Abstract
Inadequate calorie intake or starvation has been suggested as a cause of neonatal jaundice, which can further cause permanent brain damage, kernicterus. This study experimentally investigated whether additional glucose treatments induce the bilirubin-metabolizing enzyme – UDP-glucuronosyltransferase (UGT) 1A1 – to prevent the onset of neonatal hyperbilirubinemia. Neonatal humanized UGT1 (hUGT1) mice physiologically develop jaundice. In this study, UGT1A1 expression levels were determined in the liver and small intestine of neonatal hUGT1 mice that were orally treated with glucose. In the hUGT1 mice, glucose induced UGT1A1 in the small intestine, while it did not affect the expression of UGT1A1 in the liver. UGT1A1 was also induced in the human intestinal Caco-2 cells when the cells were cultured in the presence of glucose. Luciferase assays demonstrated that not only the proximal region (-1300/-7) of the UGT1A1 promoter, but also distal region (-6500/-4050) were responsible for the induction of UGT1A1 in the intestinal cells. Adequate calorie intake would lead to the sufficient expression of UGT1A1 in the small intestine to reduce serum bilirubin levels. Supplemental treatment of newborns with glucose solution can be a convenient and efficient method to treat neonatal jaundice while allowing continuous breastfeeding.
Similar content being viewed by others
Introduction
Human neonates physiologically develop mild hyperbilirubinemia, which is a result of inadequate metabolism of serum bilirubin due to a significantly low expression of a bilirubin-metabolizing enzyme – UDP-glucuronosyltransferase (UGT) 1A1. Although serum bilirubin levels in human neonates usually decrease to the normal range within a week or two after birth, hyperbilirubinemia can be severe and can cause permanent brain damage, kernicterus, which is caused by neurotoxic bilirubin accumulated in the brain1. Breast-feeding has been recognized as a risk factor for neonatal hyperbilirubinemia, as serum bilirubin levels are higher in breast-fed infants compared to those in formula-fed infants2,3,4. Neonatal hyperbilirubinemia can be caused by multiple factors such as sub-optimal intake, weight loss, increased entero-hepatic circulation, genetic polymorphism of UGT1A1 and lower gestational age5,6,7,8. While inhibition of UGT1A1 activity and suppression of its expression by breast milk have been suggested as one of the mechanisms underlying breast milk-induced neonatal jaundice9,10, the complete mechanism has not been fully understood.
Clinical intervention to treat episodes of severe hyperbilirubinemia calls for extended phototherapy treatment to photochemically reduce bilirubin or even blood transfusion. While exchange transfusions using the umbilical vein are needed far less frequently than in the past, phototherapy also has disadvantages. Skin rashes, decreased maternal-infant interaction and lack of visual sensory input are examples of potential disadvantages of this treatment11,12,13. Although breast milk can also potentially induce the risk for kernicterus14, breast-feeding still has numerous short- and long-term health benefits to growing children. Therefore, an ideal treatment of newborn infants with hyperbilirubinemia should allow continuous breast-feeding while avoiding risk of the development of kernicterus.
An interesting report of treating newborn infants to prevent hyperbilirubinemia dates back to 2001, which is when Dr. Kubota presented his work at the 16th Annual Meeting of the Japanese Society for Breastfeeding Research in Tokyo. In his clinical research, a hundred newborn infants were orally given with 5% glucose solution immediately after birth. Breast-feeding was continued and their serum bilirubin levels were determined for a week. The bilirubin levels in infants who received the glucose solution along with breast milk were significantly lower than those in infants who only received breast milk (http://www.s-kubota.net). This simple method was developed based on the facts that inadequate calorie intake or starvation has been suggested as a cause of neonatal jaundice15. However, the underlying mechanism of glucose-induced reduction of serum bilirubin remains to be cleared, which can further support an establishment of a mechanism-based therapy of human neonatal hyperbilirubinemia.
