Moreover, the novel membrane continues to exhibit inhibition to microbes even after 1320 min of protein filtration. Synthesis of self-cleaning ultrafiltration membrane with long lasting properties opens up a viable solution for bio-fouling in ultrafiltration application for wastewater purification.
Similar content being viewed by others
Introduction
One of the most pervasive problems afflicting mankind throughout the world is poor access to clean freshwater and sanitation1. With the growing demand for high quality water, many new technologies of water purification are being developed to cater for potable and non-potable use. The reliability and ease of operation of membrane-based filtration systems have led to their proliferation in wastewater treatment. Ultrafiltration (UF) is one such well-developed low pressure membrane separation process used in different applications, such as water/wastewater treatment, reverse osmosis pretreatment and separations in the food, dairy, paper, textile, pharmaceuticals, chemical and biochemical industries2,3. However, membrane fouling remains an unavoidable problem in all pressure driven membrane processes causing deterioration of the membrane performance. These problems generally originate from the accumulation of organics in the effluent water, which serves as a support for the attachment and growth of microorganisms onto the membrane surface. The resulting effects of membrane fouling such as flux reduction, rejection impairment and membrane breakage lead to high operational costs and short replacement intervals4,5,6.
Polyethersulfone (PES) is a well-known and commonly used material to produce UF membranes3,7,8 due to its excellent chemical and mechanical properties, oxidation and pH resistance and thermal tolerance9,10. However, the hydrophobic nature of PES has limited its applications especially in membrane separation processes11. Numerous research reports that the hydrophobic interactions and electrostatic interactions enhance the attachment of natural organic matters (NOMs) to the membrane surface, which is the initial step of membrane fouling12,13,14,15. It has also been reported that hydrophobic membranes suffer from more adsorptive fouling, faster flux drop and irreversible fouling than hydrophilic membranes when treating waste streams with high extracellular organic matter (EOM) content16. Thus, several techniques have been developed in order to improve the hydrophilicity of the PES membrane to increase its permeability and to reduce membrane fouling.
Sulfonation is the best way to impart more hydrophilicity to the PES membrane surface in order to make it more resistant to fouling, as demonstrated in a number of studies17,18,19,20. However, during sulfonation, PES may degrade as phenol21. Several published work have reported the enhancement of the hydrophilicity of PES ultrafiltration membranes by methods such as coating22, ultraviolet irradiation23, carboxylation24, graft polymerization25,26, nitration27, ozone treatment on membrane surface28,29, plasma treatment30 and blending additives31,32,33 in the casting solution. Among these methods, the blending of additives to the dope solution has been widely used since it is by far the simplest method34.
Polyethylene glycol (PEG) and poly (N-vinyl pyrrolidinone) (PVP) are the most commonly used hydrophilic polymer additives for the modification of PES membrane by blending method35,36. One important drawback of these additives is that they are water soluble and hence, the elution of PVP and PEG from the membrane matrix is unavoidable during backwash and harsh operations. It has been reported that, in order to prevent the elution, 1-vinylpyrrolidone can be polymerized with acrylonitrile and the final poly (1-vinylpyrrolidone-co-acrylonitrile) copolymer can be blended with polysulfone dope solution to prepare highly hydrophilic microfiltration membranes with no elution37. In addition, incorporation of silver into polymeric matrices could also reduce bio-fouling by enhancing the antimicrobial property of polymeric membrane38,39,40. In our recent study, we showed that the hydrophilicity of the PES UF membrane can be improved by blending the highly hydrophilic, water insoluble poly (acrylonitrile-co-maleic acid) (PANCMA) as a co-polymer to the PES dope solution (to introduce the carboxyl functionality in the membrane surface), which can also be used as a linker to covalently attach PEG and silver to the membrane surface to prevent elution. The modified hydrophilic membrane exhibited a lower flux drop and a stable flux during long time (4200 min) test with reservoir water41.
More recently, membrane modifications with another type of material called zwitterions, which are neutral compounds that have formal unit electrical charges of opposite sign, have been studied to mitigate membrane fouling due to proteins42,43. The preparation of zwitterionic polymers and their attachment to the membrane surfaces increased the hydrophilicity of the membrane and reduced protein adsorption on the membrane surface. Zwitterionic surfaces containing uniform chains of hydrophilic molecules with tailorable length could bind more water by hydrogen bonding, leading to a strong repulsive force to protein molecules at specific separation distances. However, zwitterionic substances do not possess any antibacterial functionality (biocides).
