Abstract
Eighty eight patients with 91 uterine leiomyomas who underwent ultrasound-guided percutaneous microwave ablation (PMWA) treatment were prospectively included in the study in order to study the dose-effect relationship parameters (DERP) of PMWA for uterine leiomyomas and its relationship with T2-weighted MR imaging (T2WI). Based on the signal intensity of T2WI, uterine leiomyomas were classified as hypointense, isointense and hyperintense. During ablation, leiomyomas were treated with quantitative microwave ablation (QMWA) energy of 50 w × 300 s or 60 w × 300 s. After QMWA, contrast-enhanced ultrasound (CEUS) was performed to evaluate DERP. No matter under 50 w × 300 s or 60 w × 300 s, quantitative microwave ablation volume (QMAV) of hyperintense leiomyoma was smaller than that of hypointense and isointense leiomyoma (P<0.016). For hypointense and isointense leiomyoma, QMAV of 60 w × 300 s was larger than that of 50 w × 300 s (P<0.05). DERPs obtained by T2WI can be used to guide the treatment of uterine leiomyoma by PMWA.
Similar content being viewed by others
Introduction
Uterine leiomyomas are benign clonal tumours that arise from the smooth-muscle cells of the human uterus. Although many of them are asymptomatic, as many as 25% patients have symptoms1 such as abnormal uterine bleeding, dysmenorrhea, pelvic pressure, low abdominal pain and infertility. Hysterectomy has long been the main mode of therapy for leiomyomas, but it is associated with significant morbidity and guarantees infertility2,3. Even women without a desire for future pregnancies might not wish to lose their uterus for various reasons. However, medical technology advancements have made less invasive treatment options available, such as uterine artery embolization4,5,6, high intensity focused ultrasound7,8,9, radiofrequency10,11 and percutaneous microwave ablation (PMWA)12,13. PMWA is a minimally invasive technique for treatment of uterine leiomyomas by inducing tissue necrosis through thermal damage. Previous reports12,13 have indicated that PMWA provides a feasible, safe and reliable alternative for the treatment of uterine leiomyomas. However, until now, the dose-effect relationship parameters (DERP) of PMWA for uterine leiomyomas are still unclear. In this study, we sought to investigate the DERPs of PMWA for uterine leiomyomas and the relationship with T2-weighted MR imaging (T2WI) to make the PMWA procedures quantized and to guide better clinical treatment.
Methods
The study was approved by the institutional ethics committee. Informed consent was obtained from all participants. And all experiments were performed in accordance with relevant guidelines and regulations.
Patients
From July 2012 to December 2013, 88 premenopausal women with symptomatic uterine leiomyomas who underwent PMWA treatment at PLA General Hospital were prospectively analyzed.
The inclusion criteria were as follows: (1) Patients had been diagnosed with uterine leiomyomas by ultrasonography and ceMRI in our hospital; patients had experienced one of the following symptoms for more than 1 year: menorrhagia or metrorrhagia, dysmenorrhea, lower abdominal pain, bulk pressure, or urinary frequency; (2) Patients were above 18 years of age and in premenopausal status; (3) Patients with no reproductive needs; (4) The average diameter was ≥5 cm (5) Patients underwent ceMRI before and after PMWA treatment within 5 days; (6) Patients had never received other treatments (such as myomectomy, HIFU, UAE, cryoablation, radiofrequency and ethanol injection) before PMWA;
Exclusion criteria: (1) menstruating, pregnant or breastfeeding women; (2) patients with pelvic infection, coagulation disorders, heart or brain diseases or malignant tumors confirmed by pathology.
Pretreatment MRI
A series of standard T1-weighted imaging, T2-weighted imaging and enhanced T1-weighted imaging were performed in all patients with a 1.5-T clinical MR system (TwinSpeed Signa EXCITE HD; GE Healthcare, Milwaukee, WI, USA) before and after treatment within 5 days.
The number, size and location of the uterine leiomyomas were analyzed by MRI. Uterine leiomyomas were classified as three types on pretreatment T2-weighted MRI14: (1) hypointense, signal intensity like skeletal muscle; (2) isointense, signal intensity is lower than myometrium but higher than that of skeletal muscle; (3) hyperintense, signal intensity is the same as or higher than myometrium.
