Abstract
Inspired by Taichi, we proposed rigid-flexible coupling concept and herein developed a highly promising solid polymer electrolyte comprised of poly (ethylene oxide), poly (cyano acrylate), lithium bis(oxalate)borate and robust cellulose nonwoven. Our investigation revealed that this new class solid polymer electrolyte possessed comprehensive properties in high mechanical integrity strength, sufficient ionic conductivity (3 × 10−4 S cm−1) at 60°C and improved dimensional thermostability (up to 160°C). In addition, the lithium iron phosphate (LiFePO4)/lithium (Li) cell using such solid polymer electrolyte displayed superior rate capacity (up to 6 C) and stable cycle performance at 80°C. Furthermore, the LiFePO4/Li battery could also operate very well even at an elevated temperature of 160°C, thus improving enhanced safety performance of lithium batteries. The use of this solid polymer electrolyte mitigates the safety risk and widens the operation temperature range of lithium batteries. Thus, this fascinating study demonstrates a proof of concept of the use of rigid-flexible coupling solid polymer electrolyte toward practical lithium battery applications with improved reliability and safety.
Similar content being viewed by others
Introduction
Lithium batteries (LIBs) are ubiquitous and widely implemented in various portable devices, electric vehicles and grid storage systems because of the extremely serious climate change and the massive consumption of fossil energy1,2,3,4,5,6,7,8,9,10,11,12,13. Nevertheless, most commercial lithium batteries containing liquid organic electrolytes often suffered from potential safety risks due to leakage, volatility, spontaneous combustion of the electrolytes, limited temperature range of operation and lack of mechanical stability. Above-mentioned safety hazards will undermine the confidence of consumers and obstruct the commercialization of electric vehicles or hybrid electric vehicles. Thus, the safety issue of lithium batteries merits further study. Solid polymer electrolytes have been widely used as advanced and safe polymer electrolyte for application in solvent-free lithium batteries due to their attractive features of high safety, high flexibility and simple technological process. Poly (ethylene oxide) (PEO)/lithium salt systems were intensively studied14,15,16,17,18,19,20,21. In this system, the ionic conductivity increases significantly (10−4–10−3 S cm−1) when the operating temperature is above the melting temperature of PEO. Nevertheless, the batteries using pristine PEO-based solid polymer electrolytes often encountered the following safety hazards: (1) poor mechanical strength; (2) poor electrochemical window; (3) inferior dimensional thermostability; (4) low ionic conductivity22,23,24. To address the above issues, many endeavours have been carried out to boost the performance over the past two decades, such as composite polymer electrolytes25,26,27,28,29,30,31, block copolymer electrolytes32,33,34 and interpenetrating network polymer electrolytes35. Despite these great advances, it is still challenging to make attempts especially in new design concept to improve comprehensive performance of solid polymer electrolyte. Therefore, it is essential to fabricate advanced solid polymer electrolyte with excellent mechanical strength, superior heat resistance, enlarged electrochemical window and high ionic conductivity.
It is well-known that cellulose is one of the renewable materials and possesses outstanding properties such as desired chemical stability, environmental benignancy and dimensional thermostability (above 270°C)36. Furthermore, we have developed a flame-retardant and thermal resistant cellulose-based composite nonwoven separator37. In the present study, by virtue of its attractive properties, flame-retardant cellulose nonwoven has been chosen as a polymer support to fabricate solid polymer electrolyte with improved mechanical strength.
Taichi, a kind of traditional Chinese boxing, is called as “the Quintessence of the Chinese Culture”. The so-called “Taichi” mainly refers to “yin” and “yang”. The conflicting and interdependent relations between the “yin” and “yang” are the general rules in the material world. Inspired by Taichi, we herein put forward a scalable and smart strategy to exploiting a new class of rigid-flexible coupling solid polymer electrolyte (hereinafter, abbreviated as “CCPL solid polymer electrolyte”) and envisage that it would be reasonable to achieve a balance among mechanical strength, dimensional thermostability, electrochemical window and ionic conductivity for high-performance LIBs application. It was demonstrated that such solid polymer electrolyte reinforced mechanical strength, improved thermal resistance, enlarged electrochemical windows and the enhanced safety issues, which was further corroborated by the fact that lithium batteries can also operate well even at an elevated temperature of 160°C.