As mentioned earlier, in the current state of the art, membranes are modified either with a hydrophilic additives or modified with an antibacterial compound (most commonly silver). It has been proven that, silver can kill the bacteria by rupturing the plasma membrane. Once the plasma membrane ruptures, the negatively charged protoplasm compounds deposit onto the positive surface of the membrane44. The presence of negatively charged –NH2, -COOH and –OH functional groups on the membrane surface has been shown to be effective in combatting the deposition of the negatively charged colloidal particles, proteins, lipids and amino acids etc. via an electrostatic repulsion mechanism45,46.
In this study, we propose and practise a novel concept to prevent bio-fouling by developing a killing and self-cleaning membrane surface having additional enhanced hydrophilicity via water insoluble PANCMA blended with the membrane matrix. In order to develop a self-cleaning surface the functional groups/compounds on the surfaces were carefully chosen. Negatively charged, acid rich, hydrophilic and water insoluble PANCMA was chosen as a base additive to anchor the amine rich antimicrobial silver nanoparticle capped polyethyleneimine (PEI). This Ag capped PEI attached PANCMA was then blended with PES dope solution to prepare the hollow fiber ultrafiltration membranes by dry wet spinning process.
The anti-bio-fouling mechanism of the novel membrane is illustrated in Figure 1. From the figure, it can be easily understood that, when the bacteria approaches the membrane surface, the antimicrobial silver will kill them by rupturing their negatively charged plasma wall (which leads to the leakage of negatively charged protein molecules through the ruptured bacterial cell wall), whereas the highly hydrophilic, negatively charged acid, hydroxyl and amine functionalities will prevent the deposition of these protein molecules onto the membrane surface by electrostatic repulsion. Thereby, the membrane can exhibit the self-cleaning property. In addition, since the silver nanoparticles (AgNPs) are embedded (covalently attached) in PEI matrix, the leaching of AgNPs is completely prevented.
Results
Synthesis of Ag capped PEI colloid
Silver capped PEI was synthesized by adding 5 ml of 0.1 M AgNO3 solution to 50 ml of aqueous PEI solution. The solution was then reduced by adding freshly prepared NaBH4 solution dropwise with constant stirring. The resulting dark yellowish green solution was then stirred continuously for about 3 hours at 80°C. Silver capped PEI was then separated out by centrifugation followed by repeated washing with water. Figure 2a shows the TEM picture of the synthesized PEI-Ag. The nanoparticles are spherical in shape with sizes in the range of 3 to 6 nm. The nanoparticles also show a uniform distribution in the PEI matrix. A schematic representation of the silver capped PEI is shown in Figure 2b.
Characterization of poly (acrylonitrile co maleic anhydride) (PANCMA) and PEI-Ag anchored to it
PANCMA was synthesized by a free radical polymerization reaction and the structure of the polymer was confirmed by FTIR spectrophotometer as shown in Figure 3.
The presence of CN stretching vibration at 2243 cm−1 and the imide stretching vibrations at 1784 cm−1 and 1707 cm−1 confirms the copolymer formation in anhydride form. The obtained polymer was further treated with Ag capped PEI and the attachment of PEI-Ag to the copolymer was also confirmed with FTIR. The –OH stretching vibration of the carboxylic acid was observed as a broad band at 3550 cm−1, the nitrile stretching vibration was found at 2245 cm−1, the carbonyl stretching vibration was observed as a strong band at 1708 cm−1 and the attachment of PEI to the copolymer was confirmed by the presence of a strong –C-N-C stretching vibration at 1352 cm−1 and –NH bending vibration at 1585 cm−1.
Membrane Characterization
PES, PES/PANCMA and PES/PANCMA-PEI-Ag hybrid hollow fibers were successfully fabricated by using the dry-wet spinning process.