Because UGT1A1 is the sole enzyme that can metabolize hydrophobic and neurotoxic bilirubin16, it can be speculated that the supplemental treatment of newborn infants with glucose solution might have enhanced the function of UGT1A1 to reduce serum bilirubin. In the present study, therefore, the effect of glucose on UGT1A1 expression was investigated in breast milk-induced neonatal jaundice model mice and in cultured cells to experimentally prove the role of intestinal UGT1A1 and its regulation in breast milk-induced jaundice.
Results
Effect of oral-treated glucose on UGT1A1 expression in hUGT1 mice
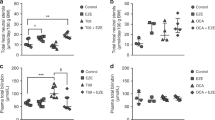
Humanized UGT1 (hUGT1) mice were previously developed in a C57BL/6 background. It was demonstrated that neonatal hUGT1 mice physiologically develop mild hyperbilirubinemia the same as human newborns10,17. In was further demonstrated that early human breast milk had an ability to suppress the expression of intestinal UGT1A1 in hUGT1 mice10. To investigate the effect of an oral glucose treatment on UGT1A1 expression, two most important tissues for bilirubin metabolism, liver and small intestine, were isolated from control and glucose-treated hUGT1 mice and then Q-RT-PCR was carried out for UGT1A1 using UGT1A1-specific primers18. The expression level of UGT1A1 was dramatically low in the liver and the expression was not affected by the glucose treatment (Fig. 1A). The expression level of UGT1A1 was much higher in the small intestine of hUGT1 mice (Fig. 1B). This observation, which is lower UGT1A1 expression in the liver and higher UGT1A1 expression in the small intestine, is in agreement with our previous finding17. Interestingly, the UGT1A1 level in the glucose-treated hUGT1 mice was statistically higher than that in the control mice (Fig. 1B). This indicates that the reduced bilirubin levels in glucose-treated human newborns might have been attributed to increased bilirubin metabolism in the small intestine.
Effects of oral glucose treatments on UGT1A1 expression in hUGT1 mice.
Glucose solution (50%, 150 µL) was orally administered to 14-day-old mice. Three hours after the treatment, liver (A) and small intestine (B) were isolated and used for the Q-RT-PCR analysis. The expression level of UGT1A1 was normalized with that of mouse CPH. *, P < 0.05 compared to the expression level in control mice. (n = 14 for control and n = 17 for glucose treatments).
Induction of UGT1A1 by glucose in Caco-2 cells
Caco-2 cells are a human small intestinal cell line that was isolated from a colon carcinoma19. Caco-2 cells have been used in a wide variety of research areas, such as drug metabolism and drug absorption. It is because Caco-2 cells are known to mimic human gut20,21,22,23. To examine the inducibility of UGT1A1 in Caco-2 cells, the cells were treated with chrysin, forskolin and 6,7-dimethylesculetin, which are known activators of AhR, PXR and CAR and the UGT1A1 expression levels were determined by Q-RT-PCR. It was shown that UGT1A1 was greatly induced with chrysin, while the induction of UGT1A1 by forskolin and 6,7-dimethylesculetin was moderate (Fig. 2). This indicates that UGT1A1 can be transcriptionally regulated in Caco-2 cells, although its inducibility is depending on the transcription factors that are activated by chemicals used in the treatment.
To examine if the induction of UGT1A1 by glucose still occurs in Caco-2 cells, the cells were cultured in the presence or absence of glucose for several days and the expression level of UGT1A1 was determined. After Caco-2 cells were cultured for a week in regular media containing 5.5 mM glucose, the cells were seeded in a 6-well plate and cultured for 3, 5 and 7 days in glucose-free or regular media. At 3 days, UGT1A1 level was 2.4-fold higher in Caco-2 cells cultured in glucose-containing media compared to that in the cells cultured in glucose-free media (Fig. 3A). While at 5 days the UGT1A1 level was still 2.7-fold higher in the cells cultured in glucose-containing media, at 7 days the level was 6.5-fold higher than that in glucose-free media. This indicates that glucose time-dependently induces UGT1A1 in Caco-2 cells.
Effects of glucose treatments on UGT1A1 expression in Caco-2 cells.