Results
Preparation of CCPL solid polymer electrolytes
To demonstrate this preparation of CCPL solid polymer electrolyte, a rigid-flexible coupling concept has been adopted as shown in Figure 1. The preparation process of solid polymer electrolytes was as follows: A certain amount of PEO and Poly (cyano acrylate) (PCA) was dispersed in anhydrous acetonitrile with the aid of ultrasonic dispersion, followed by the addition of LiBOB. The mass ratio of PEO:PCA:LiBOB was 10:2:1. The solution was stirred at 25°C for 10 hrs. Subsequently, cellulose nonwoven membrane was prepared according to the method of our previous literature37. Finally, the homogeneous solution was then poured into cellulose nonwoven membrane and the solvent was removed in vacuum oven at 60°C for 2 hrs. The final thickness of solid polymer electrolyte membrane was around 100 μm. They were stored under argon box for subsequent measurements.
Morphological characterization and FTIR spectra analysis
Figure 2 (a–c) presented the typical SEM images of cellulose nonwoven membrane and CCPL solid polymer electrolyte. As revealed by SEM image of Figure 2a, cellulose nonwoven membrane consisted of randomly arranged nanofibers with an average diameter size of 800 nm and it possessed large-sized pores (>1 μm), which was beneficial to contain polymer electrolyte. Figures 2b and 2c described the surface and cross-section morphology of CCPL solid polymer electrolyte. It can be seen that the surface of obtained polymer electrolyte was smooth and homogenous and the thickness of the membrane was about 100 μm. In addition, it was depicted from the cross section image that the pores of the fibrous matrix of cellulose nonwoven were filled with PCA, PEO and LiBOB, demonstrating the cellulose-supported solid polymer electrolyte with continuous structure has been successfully obtained.
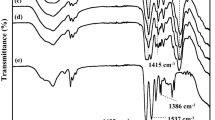
To verify our observation, FTIR was utilized to characterize the structure of solid polymer electrolyte. As shown in Figure 2d, the characteristic absorbance peak of cellulose nonwoven membrane among 3100–3600 cm−1 corresponds to O-H stretching of hydrogen bonding. After PEO was introduced onto the heat-resistant cellulose skeleton, the peak at 2862 cm−1 corresponding to the symmetric stretching of C-H bond becomes stronger. And stretching of C-O bond brought about the absorption peak at 1098 cm−1. All typical peaks of the PEO appearing in the FTIR spectra imply that solid polymer electrolyte is successfully prepared.
Mechanical property and thermal analysis
Solid polymer electrolyte of lithium batteries usually suffers from poor mechanical integrity. It is well-known that the poor mechanical performance limits the application of solid polymer electrolyte in energy storage devices. The stress-strain curves of PEO solid polymer electrolyte and CCPL solid polymer electrolyte were shown in Figure 3a. Apparently, the stress of PEO solid polymer electrolyte was only 2.5 MPa, which was consistent with previous study38. Unfortunately, the stress is too low to acceptable for lithium batteries. In our case, the mechanical strength of CCPL solid polymer electrolyte was enhanced to reach as high as 43 MPa, which was more advantageous to remain mechanical integrity when it encountered abrupt accident collision. This reason was ascribed to the synergetic effect of cellulose nonwoven, PEO and PCA kept the mechanical property of solid polymer electrolyte. In terms of actual application, the CCPL solid polymer electrolyte could deliver more reliable mechanical property and subsequently alleviate the possibility of short circuit of the batteries, thus improving the safety of lithium batteries.