FTIR analysis was used to investigate the structure of fabricated hollow fiber membrane. Figure 4 represents the FTIR spectra for neat PES membrane, PES/PANCMA (5wt%) and PES/PANCMA/PEI-Ag membranes. With the PES/PANCMA membrane, most of the anhydride moiety present in the PANCMA gets hydrolyzed to acid as the blend (dope solution) reaches the coagulation bath with water; this is confirmed from the broad band at 3560 cm−1 in the FTIR spectra. The band at 2243 cm−1 is due to the C-N stretching of the nitrile group while the band at 1708 cm−1 is the C = O stretching vibration of the acid carbonyl group present in the PANCMA. The presence of PANCMA/PEI-Ag in the PES matrix is confirmed from the nitrile, carbonyl, imide and amine vibrations at 2243 cm−1, 1716 cm−1,1770 cm−1, 1382 cm−1 and 1625 cm−1 respectively.
Morphology of Hollow Fibers
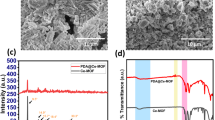
The hollow fiber membranes fabricated through dry-wet spinning process had an average inner diameter of 0.5 mm and an outer diameter of 1.0 mm. The surface morphology and cross section of the PES and modified PES hollow fiber membranes were examined using SEM and the micrographs of the Ag modified membranes are shown in Figure 5.
The fabricated hollow fibers exhibit different internal structures depending on their composition. The SEM picture of neat PES membrane shows the presence of a large number of macro voids in its internal structure. The PES membrane with 5wt% PANCMA concentration seems to have fewer macro voids (compared to control PES) but more sponge-like structures. With the PES/PANCMA/PEI-Ag membrane, the sponge-like structure dominates the macro voids and correlates well with the increase in dope solution viscosity as described in our previous paper41. The presence of silver particles was further confirmed by EDX analysis as shown in Figure 6. The presence of silver and its uniform distribution all over the membrane is evidenced from the elemental mapping shown in Figure 6b.
Leaching Test for Silver
The composite PES-PANCMA-PEI-Ag (S3) membrane samples were soaked in de-ionized (DI) water overnight and dried at ambient temperature before the leaching test. The dried membrane samples were immersed in fresh DI water and ultrasonicated at 37 kW for 120 mins. After ultra-sonication, the water was analyzed using Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES). The concentration of the leached silver in the solution was found to be less than 0.02% for all the samples. This may be due to the strong interaction of silver nanoparticles with the amine groups of the PEI, which prevents the leaching of silver from the membrane matrix.
Contact Angle
The hydrophilicity of the control PES (S1), PES-PANCMA (S2) and PES-PANCMA-PEI-Ag (S3) membrane was measured by their contact angle and the results are tabulated in Table 1. The control PES membrane (S1) showed a water contact angle of 63.2° ± 3.9°. Sample S2 with 5% PANCMA showed a water contact angle of 54.1° ± 2.8° which is about 14.4% lower than the control sample. Sample S3, with 5% of Ag capped PEI attached PANCMA, showed the lowest water contact angle of 32.7° ± 2.1°, which is about 48.3% reduction on the control sample. The effectiveness of blending PANCMA-PEI-Ag with PES in generating a more hydrophilic membrane is clearly demonstrated by these tests. The increased hydrophilicity is attributed to the incorporation of hydrophilic amine (-NH2) and acid (-COOH) groups through the attachment of PEI and PANCMA to the PES membrane matrix.
Pore Size Analysis
The average pore sizes of the control PES membrane (S1), PES-PANCMA membrane (S2) and the PES-PANCMA-PEI-Ag membrane (S3) are presented in Table 1. The experimental data indicate that the addition of the Ag capped PEI attached PANCMA does not have a significant effect on the mean pore size of the PES. The average pore sizes of sample S2 and S3 (0.08 ± 0.015 µm) are slightly lower compared to sample S1 (0.09 ± 0.02 µm). The reduction in pore size may be due to the increase in the polymer concentration (additional 5% of PANCMA for S2 and 5% PANCMA-PEI-Ag for sample S3 compared to the control membrane) which results in an increase in the viscosity of the dope solution. The rate of diffusion of coagulant into the polymer solution decreases with increase in polymer solution concentration and therefore viscosity, leading to the formation of sponge like structures with reduced pore size47.
Clean water flux
The control PES membrane (S1), PES-PANCMA membrane (S2) and the PES-PANCMA-PEI-Ag membrane (S3) were tested to evaluate the clean water flux of the membrane using a cross flow filtration setup. Table 1 shows the clean water flux for all the 3 different membranes at a constant feed water pressure of 100 kPa together with water contact angle. Even though their pore size is slightly smaller than S1, samples S2 and S3 gave higher pure water flux which may be due to the increase in hydrophilicity (represented by a decrease in contact angle) of the membrane. The highest flux achieved is 687 ± 28 LMH for sample S3 which is about 39.4% higher than that for the control membrane.