Uterine leiomyomas were classified as slight enhancement, homogeneous enhancement and inhomogeneous enhancement relative to myometrium on the basis of dynamic contrast-enhanced MRI (ceMRI): (1) Slight enhancement is that the enhancement degree was lower than that of myometrium; (2) homogeneous enhancement is defined as that of the distribution of enhanced signal is homogeneous and the enhanceement degree is the same as or higher than that of myometrium; (3) inhomogeneous enhancement is that the distribution of enhanced signal is inhomogeneous and slightly enhanced signal is interspersed among enhanced signal.
PMWA therapeutic instrument
MW tumor coagulator: A KV2000 MW tumor coagulator (Kangyou Medical Instruments, Nanjing, China) was used with frequency of 2450 MHz and was capable of continuous and pulse MW emission modes. The needle antenna was 15 gauge in diameter and 18 cm in length. The distance from the aperture of the MW emission to the needle tip was 11 mm and the emission aperture was 1 mm. For the antenna, an internal water cycle cooling system was used to lower the temperature of the needle shaft15.
PMWA procedure
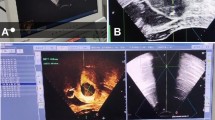
The ablation was performed under intravenous conscious sedation (intravenous infusion of flurbiprofen ester 2 mg/kg, supplemented by pumping propofol 4 mg · kg−1 · h−1). The patients were placed in a supine position. Under US guidance, the MW antenna was inserted into the lesion. The output energy of the PMWA was set at 50 w or 60 w and the ablation time was set at 300 s. Then CEUS was performed to evaluate DERP which included quantitative microwave ablation length (QMAL), quantitative microwave ablation width (QMAW), quantitative microwave ablation height (QMAH), quantitative microwave ablation volume (QMAV) and energy required for per unit volume (EPV).
Intravenous CEUS
The CEUS was performed in all patients after 50 w × 300 s or 60 w × 300 s' ablation. Informed consent was obtained from each individual participated in this study.
Contrast agent: SonoVue (manufactured by Bracco Company in Italy). Five milliliter of physiological saline was injected into a portion of 4.98 mg frozen dry powder, fully shaken and intermingled. 2.0 ml of contrast agent was injected via elbow vein quickly, followed by 5 ml of physiological saline for washing.
The DERPs was calculated with CEUS. The non-enhanced CEUS volume after 50 w × 300 s or 60 w × 300 s' ablation was defined as QMAV. The non-enhanced volume was calculated according to 4/3π (d/2)3, d = (QMAL + QMAW + QMAH)/3. The QMALs were measured along the direction of the needle, the QMAHs were measured perpendicularly to the length on the same plane and the QMAWs were measured on the plane which was perpendicular to the needle's plane. When the QMWA energy was 50 w × 300 s, EPV was calculated according to 15000 J/QMAV. When the QMWA energy was 60 w × 300 s, EPV was calculated according to 18000 J/QMAV.
Statistical analysis
Statistical analysis was performed with SPSS 13.0; measurement data were expressed with  . The non-parametric Kruskal-Wallis test was performed to test for differences between CeMRI characteristics of leiomyomas in different T2WI signal. Probability values less than 5% were considered to be significant. Statistical analysis of QMAVs and EPVs of 50 w × 300 s or 60 w × 300 s were performed using Kruskal-Wallis H rank sum test followed by Bonferroni test, Probability values of less than 1.6% were considered to be significant. Comparisons of QMAVs and EPVs of 50 w × 300 s and 60 w × 300 s were assessed using t-test or Wilcoxon test.
. The non-parametric Kruskal-Wallis test was performed to test for differences between CeMRI characteristics of leiomyomas in different T2WI signal. Probability values less than 5% were considered to be significant. Statistical analysis of QMAVs and EPVs of 50 w × 300 s or 60 w × 300 s were performed using Kruskal-Wallis H rank sum test followed by Bonferroni test, Probability values of less than 1.6% were considered to be significant. Comparisons of QMAVs and EPVs of 50 w × 300 s and 60 w × 300 s were assessed using t-test or Wilcoxon test.
Results
1. Patients and leiomyomas. A total of 88 patients with 91 uterine leiomyomas were treated with PMWA. The mean age was 40.3 ± 5.6 years (from 32 to 48 years old). The number of hypointense, isointense and hyperintense leiomyoma was 35, 29 and 27, respectively. The mean leiomyoma volume was 158.09 ± 127.28 cm3 (from 65.45 to 606.13 cm3). The mean volume of hypointense leiomyomas was 211.97 ± 193.41 cm3 (from 65.45 to 606.13 cm3), of isointense leiomyomas was 150.20 ± 99.71 cm3 (from 65.45 to 448.92 cm3) and of hyperintense leiomyomas was 148.70 ± 71.4 cm3 (from 65.45 to 268.08 cm3), respectively.