Thermal stability is also a critical intrinsic factor which influences application of solid polymer electrolyte in high-performance lithium batteries. The DSC curves of cellulose nonwoven membrane, PEO solid polymer electrolyte and CCPL solid polymer electrolyte were shown in Figure 3b. The data corresponding to DSC curves are summarized in Table 1. From the DSC data shown in Table 1, it is clear that cellulose is very stable and does not exhibit a melting point. In the case of PEO polymer electrolyte, there is a sharp endothermic peak at 52.9°C, demonstrating the melting of PEO crystallites. This suggested that lithium batteries using pristine PEO solid polymer electrolyte operating above 53°C could potentially experience deterioration and explosion initiated by short circuit owing to poor mechanical integrity. In contrast, the dimentional thermostability of CCPL solid polymer electrolyte (Figure S1) was much enhanced, which could significantly eliminate the risk of short-circuit at abused condition of lithium batteries. In addition, melting enthalpy, degree of crystallinity and Tg of CCPL solid polymer electrolyte were 64.8 J g−1, 30.7% and −28.8°C respectively, which were lower than those of pristine PEO solid polymer electrolyte. The obtained result indicated that the crystallinity of CCPL solid polymer electrolyte was lowed through introducing PCA.
Electrochemical characterizations
Apart from mechanical strength and thermal stability, electrochemical property of CCPL solid polymer electrolyte is of significant importance for its application in high-performance lithium batteries. It is crucial to evaluate the electrochemical stability of polymer electrolyte among the operating voltage for practical battery applications. The electrochemical stability of the electrolyte is studied using linear sweep voltammetry in the stainless steel/CCPL solid polymer electrolyte/Li cells at 80°C. The results are given in Figure 4a, in which the electrolyte was swept from 2.5 V and 6 V at a constant rate of 1 mV s−1. As seen from Figure 4a, the electrochemical window of CCPL solid polymer electrolyte was stable up to 4.6 V versus Li+/Li, indicating that such solid polymer electrolyte has an enhanced electrochemical stability. For a fair comparison, linear sweep voltammetry of pristine PEO solid polymer electrolyte was also characterized. It can be seen that pristine PEO-based solid polymer electrolyte exhibited lower electrochemical window (less than 4.0 V), indicating the electrochemical stability of CCPL solid polymer electrolyte was significantly improved which was ascribed to the synergetic effect between PCA and cellulose nonwoven39. The present investigation demonstrates that CCPL solid polymer electrolyte is electrochemically stable enough for the use as electrolyte for lithium batteries.
Figure 4b illustrated the dependence of ionic conductivity of PEO solid polymer electrolyte and CCPL solid polymer electrolytes at different temperatures from 20°C to 160°C. As we all know, pristine PEO polymer electrolyte will melt and lose mechanical integrity when the temperature was higher than 80°C25. Therefore, the ionic conductivity of PEO solid polymer electrolyte cannot be tested at that condition, as shown in Figure 4b. It was noticed that ionic conductivity of PEO solid polymer electrolyte and CCPL solid polymer electrolyte was 2 × 10−5 S cm−1 and 1.3 × 10−5 S cm−1 at 20°C, respectively. As the temperature increases, the ionic conductivity increases accordingly40. The ionic conductivity of CCPL solid polymer electrolyte was a little lower than that of PEO solid polymer electrolyte (<80°C), which might be attributed to the cellulose skeleton in CCPL solid polymer electrolyte. Taking into account other factors, such as mechanical property and electrochemical stability, CCPL solid polymer electrolyte would be more beneficial to improve comprehensive performance of the cells. Moreover, in the temperature range investigated, the CCPL solid polymer electrolyte presents an ionic conductivity of 1.4 × 10−3 S cm−1 at 160°C. It is worthy to note that such solid polymer electrolyte can work at elevated temperature up to 160°C without internal short-circuit.