Discussion
Zone inhibition test
The antibacterial activities of the control PES membrane (S1), PES-PANCMA membrane (S2) and the PES-PANCMA-PEI-Ag membrane (S3) were determined qualitatively using the zone inhibition test. As shown in Figure 7(a), sample S3 exhibited the best results with a clear inhibition zone of width 2.5–3.0 mm around the membrane in stark contrast to sample S1 and S2 which did not show any inhibition zone. This is due to the bactericidal property of silver nanoparticles in the composite membrane S3.
In order to study the self-cleaning property of the membrane, all three membrane samples were filtered with 10 ppm sodium alginate solution (the alginate solution is representative of the negatively charged protoplasm compounds that would be released by lysed bacteria) for 1320 min and then tested for their antibacterial property. The results are presented in Figure 7(b).
Sample S3 exhibited positive results with a clear inhibition zone around the membrane even after a long period of continuous filtration of protein solution. This result confirms that the negatively charged functional groups present in the modified membrane matrix prevent the deposition of sodium alginate on the membrane surface. This finding is in agreement with previously reported work indicating that membranes with hydrophilic negatively charged functional groups on the surface can repel proteins which make up extra cellular polymeric substances (EPS)48.
Long time performance and the fouling evaluation with sodium alginate
To evaluate the antifouling efficiency of the membrane, a long time (1320 min) filtration of 10ppm sodium alginate in DI water was carried out for the control PES membrane (S1), PES-PANCMA membrane (S2) and the PES-PANCMA-PEI-Ag membrane (S3) individually and the results are summarized in Figure 8a. It is observed that the modified PES-PANCMA-PEI-Ag membrane (S3) gives stable flux compared to the other two membrane samples S1 and S2. The flux drop of the membranes is shown in Figure 8b. The flux drop for the modified PES-PANCMA-PEI-Ag membrane (S3) is lower (16.3% of the initial flux) than the control membrane sample S1 (55.3% of the initial flux) and the PES-PANCMA membrane sample S2 (33% of the initial flux). The obtained results indicate that there is not much decrease in the flux with time for the modified PES-PANCMA-PEI-Ag membrane (S3) showing that this membrane can efficiently repel the organic matter in the form of the sodium alginate protein from the surface of the membrane.
In order to study the filtration efficiency of the membrane, the total organic carbon (TOC) of the feed water and permeate water were measured (4 samples were collected every 60 mins and the TOC was measured in order to get the average TOC removal efficiency over time). Percentage of TOC rejection was calculated and plotted in Figure 8c. It was found that the TOC removal of the modified PES-PANCMA-PEI-Ag membrane (S3) is higher compared to samples S1 and S2. It is also observed that the modified membrane gives comparatively stable rejection of TOC when compared to the other two samples. This is due to the hydrophilicity and the presence of highly negative charged –NH2, -COOH and –OH on the membrane surface. The negative surface charge of the membrane prevents the deposition of the negatively charged colloidal particles (proteins, lipids and amino acids etc.,) on the membrane surface by electrostatic repulsion, which can slow down the membrane fouling process49. It is also reported that the membrane fouling can be reduced by increasing the negative surface density of the membrane50. TOC removal efficiency of the control membrane was also increased with time, which may due to the pore constriction or development of a cake layer (fouling) on the membrane surface51.
Methods
Materials and chemicals
Polyethersulphone (PES) k-3010 powder was purchased from Sumitomo chemicals pte ltd, Japan. Acrylonitrile, maleic anhydride, azoisobisbutyronitrile (AIBN), silver nitrate (AgNO3), sodium borohydride (NaBH4) and polyethylenimine (PEI) were purchased from Sigma Aldrich with 99% purity. High purity ethanol, N-methyl-2- pyrolidone (NMP), N,N-Dimethyl acetamide (DMAc), Polyvinyl pyrolidone (PVPK30) and Diethylene glycol (DEG) were also purchased from Sigma Aldrich and used as received. The water used for the reaction was distilled and de-ionized (DI) with a Milli-Q plus system from Millipore, Bedford, MA, USA.