(A), Caco-2 cells were cultured in the normal or glucose-free medium for 3 to 7 days. RNA was isolated from the cells and UGT1A1 levels were determined with Q-RT-PCR. Relative expression level of UGT1A1 (UGT1A1 level in normal medium/UGT1A1 level in glucose-free medium) was shown. (B), Caco-2 cells were cultured in the medium with 0, 5.5, or 27.5 mM glucose for 3. RNA was isolated from the cells and UGT1A1 levels were determined with Q-RT-PCR. The expression level of UGT1A1 was normalized with that of GAPDH.*, P < 0.05.
To examine the concentration-dependent effect of glucose on the UGT1A1 expression in Caco-2 cells, UGT1A1 levels were determined in Caco-2 cells that were cultured in glucose-free, low glucose and high glucose media. The UGT1A1 level was two-fold higher in the cells that were cultured with a lower concentration of glucose compared to that in the cells cultured in glucose-free media (Fig. 3B), which was in agreement with our previous finding (Fig. 3A). A higher concentration of glucose (27.5 mM) exhibited slightly stronger inducibility against UGT1A1 in Caco-2 cells (Fig. 3B). These findings indicate that glucose is capable to time- and concentration-dependently induce UGT1A1 in Caco-2 cells.
Promoter region of human UGT1A1 contains putative binding sites for Specificity Protein 1 (SP1)
It has been suggested that glucose can increase transcription of certain genes via activating a transcription factor, SP124. To examine if there is a possibility that SP1 is the central factor of inducing UGT1A1 by the glucose treatment, an in silico sequence analysis was carried out to search SP1 binding sites on the promoter region of human UGT1A1. SP1 binding sites were identifies using a program TFSEARCH (http://www.cbrc.jp/research/db/TFSEARCH.html). The in silico analysis revealed that there are seven binding sites for SP1 on the promoter region of UGT1A1 (-10,000 to -1) with a threshold score of 85.0 (Fig. 4A and B).
Role of SP1 in basal function of the UGT1A1 promoter
To investigate the effect of SP1 sites on the induction of UGT1A1 by glucose, three reporter vectors containing the promoter region of UGT1A1 were established (Fig. 4C). Each promoter region included in the reporter vectors contains at least one SP1 site. These vectors were transiently introduced into Caco-2 cells and the basal promoter activities were examined by a luciferase assay. Compared to the control vector, which has a minimal promoter, the vectors containing the promoter regions of UGT1A1 had much greater luminescence intensities (Fig. 5A). Among them, UGT1A1-1300/-7-Luc had the highest promoter activity, indicating that the promoter region, -1300 to +1, of UGT1A1 plays an important role in the basal expression of UGT1A1 in the intestinal cells.
Luciferase assays using promoter vectors containing the promoter regions of human UGT1A1.
(A), Reporter vectors were transiently introduced to Caco-2 cells. The cells were cultured for three days in the normal DMEM medium and then luciferase assays were conducted. (B), The vector-introduced Caco-2 cells were cultured for three days in the presence or absence of glucose. Luciferase assays were conducted and fold increase of the luminescence intensities (intensity in normal medium/intensity in glucose-free medium) was shown. Error bars show SD, n > 3. *, P < 0.05; **, P < 0.01 compared to the Min P-Luc-introduced cells.
Role of SP1 in glucose-induced regulation of UGT1A1
The promoter vector-introduced cells were cultured in the presence or absence of glucose for three days to investigate the role of SP1 sites in the transcriptional regulation of UGT1A1. The UGT1A1-1300/-7-Luc-introduced cells showed a two-fold higher luminescence intensity when cultured in the presence of glucose compared to when cultured in the absence of glucose (Fig. 5B). The luminescence intensity in the UGT1A1-3300/-2800-Luc-introduced cells was not different between the presence and absence of glucose. In contrast, the luminescence intensity in the UGT1A1-5500/-4500-Luc-introduced cells was 2.5-fold higher when cultured in the presence of glucose, compared to when cultured in the absence of glucose. These data indicate that the promoter regions, -1300 to -7 and -5500 to -4500, of UGT1A1 play an important role in the induction of UGT1A1 by glucose.