For more convenient use of this solid polymer electrolyte, the ability to avoid short-circuit of battery is highly desirable. For this purpose, we monitored the ionic conductivity at the fixed time at 160°C. As displayed in Figure S2, ionic conductivity basically maintained at 1.4 × 10−3 S cm−1 for 60 h, indicative of an excellent dimentional thermostability. In view of the exciting and outstanding performance, we can speculate that such CCPL solid polymer electrolyte can be applied to lithium batteries running at elevated temperature.
Discussion
Typical charge-discharge profiles of LiFePO4/Li batteries obtained at various rates at the temperature of 80°C were shown in Figure 5a. In 0.1 C/0.1 C, the charge and discharge voltages exhibited obvious and flat profiles41. The stable voltage profiles would be attributed to good electrochemical stability and mechanical integrity of CCPL solid polymer electrolyte33. As the current density increases, the voltage plateau and the specific discharge capacity decrease slightly. It can be found that LiFePO4/Li cell using CCPL solid polymer electrolyte exhibited excellent rate performance at the temperature. They could achieve capacities of 153, 148, 134, 118, 102, 74.4 and 58.1 mAh g−1 at various current rates of 0.1 C, 0.2 C, 0.5 C, 1 C, 2 C, 4 C and 6 C, respectively, which was better than those of the PEO polymer electrolyte16. The excellent performance at different rates may be ascribed to satisfactory ionic conductivity and favourable interfacial properties between the electrodes and the electrolyte in the cell at the temperature of 80°C.
(a) Typical charge-discharge profiles obtained at various rates, (b) cycle performance of LiFePO4/Li cell using CCPL solid polymer electrolyte at 1 C. Temperature: 80°C. (c) Nyquist plots for the LiFePO4/Li cells with CCPL solid polymer electrolyte after the first cycle and after the 1000 cycles test and (d) Illustration of aluminum-pouch-type lithium batteries using LiFePO4 as the cathode and lithium metal as the anode for powering a LED lamp. Temperature: 25°C.
Figure 5b presented cycle performance of the LiFePO4/Li cell using CCPL solid polymer electrolyte at 80°C. It can be found that 88% of the discharge capacity is retained after 1000 cycles, indicating that CCPL solid polymer electrolyte can afford a good cycling performance. This would be attributed to the improved interfacial compatibility and limited cell impedance augment during long term cycling owing to introduction of PCA.
To probe the variation of cell impedances during cycling, AC impedance measurement was carried out for LiFePO4/Li cells assembled with such solid polymer electrolyte after the first cycle and after the 1000 cycles test. As shown in Figure 5c, the intercept of the spectra with the real axis reflects the bulk resistance (Rbulk) of CCPL solid polymer electrolyte. In the middle frequency, there is an irregular semicircle attributed to the charge-transfer resistance. It can be seen from Figure 5c that the value of charge-transfer resistance after first cycle was 55 Ω. After the 1000 cycles, the value became 80 Ω, indicative of a minor augment of charge-transfer resistance. Good interfacial properties are mainly due to the flexibility of the polymer electrolyte, which can make the contact between the SPEs and electrodes more sufficient. In addition, the discharge capacity of LiFePO4/Li cells using CCPL solid polymer electrolyte at 25°C and 40°C were also listed in Table S1, Figures S3 and S4. Happily, at 25°C, CCPL solid polymer electrolyte based LiFePO4/Li cells could deliver 104.4 mAh g−1 and 48.9 mAh g−1 at 0.03 C and 0.1 C, respectively. Finally, in order to explore the feasibility of CCPL solid polymer electrolyte, illustration of aluminum pouch cell comprising LiFePO4 and lithium metal was shown in Figure 5d. It can be seen that the cell was successfully able to light up a red LED lamp at 25°C, further proving the practicability and reliability of this battery.