Synthesis of poly (acrylonitrile co maleic anhydride) (PANCMA)
PANCMA was synthesized by a free radical polymerization in solution-suspension method using DMAc as follows. To about 150 ml DMAc, 9.89 grams of maleic anhydride and 5.31 grams of acrylonitrile were added and stirred at room temperature for about 30 minutes. After complete dissolution, 1.25 grams of AIBN was added to the solution which was stirred at 60°C for further period of five hours. The polymer obtained as a suspension was separated out by filtration and washed thoroughly with diethyl ether and dried overnight in vaccum at 40°C. The schematic representation of the reaction is shown in Figure 9.
Synthesis of Ag capped PEI
Silver attached PEI was prepared by a previously reported procedure as below. To a 50 ml of aqueous PEI (50%) solution, 0.5grams of AgNO3 was added and allowed to stir at room temperature for 1 hour. About 10 ml of freshly prepared NaBH4 was then added drop-wise to the above solution with stirring over a period of 30 mins followed by continuous stirring over night at 80°C. The final dark green solution obtained was then subjected to centrifugation to remove excess reactants. The schematic representation of the reaction is shown in Figure 10.
Synthesis of Ag capped PEI attached PANCMA
Silver capped PEI was attached to PANCMA by a simple nucleophilic addition reaction as below. About 10 grams of PANCMA was added to a 250 ml flask with 100 ml DMAc. The flask was connected to a nitrogen inlet. After complete dissolution, 25 ml of silver capped PEI solution was added dropwise to the polymer solution and allowed to stir at 25°C for 8 hours. The obtained final homogeneous solution was poured into methanol to separate the Ag capped PEI attached PANCMA. The precipitated polymer was filtered, washed with methanol and dried in vacuum at 40°C overnight. The schematic representation of the reaction is shown in Figure 11.
Preparation of membranes by dry wet spinning
Polyethersulfone (PES) hollow fiber ultrafiltration membranes were prepared by dry wet spinning method. PES was used as the base polymer, NMP was the base solvent, DEG was used as a non-solvent and two additives, PVP K30 (pore forming agent) and hydrophilic and antimicrobial PANCMA-PEI-Ag polymer composite were used to improve the surface property of the membrane. The composition of the casting solution consists of 21 wt% PES, 5 wt% PVP-K-30, 5 wt% DEG, 64 ~ 69 wt% NMP, 0–5 wt% PANCMA and 0-5 wt% PANCMA-PEI-Ag, respectively. The dope compositions are presented in Table 2. PVP powder was first added into the NMP/DEG mixture in a round bottom flask and the solution was stirred by a mechanical stirrer for at least 1–1.5 hours. After complete dissolution of PVP, PANCMA/PANCMA-PEI-Ag and PES were added and allowed to stir at a constant speed of 250 ~ 350 rpm for at least 24 h at 80°C to obtain a completely dissolved homogeneous polymeric solution. The dope solution was poured into the polymer tank and degassed at a negative pressure of -0.6 bar for 15–20 min. Nitrogen gas was purged into the dope tank to create an inert atmosphere and to push the polymer towards the polymer pump. NMP and water were mixed in 80:20 volume ratio and the mixture, used as the bore liquid, was poured into the bore liquid tank. The polymer solution and the bore liquid were pumped to the spinneret (OD 1.2 mm, ID 0.6 mm). The air gap was fixed at 10 mm. The hollow fiber membranes were fabricated at around 25°C and at around 65–70% relative humidity with a take up speed of 0.25 m/s. The membranes were collected from the winder and left inside a post-coagulation water tank for a minimum of 24 hours to washout the residual NMP, DEG and PVP that were not removed from the solution during the fabrication process. The membranes were immersed into a post treatment solution of 40% ethanol and 60% glycerin before being subjected to the clean water flux tests.
References
Mar, k. A. et al. Science and technology for water purification in the coming decades. Nature 452, 301–310 (2008).
Guo, H., Wyart, Y., Perot, J., Nauleau, F. & Moulin, P. Low-pressure membrane integrity tests for drinking water treatment: a review. Water Res. 44, 41–57 (2010).
Celik, E., Park, H., Choi, H. & Choi, H. Carbon nanotube blended polyethersulfone membranes for fouling control in water treatment. Water Res. 45, 274–282 (2011).