Discussion
As liver is known as the most important organ for metabolism of endogenous and exogenous compounds, bilirubin has been reported to be mainly metabolized to its glucuronides in the liver25. However, there are contradicting findings that a genetic polymorphism of UGT1A1, which causes a significant decrease in the hepatic UGT1A1 expression, did not affect the serum bilirubin levels in human adults26. Transplantation of small intestine from Wister rats to Gunn rats, which are genetically deficient in the UGT1 locus, resulted in a reduction of serum bilirubin levels27. Our recent finding demonstrated that specific induction of UGT1A1 in skin also resulted in a reduction of serum bilirubin28. These evidences indicate that not only liver, but extrahepatic tissues also contribute to metabolism of bilirubin. In the hUGT1 mice, developmental expression of UGT1A1 in the small intestine correlated well to the developmental change of serum bilirubin levels17. Specific induction of UGT1A1 in the small intestine significantly decreased the serum bilirubin level in neonatal hUGT1 mice10. These findings suggest that UGT1A1 expressed in the small intestine might be a major contributor to bilirubin metabolism especially in the neonatal periods. In the present study, the oral glucose treatment of neonatal hUGT1 mice resulted in the induction of UGT1A1 in the small intestine, while it did not affect the expression of UGT1A1 in the liver (Fig. 1). The failure of the liver to respond to the oral administration of 50% glucose might be explained by intestinal metabolism of the glucose resulting in lower dosing effect on hepatocytes. Due to the limited availability of human samples, especially of newborn intestine, whether the expression of UGT1A1 in newborn intestine is evident or not remains to be solved. The only observation that can support our finding is that UGT1A1 was expressed in intestine of neonatal calves29. While it can be strongly postulated that the observed decrease of serum bilirubin levels in glucose-treated human newborns can be mainly attributed to an increase of UGT1A1 expression in the small intestine, further analysis is needed to fully understand the mechanism. This is because that it was also reported that supplementary water feedings in human neonates were associated with an increase in postnatal total serum bilirubin levels30,31.
While nowadays phototherapy utilizing blue light is a first choice for treating neonatal jaundice, an UGT1A1-inducing agent, phenobarbital, was used before to promote bilirubin metabolism by inducing the bilirubin-glucuronidating enzyme, UGT1A1 and thereby to treat neonatal hyperbilirubinemia. The inducibility of UGT1A1 by phenobarbital was completely abolished in hUGT1 mice in a constitutive-active/androgen receptor (CAR)-null background (hUGT1/Car-/- mice)10, confirming that CAR is responsible for the induction of UGT1A1 by phenobarbital. Rifampicin has been known as a potent agonist of pregnane X receptor (PXR) and has been shown to induce UGT1A1 in human primary hepatocytes and in human hepatocarcinoma HepG2 cell lines32,33. Other nuclear receptors also regulate the expression of UGT1A1. To date, it has been reported that glucocorticoid receptor (GR)34 and peroxisome proliferator-activated receptor (PPAR)35 induce UGT1A1, while the role of vitamin D receptor (VDR), liver X receptor (LXR) and farnesoid X receptor (FXR) in the regulation of UGT1A1 expression is still unknown. Benzo[a]pyrene (B[a]P) is a prototypical member of polycyclic aromatic hydrocarbons (PAHs), which is generated as a result of combustion and is found in significant concentrations in tobacco smoke. It has been known that B[a]P strongly activate Ah receptor, inducing its target genes, including UGT1A1, through binding their xenobiotic-responsive element (XRE)36. NF-E2-related factor 2 (Nrf2) is an oxidative stress response transcription factor. Under unstimulated conditions, Nrf2, which is sequestered by Keap1 in the cytoplasm, is rapidly degraded by the proteasome pathway. However, once oxidative stress exceeds a certain threshold, the Keap1-Nrf2 complex is disrupted and Nrf2 is translocated into the nucleus, up-regulating its target genes. UGT1A1 is known as an Nrf2-mediated oxidative stress-responsive gene37. Meanwhile, the role of SP1 in the transcriptional regulation of UGT1A1 has not been fully investigated. Current study demonstrated that glucose, possible SP1-activator, induced the intestinal UGT1A1 expression in vitro and in vivo. Therefore, activation of SP1 could be an alternative method of treating neonatal jaundice.