The rate performance comparison data of such solid electrolyte operated at 60°C, 80°C and 160°C was illustrated in Figure 6a. It was noted that the results at 60°C displayed attractive performance compared with the reported results16. To further prove the safety concern, we conducted experiment in a harsh condition. When used in lithium batteries system at an elevated temperature of 160°C, the LiFePO4/Li battery could also run very well at various rates from 0.1 C to 10 C (Figure 6b), thus indicating an improved safety performance of battery at elevated temperatures. This is a solid evidence to prove that the CCPL solid polymer electrolyte showed unprecedented improvements in safety characteristic at elevated temperatures. Future development of large electric vehicles and power grids requires lithium ion batteries with not only superior cycle performance but also high energy density. Therefore, it is vital to evaluate the electrochemical stability of polymer electrolyte among high voltage for practical battery applications. As we all know, pristine PEO-based solid polymer electrolyte exhibited lower electrochemical window (less than 4.0 V), which would limit future application. As shown in Figure 6c and 6d, the charge and discharge voltages exhibited obvious and flat profiles. In addition, the obtained capacity retention of LiMn2O4/Li cell was 97.7% after 55 cycles, indicating that CCPL solid polymer electrolyte could afford a good cycling performance at a relatively high voltage.
(a) Rate performance of LiFePO4/Li cell compared at 60°C, 80°C and 160°C. The black data was taken from ref. 16 for comparison. (b) Typical charge/discharge profiles obtained at various rates from 0.1 C to 10 C for LiFePO4/Li cell using CCPL solid polymer electrolyte. Temperature: 160°C. (c) Charge/discharge curves of LiMn2O4/Li cell using CCPL solid polymer electrolyte compared at first cycle and 55th cycle. Temperature: 60°C. (d) The capacity retention of LiMn2O4/Li cell using CCPL solid polymer electrolyte at 0.5 C. Temperature: 60°C.
LiFePO4/Li battery using such CCPL solid polymer electrolyte exhibit fascinating electrochemical performance for three aspects: (1) high capacity retention: 88% after 1000 cycles at 80°C, (2) high temperature stability: the batteries were still able to operate well in a wide temperature range even to 160°C, (3) high electrochemical window: the LiMn2O4/Li cell using CCPL solid polymer electrolyte exhibited stable charge/discharge profiles and superior cycle performance at 0.5 C.
A corollary of this is that, this would offer a kind of new concept for designing and exploiting rigid-flexible coupling solid polymer electrolyte by tuning various components for realizing superior overall performance of all-solid-state lithium batteries. Ongoing studies are being conducted to enhance the flame retardancy and ionic conductivity of solid polymer electrolyte to further improve the safety issue and battery performance.
In conclusion, from practical and fundamental viewpoint, we have successfully fabricated cellulose nonwoven/PCA-PEO/LiBOB as rigid-flexible coupling solid polymer electrolyte for high-performance lithium batteries. This solid polymer electrolyte exhibited fascinating characteristics for lithium batteries in terms of fair ionic conductivity (3 × 10−4 S cm−1) at 60°C, excellent mechanical strength as compared to pristine PEO solid polymer electrolyte and superior dimentional thermostability (up to 160°C). In addition, the LiFePO4/Li battery using such solid polymer electrolyte exhibited excellent rate capability and high cycling retention owing to its facile ion transport and excellent interfacial compatibility. Furthermore, such solid polymer electrolyte based LiFePO4/Li cell also allowed for a safe and stable operation even at an elevated temperature of 160°C. The rigid-flexible coupling design adopted in this work should inspire the development of advanced solid polymer electrolytes. In addition, this may provide a bridge to the future advanced solid polymer electrolyte for application in high-performance power lithium batteries.