Drews, A. Membrane fouling in membrane bioreactors e characterization, contradictions, cause and cures. J. Membr. Sci. 363, 1e28 (2010).
Asatekin, A., Kang, S., Elimelech, M. & Mayes, A. M. Anti-fouling ultrafiltration membranes containing polyacrylonitrile graft-poly (ethylene oxide) comb copolymer additives. J. Membr. Sci. 298, 136–146 (2007).
Jarusutthira, K. C. & Amy, G. Role of soluble microbial products (SMP) in membrane fouling and flux decline. Environ. Sci. Technol. 40, 969–974 (2006).
Nan, J. et al. Preparation and characterization of PES-SiO2 organic–organic composite ultrafiltration membranes for raw water treatment. Chem. Eng. J. 168, 1272–1278 (2011).
Boussu, K., Vandecasteele, C. & Van der Bruggen, B. Study of the characteristics and the performance of self-made nano porous polyethersulfone membranes. Polymer 47, 3464–3476 (2006).
Razmjou, A., Mansouri, J. & Chen, V. The effects of mechanical and chemical modification of TiO2 nanoparticles on the surface chemistry, structure and fouling performance of PES ultrafiltration membranes. J. Membr. Sci. 378, 73–84 (2011).
Al Malek, S. A., Abu, M. N., Seman, D. & Johnson, N. Hilal, Formation and characterization of polyethersulfone membranes using different concentrations of polyvinlylpyrrolidone. Desalination 288, 31–39 (2012).
Ng, L. Y., Ahmad, A. & Mohammad, A. W. Alteration of polyethersulphone membranes through UV-induced modification using various materials: A brief review. Arabian Journal of Chemistry (2013) (article in press).
Kelly, S. T. & Zydney, A. L. Mechanisms for BSA fouling during microfiltration. J.Membr. Sci. 107, 115–127 (1995).
Nystrom, M., Pihlajamaki, A. & Ehsani, N. Characterization of Ultrafiltration Membranes by Simultaneous Streaming Potential and Flux Measurements. J.Membr. Sci. 87, 245–256 (1994).
Donogh, Mc., Bauser, R. M., Stroh, H. & Chmiel, H. Concentration Polarisation and Adsorption Effects in Cross-Ultrafiltration of Proteins. Desalination 79, 217–231 (1990).
Ricq, L., Narcom, S., Reggiani, J. C. & Pagetti, J. Streaming Potential and Protein Transmission Ultrafiltration of Single Proteins and Proteins in Mixture: B-lactoglobulin and Lysozyme. J. Membr. Sci. 156, 81–96 (1999).
Fangshu, Qu. et al. Ultrafiltration membrane fouling caused by extracellular organic matter (EOM) from Microcystisaeruginosa: Effects of membrane pore size and surface hydrophobicity. J.Membr. Sci. 449, 58–66 (2014).
Klaysom, C., Ladewig, B. P., Lu, G. Q. M. & Wang, L. Preparation and characterization of sulfonated polyethersulfone for cation exchange membranes. J. Membr. Sci. 368, 48–53 (2011).
Baron, G. N. B., Cha, B. J. & Jung, B. Negatively charged poly(vinylidene fluoride) microfiltration membranes by sulfonation. J. Membr. Sci. 290, 46–54 (2007).
Lau, W. J. & Ismail, A. F. Effect of SPEEK content on the morphological and electrical properties of PES/SPEEK blend nanofiltration membranes. Desalination 249, 996–1005 (2009).
Rahimpour, A., Madaeni, S. S., Ghorbani, S., Shockravi, A. & Mansourpanah, Y. The influence of sulfonated polyethersulfone (SPES) on surface nano-morphology and performance of polyethersulfone (PES) membrane. Appl. Surf. Sci. 256, 1825–1831 (2010a).
Blanco, J. F., Nguyen, Q. T. & Schaetzel, P. Novel hydropilic membrane materials: sulfonated polyethersulfone Cardo. J. Membr. Sci. 186, 267–279 (2001).
Reddy, A. V. R., Mohan, D. J., Bhattacharya, A., Shah, V. J. & Ghosh, P. K. Surface modification of ultrafiltration membranes by pre adsorption of a negatively charged polymer I. Permeation of water soluble polymers and inorganic salt solutions and fouling resistance properties. J. Membr. Sci. 214, 211–221 (2003).