2. Contrast-enhancement characteristics of leiomyomas with different T2WI signal intensity were showed in Table 1. Homogeneous enhancement were showed (10/35) 28.57% in hypointense leiomyomas versus (17/27) 62.96% in hyperintense leiomyomas. Inhomogeneous enhancement or light enhancement were showed (25/35) 71.43% in hypointense leiomyomas versus (10/27) 37.04% in hyperintense leiomyomas. Significant difference was observed (X2 = 7.33,P = 0.01) (Table 1).
3. QMWLs, QMWWs, QMWHs, QMAVs and EPVs of 50 w × 300 s and 60 w × 300 s for different signal intensity were listed in Table 2 and Table 3. No matter for 50 w × 300 s or 60 w × 300 s, QMAVs of hyperintense leiomyomas were smaller than that of the hypointense and isointense leiomyomas. (Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6) (p < 0.016).
4. By comparison, QMAVs of 50 w × 300 s for hypointense and isointense leiomyomas were smaller than that of 60 w × 300 s with statistically significant difference (Table 4). For the EPVs of 50 w × 300 s and 60 w × 300 s for different signal intensity, there was no difference was observed (Table 5).
Discussion
Uterine leiomyomas are the most common tumor in the female reproductives system. Since the first case of uterine leiomyoma treatment with PMWA was reported in 200816 in China, a series of studies12,13 have indicated that PMWA provides a feasible, safe and reliable alternative for the treatment of uterine leiomyomas. In these treatments, they used the DERP of MW ablation in porcine musculatureex in vivo and hyperecho (caused by microbubbles generated during MW emission and representing roughly the ablation zone17) to guide the ablation of leiomyomas12. On the one hand, the DERP which was got from in vitro porcine muscle did not consider the influence of blood flow to ablation effect. Moreover, the proportion and arrangment of the cells in porcine muscle and leiomyoma are different. So the DERP of in vitro porcine muscle could not predict the DEPR of leiomyoma precisely. On the other hand, the study of Fang Wang et al.13 showed that measurements of the hyperechoic range on ultrasonography is strongly correlated to the no enhancement area by contrast-enhanced ultrasonography. But variations of the hyperecho needed to be monitored by real-time ultrasonography, which could not be used to predict the ablation area before treatment, so as to do treatment planning. Therefore, the study of DEPR of in vivo uterine leiomyomas is necessary.
MRI is the most accurate imaging technique for detection and evaluation of leiomyomas and therefore it has become the imaging modality of choice before and after PMWA. The study of Schwartz18 thought leiomyoma subtypes can be diagnosed accurately by MRI. It had 95% sensitivity and 72% specificity to diagnose an ordinary uterine leiomyoma and 10% sensitivity and 100% specificity for a cellular uterine leiomyoma, respectively. Specific sequence imaging can provide some information about tissue directly19,20 and can reveal the pathological character of lesions to a certain extent21. The study of Swe et al.22 has shown that uterine leiomyomas with T2W1 hypointensity or isointensity indicating more fiber and fewer cellular content or lesion with less blood supply. To the contrary, uterine leiomyomas with T2W1 hyperintensity indicated cellular leiomyomas or vascularization. DERP of uterine leiomyoma is closely related to pathological character. Previous study found that EPVs of different T2WI signal intensity were different23. MRI signal intensity of uterine leiomyomas can be used to predict PMWA energy. Therefore, we further studied the DERP of QMWA for uterine leiomyomas of different T2W1 signal intensity. The study of DERP of QMWA for in vitro porcine muscle indicated that a spherical ablation shape can be created most likely, when the microwave power is 50 w or 60 w. And the two powers can be used as clinical treatment power. Therefore, in the study, a QMWA of 50 w/60 w for 300 s were performed for uterine leiomyomas of different T2WI signal intensity. And DERP was summarized and analyzed (Table 2, Table 3) in order to be used to predict PMWA energy of uterine leiomyoma before treatment and to predict the number of antennas and their placement.