Methods
Sample collection
Poly (ethylene oxide) (PEO, Mw = 300,000, Alfa Aesar Company) was dried at 50°C for 10 hrs under vacuum condition before use; Poly (cyano acrylate) (PCA) was purchased from Beijing Chemical Company; Lithium bis(oxalate)borate (LiBOB) was supplied by Suzhou Fotai New Materials Co., Ltd. Acetonitrile was obtained from Tianjin Fuyu Fine Chemical Co., Ltd.
Samples characterization
A field emission scanning electron microscope (Hitachi S-4800, operating at 3 kV) was used to observe morphology of the obtained solid polymer electrolyte. The chemical structure of the membranes was characterized by Fourier transform infrared spectroscopy (FT-IR, Bruker VERTEX 70). The stress-strain curves were measured using an Inston-3300 universal testing machine (USA) at a stretching speed of 1.66 mm sec−1 with the sample straps of about 1 cm wide and 8 cm long. Differential scanning calorimeter (DSC, PerkinElmer) was carried out to measure the thermal properties of the polymer electrolyte at a heating rate of 10°C/min under nitrogen atmosphere.
Electrochemical evaluation
The ionic conductivity of the samples was evaluated by sandwiching the samples between the two stainless steel electrodes via AC impedance techniques. The measurements were performed using an electrochemical workstation over a frequency range from 1 Hz to 106 Hz at various temperatures ranging from 20°C to 160°C. Linear sweep voltammetry of polymer electrolyte was carried out on electrochemical workstation with a sweep rate of 1 mV/s between 2.5 V and 6.0 V. The LiFePO4 cathode was composed of 60 wt% LiFePO4, 32 wt% PEO-PCA-LiBOB and 8 wt% carbon black. The LiFePO4/Li battery was charged and discharged between 2.5 V–4.0 V at various currents. The C-rates in all of the electrochemical measurements are defined based on 1 C = 150 mA g−1. The LiMn2O4 cathode was composed of 80 wt% Li Mn2O4, 15 wt% PEO-PCA-LiBOB and 5 wt% carbon black. The LiMn2O4/Li battery was charged and discharged between 3 V–4.3 V at 0.5 C (1 C = 120 mA g−1). Before the measurements, all the cells were kept in the oven at the testing temperature for 3 hrs to reach thermal equilibrium.
References
Arora, P. & Zhang, Z. M. Battery separators. Chem. Rev. 104, 4419–4462 (2004).
Tarascon, J. M. & Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 414, 359–367 (2001).
Lee, E. S., Huq, A., Chang, H. Y. & Manthiram, A. High-voltage, high-energy layered-spinel composite cathodes with superior cycle life for Lithium-ion batteries. Chem. Mater. 24, 600–612 (2012)
Cui, G. L. et al. A germanium–carbon nanocomposite material for lithium batteries. Adv. Mater. 20, 3079–3083 (2008).
Palacin, M. R. Recent advances in rechargeable battery materials: a chemist's perspective. Chem. Soc. Rev. 38, 2565–2575 (2009).
Pfaffenhuber, C., Göbel, M., Popovic, J. & Maier J. Soggy-sand electrolytes: status and perspectives. Phys. Chem. Chem. Phys. 15, 18318–18335 (2013).
Liang, C. D., Dudney, N. J. & Howe, J. Y. Hierarchically structured sulfur/carbon Nanocomposite material for high-energy lithium battery. Chem. Mater. 21, 4724–4730 (2009).
Zhou, X. S., Wan, L. J. & Guo, Y. G. Binding SnO2 nanocrystals in nitrogen-doped grapheme sheets as anode materials for lithium-ion batteries. Adv. Mater. 25, 2152–2157 (2013).
Suo, L. M., Hu, Y. S., Li, H., Armand, M. & Chen, L. Q. A new class of solvent-in-salt electrolyte for high-energy rechargeable metallic lithium batteries. Nat. Commun. 4, 1481 (2013).
Maier, J. Thermodynamics of electrochemical lithium storage. Angew. Chem. Int. Ed. 52, 4998–5026 (2013).