Since bilirubin is a neurotoxic compound, its accumulation in the brain can result in a development of kernicterus, which is a permanent brain damage. However, do hyperbilirubinemic human infants truly require immediate intervention to reduce serum bilirubin levels? Mammals have evolved an enzymatic step to convert nontoxic biliverdin to toxic bilirubin in the body38,39. While all primates manifest neonatal jaundice and hyperbilirubinemia40,41, humans uniquely develop severe hyperbilirubinemia during neonatal life. Why would nature develop a system that generates elevated bilirubin levels in a high proportion of all neonates even with taking a risk of death from accumulated bilirubin in the brain? It has been reported that bilirubin is a potent antioxidant and that increased bilirubin levels correlated well with development of various benefits including a decreased risk of cancer42. While there is no report investigating the relationship between bilirubin levels during neonatal periods and their effect on long-term health benefits, such study in the future might influence therapeutic strategy for neonatal jaundice.
Breast milk contains numerous ingredients that are necessary for childhood development. However, once neonates are diagnosed with jaundice, they need to be isolated from mothers to receive phototherapy, which can cause minor but disadvantages. Supplemental treatment of newborns with glucose solution can be a convenient and efficient method to treat neonatal jaundice while allowing continuous maternal-infant interaction.
Methods
Chemicals and Reagents
6,7-Dimethoxycoumarin and chrysin were purchased from Sigma–Aldrich (St Louis, MO). Forskolin was obtained from Wako Pure Chemical (Osaka, Japan). Promoter vectors (pGL4.26, pGL4.50 and pGL4.74) and a Dual-Glo luciferase assay system were purchased from Promega (Madison, WI). All other chemicals and solvents were of analytical grade or the highest grade commercially available.
Animals and treatments
Tg(UGT1A1*28)Ugt1−/− (hUGT1) mice were developed previously in a C57BL/6 background10,17. All animals received food and water ad libitum and mouse handling and experimental procedures were conducted in accordance with our animal care protocol, which was previously approved by Kitasato University. Glucose solution (50%, 150 µL) was orally administered to 14-day-old mice. Three hours after the treatment, liver and small intestine were isolated. For tissue collections, mice were anesthetized by diethyl ether inhalation and the liver was perfused with ice-cold 1.15% KCl. The small intestine and liver were rinsed in cold 1.15% KCl and stored at −80°C.
Quantitative (Q) reverse transcription (RT)-PCR
Total RNA was extracted from tissues with Trizol reagent (Invitrogen). The cDNA was synthesized from total RNA using ReverTra Ace (TOYOBO, Osaka, Japan) according to the manufacturer's protocol. Primers for human UGT1A1, GAPDH and mouse cyclophilin B (CPH) were developed previously10,18. Q-RT-PCR was performed with Ssofast Evagreen Supermix and the reactions were run in a CFX96™ Real-Time PCR Detection System (BioRad). Expression of GAPDH mRNA was used as an internal control for the cDNA quantity and quality.
Cell culture and treatments
Caco-2 cells were obtained from American Type Culture Collection (Rockville, MD, U.S.A.). Cells were cultured on flasks (150 cm2; Falcon, Becton Dickinson Co., Ltd., Oxnard, CA) and maintained at 37°C in a humidified atmosphere containing 5% of CO2. The culture medium consisted of Dulbecco's modified Eagle's medium, 1% nonessential amino acids, 2% Gluta MAX-1 and 10% fetal bovine serum, which were all obtained from Life Technologies (Gaithersburg, MD). Before the glucose treatment, Caco-2 cells were seeded into six-well plates at 1 × 106 cells/well. After 24 hours, the culture medium was changed to a normal DMEM medium or glucose free DMEM medium and subsequently cells were maintained for 24 to 72 hours until harvesting. Cells were also co-treated with 6,7-Dimethoxycoumarin, chrysin, or forskolin for 72 hours. RNA was isolated from the cells by the method described above and was used for the Q-RT-PCR analysis.