Rahimpour, A. UV photo grafting of hydrophilic monomers onto the surface of nano-porous PES membranes for improving surface properties. Desalination 265, 93–101 (2011).
Wang, D., Zou, W., Li, L., Wei, Q., Sun, S. & Zhao, C. Preparation and characterization of functional carboxylic polyethersulfone membrane. J. Membr. Sci. 374, 93–101 (2011).
Peeva, P. D., Million, N. & Ulbricht, M. Factors affecting the sieving behavior of antifouling thin-layer cross-linked hydrogel polyethersulfone composite ultrafiltration membranes. J. Membr. Sci. 390–391, 99–112 (2012).
Yune, P. S., Kilduff, J. E. & Belfort, G. Fouling-resistant properties of a surface-modified poly (ether sulfone) ultrafiltration membrane grafted with poly(ethylene glycol)- amide binary monomers. J. Membr. Sci. 377, 159–166 (2011).
Conceic, T. et al. Poly (ether ether ketone) derivatives: synthetic route and characterization of nitrated and sulfonated polymers. Mater. Sci. Eng. C 29, 575–582 (2009).
Geluwe, S. V., Braeken, L. & Van der Bruggen, B. Ozone oxidation for the alleviation of membrane fouling by natural organic matter: a review. Water Res. 45, 3551–3570 (2011).
Gao, W. et al. Membrane fouling control in ultrafiltration technology for drinking water production: a review. Desalination 272, 1–8 (2011).
Kull, K. R., Steen, M. L. & Fisher, E. R. Surface modification with nitrogen-containing plasmas to produce hydrophilic, low fouling membranes. J. Membr. Sci. 246, 203–215 (2005).
Zhao, S. et al. Performance improvement of polysulfone ultrafiltration membrane using PANiEB as both pore forming agent and hydrophilic modifier. J. Membr. Sci. 385–386, 251–262 (2011).
Arthanareeswaran, G., Mohan, D. & Raajenthiren, M. Preparation, characterization and performance studies of ultrafiltration membranes with polymeric additive. J. Membr. Sci. 350, 130–138 (2010).
Li, L. L. et al. Preparation and characterization of poly (acrylonitrile-acrylic acid-N-vinyl pyrrolidinone) terpolymer blended polyethersulfone membranes. J. Membr. Sci. 349, 56–64 (2010).
Van der Bruggen, B. Chemical modification of polyethersulfone nanofiltration membranes: a review. J. Appl. Polym. Sci. 114, 630–642 (2009).
Idris, A., Mat Zain, N. & Noordin, M. Y. Synthesis, characterization and performance of asymmetric polyethersulfone (PES) ultrafiltration membranes with polyethylene glycol of different molecular weights as additives. Desalination 207, 324–339 (2007).
Marchese, J. et al. Fouling behavior of polyethersulfone UF membranes made with different PVP. J. Membr. Sci. 211, 1–11 (2003).
Moon, E. J., Kim, J. W. & Kim, C. K. Fabrication of membranes for the liquid separation: Part 2: microfiltration membranes prepared from immiscible blends containing polysulfone and poly(1-vinylpyrrolidone-co-acrylonitrile) copolymers. J. Membr. Sci. 274, 244–251 (2006).
Kim, Y., Rana, D., Matsuura, T. & Chung, W. J. Towards antibiofouling ultrafiltration membranes by blending silver containing surface modifying macromolecules. Chem. Commun. 48, 693–695 (2012).
Diagne, F. et al. Polyelectrolyte and silver nanoparticle modification of microfiltration membranes to mitigate organic and bacterial fouling. Environ. Sci. Technol. 46, 4025–4033 (2012).
Zhang, M., Zhang, K., De Gusseme, B. & Verstraete, W. Biogenic silver nanoparticles (bio-Ag0) decrease biofouling of bio- Ag0/PES nanocomposite membranes. Water Res. 46, 2077–2087 (2012).
Prince, J. A., Bhuvana, S., Boodhoo, K. V. K., Anbharasi, V. & Singh, G. Synthesis and characterization of PEG-Ag immobilized PES hollow fiber ultrafiltration membranes with long lasting antifouling properties. J. Membr. Sci. 454, 538–548 (2014).