Goodenough, J. B. & Park, K. S. The Li-ion rechargeable battery: a perspective. J. Am. Chem. Soc. 135, 1167–1176 (2013).
Kamaya, N. et al. A lithium superionic conductor. Nat. Mater. 10, 682–686 (2011).
Li, H., Wang, Z. X., Chen, L. Q. & Huang, X. J. Research on advanced materials for Li-ion batteries. Adv. Mater. 21, 4593–4607 (2009).
Croce, F., Appetecchi, G. B., Persi, L. & Scrosati, B. Nanocomposite polymer electrolytes for lithium batteries. Nature 394, 456–458 (1998).
Nguyen, C. A., Argun, A. A., Hammond, P. T., Lu, X. H. & Lee, P. S. Layer-by-layer assembled solid polymer electrolyte for electrochromic devices. Chem. Mater. 23, 2142–2149 (2011).
Croce, F., Sacchetti, S. & Scrosati B. Advanced, lithium batteries based on high-performance composite polymer electrolytes. J. Power Sources 162, 685–689 (2006).
Christie, A. M., Lilley, S. J., Staunton, E., Andreev, Y. G. & Bruce, P. G. Increasing the conductivity of crystalline polymer electrolytes. Nature 433, 50–53 (2005).
Lightfoot, P., Mehta, M. A. & Bruce, P. G. Crystal-structure of the polymer electrolyte poly (ethylene oxide) 3: LiCF3SO3 . Science 262, 883–883 (1993).
Wang, L. S., Li, X. W. & Yang, W. S. Enhanced of electrochemical properties of hot-pressed poly(ethylene oxide) based nanocomposite polymer electrolyte films for all-solid-state lithium polymer batteries. Electrochim. Acta 55, 1895–1899 (2010).
Bronstein, L. M., Joo, C., Karlinsey, R., Ryder, A. & Zwanziger, J. W. Nanostructured inorganic-organic composites as a basis for solid polymer electrolytes with enhanced properties. Chem. Mater. 13, 3678–3684 (2001).
Nakayama, M., Wada, S., Kuroki, S. & Nogami, M. Factors affecting cyclic durability of all-solid-state lithium polymer batteries using poly(ethylene oxide)-based solid polymer electrolytes. Energy Environ. Sci. 3, 1995–2002 (2010).
Wu, X. L. et al. Enhanced working temperature of PEO-based polymer electrolyte via porous PTFE film as an efficient heat resister. Solid State Ionics 245–246, 1-7 (2013).
Lu, Q. W. et al. A novel solid composite polymer electrolyte based on poly(ethyleneoxide) segmented polysulfone copolymers for rechargeable lithium batteries. J. Membrane Sci. 425–426, 105–112 (2013).
Li, Y. H. et al. A novel polymer electrolyte with improved high-temperature tolerance up to 170°C for high-temperature lithium-ion batteries. J. Power Sources 244, 234–239 (2013).
Barbosa, P. C., Silva, M. M., Smith, M. J., Goncalves, A. & Fortunato, E. Studies of solid-state electrochromic devices based on PEO/siliceous hybrids doped with lithium perchlorate. Electrochim. Acta 52, 2938–2943 (2007).
Jarosik, A., Pfaffenhuber, C., Bunde, A. & and Maier, J. Electrochemical investigations of polyethylene glycol-based “soggy sand” electrolytes from the local mechanism to the overall conduction. Adv. Funct. Mater. 21, 3961–3966 (2011).
Barbosa, P. C. et al. Solid-state electrochromic devices using PTMC/PEO blends as polymer electrolytes. Electrochim. Acta 55, 1495–1502 (2010).
Tong, Y. F., Chen, L., He, X. H. & Chen, Y. W. Free mesogen assisted assembly of the star-shaped liquid-crystalline copolymer/polyethylene oxide solid electrolytes for lithium ion batteries. Electrochim. Acta 118, 33–40 (2014).