Establishment reporter vectors
To construct pGL4.26 vectors containing 5′-flanking regions of human UGT1A1, promoter regions of UGT1A1 were obtained by PCR reaction with primers and were subcloned into the vector. The forward and reverse primers were 5′-CCG CTC GAG CTT GGG CCA GTG GAA TGA GT -3′ and 5′-CCC AAG CTT TTT GCT CCT GCC AGA GGT TC-3′ for UGT1A1-1300/-7 and 5′-CCG CTC GAG AAT GAG CTT GGA CAG GTG GG-3′ and 5′-CCC AAG CTT GCC ACC TTC CTT GAA TCC CT-3′ for UGT1A1-3159/-2587 and 5′-CCC CAA GCA CAG CAA TAG GA-3′ and 5′-TGG TAT GGG GAA GGA GGG TT-3′ for UGT1A1-6500/-4050. UGT1A1-1300/-7 and UGT1A1-3159/-2587 were subcloned into the vector using restriction enzymes XhoI and HindIII.
Transfection and luciferase assay
Reporter vectors were transiently transfected into Caco-2 cells using Lipofectamine 2000 (Life Technologies). The vector transfected-cells were cultured for 24 to 72 hours in the DMEM medium in the presence or absence of glucose. The cells were suspended in the lysis buffer and then the luciferase activity was measured with a luminometer (ATTO, Tokyo, Japan) using the dual-luciferase reporter assay system.
Statistical analyses
Statistical significances were determined by analysis of variance followed by Dunnett's test. A value of P < 0.05 was considered statistically significant.
References
Gourley, G. R. Bilirubin metabolism and kernicterus. Adv. Pediatr. 44, 173–229 (1997).
Newman, A. J. & Gross, S. Hyperbilirubinemia in breast-fed infants. Pediatrics 32, 995–1001 (1963).
Arias, I. M., Gartner, L. M., Seifter, S. & Furman, M. Prolonged neonatal unconjugated hyperbilirubinemia associated with breast feeding and a steriod, pregnane-3(alpha), 20(beta)-diol, in maternal milk that inhibits glucuronide formation in vitro. J. Clin. Invest. 43, 2037–2047 (1964).
Schneider, A. P. Breast milk jaundice in the newborn: A real entity. JAMA 255, 3270–3274 (1986).
American Academy of Pediatrics. Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 114, 297–316 (2004).
Ip, S. et al. An Evidence-Based Review of Important Issues Concerning Neonatal Hyperbilirubinemia. Pediatrics 114, e130–e153 (2004).
Maisels, M. J. et al. Hyperbilirubinemia in the newborn infant > or = 35 weeks' gestation: an update with clarifications. Pediatrics 124, 1193–1198 (2009).
Maruo, Y. et al. Bilirubin uridine diphosphate-glucuronosyltransferase variation is a genetic basis of breast milk jaundice. J Pediatr. 165, 36–41 (2014).
Shibuya, A., Itoh, T., Tukey, R. H. & Fujiwara, R. Impact of fatty acids on human UDP-glucuronosyltransferase 1A1 activity and its expression in neonatal hyperbilirubinemia. Sci. Rep. 3, 2903 (2013).
Fujiwara, R., Chen, S., Karin, M. & Tukey, R. H. Reduced expression of UGT1A1 in intestines of humanized UGT1 mice via inactivation of NF-kB leads to hyperbilirubinemia. Gastroenterology 142, 109–18 (2012).
Tan, K. L. The nature of the dose-response relationship of phototherapy for neonatal hyperbilirubinemia. J. Pediatr. 90, 448–452 (1977).
Wananukul, S. & Praisuwanna, P. Clear topical ointment decreases transepidermal water loss in jaundiced preterm infants receiving phototherapy. J. Med. Assoc. Thai. 85, 102–106 (2002).