Ishihara, K. et al. Why do phospholipid polymers reduce protein adsorption? J. Biomed. Mater. Res. 39, 323–330 (1998).
Liu, P. S., Chen, Q., Wu, S. S., Shen, J. & Lin, S. C. Surface modification of cellulose membranes with zwitterionic polymers for resistance to protein adsorption and platelet adhesion. J. Membr. Sci. 350, 387–394 (2010).
Guggenbichler, J. P., Boswald, M., Lugauer, S. & Krall, T. A new technology of microdispersed silver in polyurethane induces antimicrobial activity in central venous catheters. Infection 27 (Suppl. 1), S16–S23 (1999).
Kang, S. T. et al. Direct observation of biofouling in cross-flow microfiltration: mechanisms of deposition and release. J. Membr. Sci. 244, 151–165 (2004).
Kang, S., Hoek, E. M. V., Choi, H. & Shin, H. Effect of membrane surface properties during the fast evaluation of cell attachment. Sep. Sci. Technol. 41, 1475–1487 (2006).
Young, T. H., Cheng, L. P., Lin, D. J., Fane, L. & Chuang, W. Y. Mechanisms of PVDF membrane formation by immersion-precipitation in soft (1-octanol) and harsh (water) non solvents. Polymer 40, 5315–5323 (1999).
Subramani, A. & Hoek, E. M. V. Direct observation of initial microbial deposition onto reverse osmosis and nanofiltration membranes. J. Membr. Sci. 319, 111–125 (2008).
Knoell, T. et al. Biofouling potentials of microporous polysulfone membranes containing a sulfonated polyether–ethersulfone/polyethersulfone block copolymer: correlation of membrane surface properties with bacterial attachment. J. Membr. Sci. 157 (1), 117–138 (1999).
Khulbe, K. C., Feng, C. & Matsuura, T. The Art of Surface Modification of Synthetic Polymeric Membranes, Wiley Inter Science- J of Appl. Polym Sci. 10.1002/app.31108 (2009).
Katsoufidou, K., Yiantsios, S. G. & Karabelas, A. J. Experimental study of ultrafiltration membrane fouling by sodium alginate and flux recovery by backwashing. J. Membr. Sci. 300, 137–146 (2007).
Acknowledgements
This work was supported from funding provided by Ministry of Education, Singapore under the Innovation Fund. The authors also gratefully acknowledge the support from Newcastle University, United Kingdom, Environmental & Water Technology – Centre of Innovation, Ngee Ann Polytechnic, Singapore and Singapore Institute of Technology for the NU-Poly-SIT scholarship awarded to J.A.P.
Author information
Authors and Affiliations
Contributions
J.A.P. and S.B. conceived the project and planned the experiments and synthesized the polymer composite. J.A.P. and N.A. prepared the hollow fiber membrane samples and carried out most of the experiments (contact angle measurement, clean water flux and long time performance study with sodium alginate). J.A.P., B.S. and V.A. carried out the FTIR, SEM and SEM-EDS elemental mapping measurements.V.A. carried out the antibacterial studies. J.A.P., S.B., G.S. and K.V.K.B. analyzed the data and wrote the paper. All authors discussed the results and commented on the paper.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Prince, J., Bhuvana, S., Anbharasi, V. et al. Self-cleaning Metal Organic Framework (MOF) based ultra filtration membranes - A solution to bio-fouling in membrane separation processes. Sci Rep 4, 6555 (2014). https://doi.org/10.1038/srep06555
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep06555
This article is cited by
-
Industrial oily wastewater treatment by microfiltration using silver nanoparticle-incorporated poly (acrylonitrile-styrene) membrane
Environmental Sciences Europe (2023)
-
Acute toxicity of nanoscale zeolitic imidazolate framework 8 (ZIF-8) to saltwater planktonic species Artemia salina and Nannochloropsis oculata
Environmental Science and Pollution Research (2023)
-
Phosphate group functionalized magnetic metal–organic framework nanocomposite for highly efficient removal of U(VI) from aqueous solution
Scientific Reports (2021)
-
Metal Organic Framework in Membrane Separation for Wastewater Treatment: Potential and Way Forward
Arabian Journal for Science and Engineering (2021)
-
Testing the self-cleaning properties of a coordination polymer surface
Adsorption (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.