The QMAVs for hypo-, iso- and hyper-intensity uterine leiomyomas under 50 w × 300 s were respectively 46.48 ± 25.63 cm3, 44.46 ± 16.72 cm3 and 23.58 ± 11.85 cm3; the QMAVs for hypo-, iso- and hyper-intensity uterine leiomyomas under 60 w × 300 s were respectively 54.29 ± 22.46 cm3, 51.36 ± 8.63 cm3, 22.54 ± 2.98 cm3. No matter for 50 w × 300 s or 60 w × 300 s, QMAVs of hyperintense leiomyomas were smaller than that of hypointense and isointense leiomyomas. These were statistically significance difference. This study analyzed the ceMRI characteristics for hypointensity, isointensity and hyperintensity uterine leiomyomas, which indicates that uterine leiomyomas of hypointensity are mainly slight or inhomogeneous enhancement and uterine leiomyomas of hyperintensity were mainly homogeneous enhancement, between which there was statistical significance. According to Harman's study24, increased contrast enhancement of leiomyoma is presumably indicative of a lesion with increased vascularity. As high perfusion decreases heat accumulation through the vascular cooling effect, more abundant blood supply, more heat will be taken away during the ablation process, smaller microwave ablation region will be obtained25,26. In our study, hyperintense leiomyomas enhanced better than that of hypointense leiomyomas, which was consistent with Swe's study. And the QMAV of uterine leiomyomas with T2W1 hyperintensity is smaller than that of hypointensity.
For hypointense and isointense leiomyomas, QMAVs of 60 w × 300 s were larger than that of 50 w × 300 s, with statistically significant difference. Comparing with 50 w × 300 s, 60 w × 300 s may extend the ablation range. For larger leiomyomas, treatment with 60 w × 300 s is more time-saving than 50 w × 300 s.
The ablation EPVs for hypo-, iso- and hyper-intensity uterine leiomyomas under 50 w × 300 s were respectively 381.91 ± 120.74 J/cm3, 393.00 ± 171.86 J/cm3, 843.80 ± 592.09 J/cm3. The ablation EPVs for hypo-, iso- and hyper-intensity uterine leiomyomas under 60 w × 300 s were respectively 373.79 ± 119.26 J/cm3, 368.54 ± 49.26 J/cm3, 807.81 ± 102.87 J/cm3. EPVs of 50 w × 300 s is greater than that of 60 w × 300 s, but the difference was not statistically significant. In the study, we can not conclude that treatment with 60 w × 300 s is more energy-saving than that with 50 w × 300 s.
In conclusion
This research prospectively studied the DERPs of PMWA for uterine leiomyomas and compared the QMAVs and EPVs in different T2WI signal intensity. The parameters obtained can be used to guide treatment planning. This can effectively reduce the unnecessary microwave radiation time, increase the MW ablatioin safety and promote the widespread utilization of MWA technique for uterine leiomyomas in clinical.
References
Wallach, E. E. & Vlahos, N. F. Uterine myomas: an overview of development, clinical features and management. Obstet Gynecol. 104, 393–406 (2004).
Isonishi, S. et al. Analysis of prognostic factors for patients with leiomyoma treated with uterine arterial embolization. Am J Obstet Gynecol. 198, 270.e1–e6 (2008).
Van der Kooij, S. M., Ankum, W. M. & Hehenkamp, W. J. Review of nonsurgical/minimally invasive treatments for uterine fibroids. Curr Opin Obstet Gyneclo. 24, 368–375 (2012).
Chang, S. et al. Uterine Artery Embolization for Symptomatic Fibroids with High Signal Intensity on T2-Weighted MR Imaging. Korean J Radiol. 13, 618–624 (2012).
Moss, J. G. et al. Randomised comparison of uterine artery embolisation (UAE) with surgical treatment in patients with symptomatic uterine fibroids (REST trial): 5-year results. BJOG. 118, 936–944 (2011).
Toor, S. S. et al. Clinical failure after uterine artery embolization: evaluation of patient and MR imaging characteristics. J Vasc Interv Radiol. 19, 662–667 (2008).
Stewart, E. A. et al. Clinical outcomes of focused ultrasound surgery for the treatment of uterine fibroids. Fertil Steril. 85, 22–29 (2006).
Gorny, K. R. et al. Magnetic resonance-guided focused ultrasound of uterine leiomyomas: review of a 12-month outcome of 130 clinical patients. JVIR. 22, 857–864 (2011).
Lenard, Z. M. et al. Uterine leiomyomas: MR imaging-guided focused ultrasound surgery--imaging predictors of success. Radiology. 249, 187–194 (2008).
Guido, R. S. et al. Radiofrequency volumetric thermal ablation of fibroids: a prospective, clinical analysis of two years' outcome from the Halt trial. Health Qual Life Outcomes. 11, 139 (2013).