Kumar, P. et al. Light emitting diodes versus compact fluorescent tubes for phototherapy in neonatal jaundice: a multi center randomized controlled trial. Indian. Pediatr. 47, 131–137 (2010).
Newman, A. J. & Gross, S. Hyperbilirubinemia in breast-fed infants. Pediatrics. 32, 995–1001 (1963).
Sato, H. et al. Association of breast-fed neonatal hyperbilirubinemia with UGT1A1 polymorphisms: 211G>A (G71R) mutation becomes a risk factor under inadequate feeding. J. Hum. Genet. 58, 7–10 (2013).
Bosma, P. J. et al. Bilirubin UDP-glucuronosyltransferase 1 is the only relevant bilirubin glucuronidating isoform in man. J. Biol. Chem. 269, 17960–17964 (1994).
Fujiwara, R., Nguyen, N., Chen, S. & Tukey, R. H. Developmental hyperbilirubinemia and CNS toxicity in mice humanized with the UDP glucuronosyltransferase 1 (UGT1) locus. Proc. Natl. Acad. Sci. U.S.A. 107, 5024–9 (2010).
Nakamura, A., Nakajima, M., Yamanaka, H., Fujiwara, R. & Yokoi, T. Expression of UGT1A and UGT2B mRNA in human normal tissues and various cell lines. Drug Metab. Dispos. 36, 1461–1464 (2008).
Tom, B. H. et al. Human colonic adenocarcinoma cells. I. Establishment and description of a new line. In Vitro 12, 180–91 (1976).
Artursson, P. Epithelial transport of drugs in cell culture. I: A model for studying the passive diffusion of drugs over intestinal absorptive (Caco-2) cells. J. Pharm. Sci. 79, 476–82 (1990).
Hunter, J., Jepson, M. A., Tsuruo, T., Simmons, N. L. & Hirst, B. H. Functional expression of P-glycoprotein in apical membranes of human intestinal Caco-2 cells. Kinetics of vinblastine secretion and interaction with modulators. J. Biol. Chem. 268, 14991–14997 (1993).
Meunier, V., Bourrié, M., Berger, Y. & Fabre, G. The human intestinal epithelial cell line Caco-2; pharmacological and pharmacokinetic applications. Cell Biol. Toxicol. 11, 187–94 (1995).
Walle, U. K., Galijatovic, A. & Walle, T. Transport of the flavonoid chrysin and its conjugated metabolites by the human intestinal cell line Caco-2. Biochem Pharmacol. 58, 431–8 (1999).
Li, T., Bai, L., Li, J., Igarashi, S. & Ghishan, F. K. Sp1 is required for glucose-induced transcriptional regulation of mouse vesicular glutamate transporter 2 gene. Gastroenterology. 134, 1994–2003 (2008).
Kamisako, T. et al. Recent advances in bilirubin metabolism research: the molecular mechanism of hepatocyte bilirubin transport and its clinical relevance. J Gastroenterol. 35, 659–664 (2000).
Beutler, E., Gelbart, T. & Demina, A. Racial variability in the UDP-glucuronosyltransferase 1 (UGT1A1) promoter: a balanced polymorphism for regulation of bilirubin metabolism? Proc. Natl. Acad. Sci. U.S.A. 95, 8170–4 (1998).
Medley, M. M., Hooker, R. L., Rabinowitz, S., Holton, R. & Jaffe, B. M. Correction of congenital indirect hyperbilirubinemia by small intestinal transplantation. Am. J. Surg. 169, 20–27 (1995).
Sumida, K. et al. Importance of UDP-glucuronosyltransferase 1A1 expression in skin and its induction by UVB in neonatal hyperbilirubinemia. Mol. Pharmacol. 84, 679–86 (2013).
Krüger, K. A., Blum, J. W. & Greger, D. L. Expression of nuclear receptor and target genes in liver and intestine of neonatal calves fed colostrum and vitamin A. J. Dairy Sci. 88, 3971–81 (2005).
de Carvalho, M., Hall, M. & Harvey, D. Effects of water supplementation on physiological jaundice in breast-fed babies. Arch. Dis. Child. 56, 568–9 (1981).
Nicoll, A., Ginsburg, R. & Tripp, J. H. Supplementary feeding and jaundice in newborns. Acta. Paediatr. Scand. 71, 759–61 (1982).
Maglich, J. M. et al. Nuclear pregnane X receptor and constitutive androstane receptor regulate overlapping but distinct sets of genes involved in xenobiotic detoxification. Mol. Pharmacol. 62, 638–646 (2002).
Sugatani, J. et al. Transcriptional regulation of human UGT1A1 gene expression: activated glucocorticoid receptor enhances constitutive androstane receptor/pregnane X receptor-mediated UDP-glucuronosyltransferase 1A1 regulation with glucocorticoid receptor-interacting protein 1. Mol. Pharmacol. 67, 845–855 (2005).
Usui, T., Kuno, T. & Mizutani, T. Induction of human UDP-glucuronosyltransferase 1A1 by cortisol-GR. Mol. Biol. Rep. 33, 91–96 (2006).
Senekeo-Effenberger, K. et al. Expression of the Human UGT1 Locus in Transgenic Mice by 4-Chloro-6-(2,3-xylidino)-2-pyrimidinylthioacetic Acid (WY-14643) and Implications on Drug Metabolism through Peroxisome Proliferator-Activated Receptor α Activation. Drug Metab. Dispos. 35, 419–27 (2007).
Landers, J. P. & Bunce, N. J. The Ah receptor and the mechanism of dioxin toxicity. Biochem. J. 276, 273–287 (1991).
Yueh, M. F. & Tukey, R. H. Nrf2-Keap1 Signaling Pathway Regulates Human UGT1A1 Expression in Vitro and in Transgenic UGT1 Mice. J. Biol. Chem. 282, 8749–8758 (2007).
McDonagh, A. F. Bile pigments: bilatrienes and 5,15-biladienes. In: Dolphin D. (ed). The Porphyrins. New York, NY: Academic Press 293–491 (1979).
Tenhunen, R., Ross, M. E., Marver, H. S. & Schmid, R. Reduced nicotinamide-adenine dinucleotide phosphate dependent biliverdin reductase: partial purification and characterization. Biochemistry. 9, 298–303 (1970).
Gartner, L. M., Lee, K. S., Vaisman, S., Lane, D. & Zarafu, I. Development of bilirubin transport and metabolism in the newborn rhesus monkey. J Pediatr. 90, 513–31 (1977).
Gartner, L. M. & Lane, D. L. The physiology of physiologic hyperbilirubinemia of the newborn. Medical primatology. 237–247 (1972).
Temme, E. H., Zhang, J., Schouten, E. G. & Kesteloot, H. Serum bilirubin and 10-year mortality risk in a Belgian population. Cancer Causes Control. 12, 887–894 (2001).
Acknowledgements
This work was supported by a Grant-in-Aid for Research Activity Start-up [24890224] and a Grant-in-Aid for Encouragement of Young Scientists B [26870562] (R.F.). This work was also supported by the US Public Health Service [ES010337, GM086713 and GM100481] (R.H.T.).
Author information
Authors and Affiliations
Contributions
T.I., R.H.T. and R.F. wrote the main manuscript text and N.A., Y.F. and R.F. conducted experiments and prepared figures. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Aoshima, N., Fujie, Y., Itoh, T. et al. Glucose induces intestinal human UDP-glucuronosyltransferase (UGT) 1A1 to prevent neonatal hyperbilirubinemia. Sci Rep 4, 6343 (2014). https://doi.org/10.1038/srep06343
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep06343
This article is cited by
-
Potential of therapeutic bile acids in the treatment of neonatal Hyperbilirubinemia
Scientific Reports (2021)
-
Hepatic expression of transcription factors affecting developmental regulation of UGT1A1 in the Han Chinese population
European Journal of Clinical Pharmacology (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.