Iversen, H., Lenz, S. & Dueholm, M. Ultrasound-guided radiofrequency ablation of symptomatic uterine fibroids: short-term evaluation of effect of treatment on quality of life and symptom severity. Ultrasound Obstet Gynecol. 40, 445–451 (2012).
Zhang, J. et al. Ultrasound-guided percutaneous microwave ablation for symptomatic uterine fibroid treatment--a clinical study. Int J Hyperthermia. 27, 510–516 (2011).
Wang, F. et al. Imaging manifestation of conventional and contrast-enhanced ultrasonography in percutaneous microwave ablation for the treatment of uterine fibroids. Eur J Radiol. 81, 2947–2952 (2012).
Funaki, K., Sawada, K., Maeda, F. & Nagai, S. Subjective effect of magnetic resonance- guided focused ultrasound surgery for uterine fibroids. J Obstet Gynaecol Res. 33, 834–839 (2007).
Lu, Y., Nan, Q., Li, L. & Liu, Y. Numerical study on thermal field of microwave ablation with water-cooled antenna. Int J Hyperthermia. 25, 108–15 (2009).
Zhang, J. et al. Ultrasound-guided percutaneous microwave ablation for management of uterine fibroid-a case report. Chin J Ultrasongry. 17, 326 (2008).
Zhang, J., Zhang, B. S., Feng, L., Jiang, X. & Ren, J. T. Experimental study of microwave ablation for muscular tissues with water-cooling single needle antenna. Chinese J Med Ultrasound. 4, 22–25 (2009).
Schwartz, L. B., Zawin, M., Carcangiu, M. L., Lange, R. & McCarthy, S. Does pelvic magnetic resonance imaging differentiate among the histologic subtypes of uterine leiomyomata? Fertil Steril. 70, 580–587 (1998).
Kishi, Y. et al. Four subtypes of adenomyosis assessed by magnetic resonance imaging and their specification. Am J Obstet Gynecol. 207, 114 e1–7 (2012).
Song, S. E. et al. MR imaging features of uterine adenomyomas. Abdom Imaging. 36, 483–488 (2011).
Murase, E., Siegelman, E. S., Outwater, E. K., Perez-Jaffe, L. A. & Tureck, R. W. Uterine leiomyomas: histopathologic features, MR imaging findings, differential diagnosis and treatment. Radiographics. 19, 1179–1197 (1999).
Swe, T. T. et al. Uterine leiomyoma: correlation between signal intensity on magnetic resonance imaging and pathologic characteristics. Radiat Med. 10, 235–242 (1992).
Ma, X. et al. Feasibility of predicting the microwave ablation energy for uterine leiomyomas with MRI signal intensity. CJMIT. 29, 1359–1362 (2013).
Harman, M., Zeteroğlu, S., Arslan, H., Sengül, M. & Etlik, O. Predictive value of magnetic resonance imaging signal and contrast-enhancement characteristics on post- embolization volume reduction of uterine fibroids. Acta Radiol. 47, 427–435 (2006).
Shitzer, A. & Kleiner, M. K. On the relationship between blood perfusion, metabolism and temperature in biological tissue heat balance. J Biomech Eng. 102, 162–169 (1980).
Pennes, H. H. Analysis of tissue and arterial blood temperatures in the resting human forearm. J Appl Physiol. 1, 93–122 (1948).
Acknowledgements
This study was supported by the Fund of PLA 12th Five Years Plan (CWS11J310) and the Capital Characteristic Clinical Application Research (Z131107002213144).
Author information
Authors and Affiliations
Contributions
M.X., Z.J., H.Z.Y., X.R.F. and D.B.W. wrote the main manuscript text, H.Y.L. and Y.Y. prepared figures 1–3, X.C.T. and Z.B.S. prepared figures 4–6. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Xia, M., Jing, Z., Zhi-yu, H. et al. Research of dose-effect relationship parameters of percutaneous microwave ablation for uterine leiomyomas - a quantitative study. Sci Rep 4, 6469 (2014). https://doi.org/10.1038/srep06469
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep06469
This article is cited by
-
Unplanned pregnancy after ultrasound-guided percutaneous microwave ablation of uterine fibroids: A follow-up study
Scientific Reports (2016)
-
Feasibility of Using Wideband Microwave System for Non-Invasive Detection and Monitoring of Pulmonary Oedema
Scientific Reports (2015)
-
Ultrasound-guided percutaneous microwave ablation for adenomyosis: efficacy of treatment and effect on ovarian function
Scientific Reports (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.