Park, Y. W. & Lee, D. S. The fabrication and properties of solid polymer electrolytes based on PEO/PVP blends. J. Non-Cryst. Solids 351, 144–148 (2005).
Croce, F., Sacchetti, S. & Scrosati, B. Advanced, high-performance composite polymer electrolytes for lithium batteries. J. Power Sources 161, 560–564 (2006).
Ji, J. Y., Keen, J. & Zhong, W. H. Simultaneous improvement in ionic conductivity and mechanical properties of multi-functional block-copolymer modified solid polymer electrolytes for lithium ion batteries. J. Power Sources 196, 10163–10168 (2011).
Schulze, M. W., McIntosh, L. D., Hillmyer, M. A. & Lodge, T. P. High-modulus, high-conductivity nanostructured polymer electrolyte membranes via polymerization-induced phase separation. Nano Lett. 14, 122–126 (2014).
Kim, D. G. et al. Preparation of solid-state composite electrolytes based on organic/inorganic hybrid star-shaped polymer and PEG-functionalized POSS for all-solid-state lithium battery applications. Polymer 54, 5812–5820 (2013).
Zuo, X. et al. A novel all-solid electrolyte based on a co-polymer of poly-(methoxy/hexadecal-poly(ethylene glycol) methacrylate) for lithium-ion cell. J. Mater. Chem. 22, 22265–22271 (2012).
He, D., Kim, D. W., Park, J. S., Cho, S. Y. & Kang, Y. K. Electrochemical properties of semi-interpenetrating polymer network solid polymer electrolytes based on multi-armed oligo(ethyleneoxy) phosphate. J. Power Sources 244, 170–176 (2013).
Zhang, J. J. et al. Renewable and superior thermal-resistant cellulose-based composite nonwoven as lithium-ion battery separator. ACS Appl. Mater. Interfaces 5, 128–134 (2013).
Zhang, J. J. et al. Sustainable, heat-resistant and flame-retardant cellulose-based composite separator for high-performance lithium ion battery. Sci. Rep. 4, 3935 (2014).
Bouchet, R. et al. Single-ion BAB triblock copolymers as highly efficient electrolytes for lithium-metal batteries. Nat. Mater. 5, 452–457 (2013).
Shao, N., Sun, X. G., Dai, S. & Jiang, D. E. Oxidation potentials of functionalized sulfone solvents for high-voltage Li-ion batteries: a computational study. J. Phys. Chem. B 116, 3235–3238 (2012).
Wu, C. G., Wu, C. Hui., Lu, M. I. & Chuang, H. J. New solid polymer electrolytes based on PEO/PAN hybrids. J. Appl. Polym. Sci. 99, 1530–1540 (2006).
Yamada, A. et al. Room-temperature miscibility gap in LixFePO4 . Nat. Mater. 5, 357–360 (2006).
Acknowledgements
This work was financially supported by Key Basic Research Project of China (973 Program) (No. MOST2011CB935700), the Instrument Developing Project of the Chinese Academy of Sciences (No. YZ201137), the National High Technology Research and Development Program of China (863 Program, No.2013AA050905) and “135” Projects Fund of CAS-QIBEBT Director Innovation Foundation.
Author information
Authors and Affiliations
Contributions
J.J.Z., G.L.C. and L.Q.C. devised the original concept, designed the experiments and discussed the interpretation of results. J.J.Z. and L.P.Y. and co-wrote the paper. P.H., Z.H.L., B.S.Q. and B.Z. fabricated the solid polymer electrolyte. Q.F.W., G.L.D. and C.J.Z. optimized the solid polymer electrolyte. X.H.Z. and J.H.Y. performed the electrochemical experiments. All authors discussed the results and participated in manuscript revision.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Zhang, J., Yue, L., Hu, P. et al. Taichi-inspired rigid-flexible coupling cellulose-supported solid polymer electrolyte for high-performance lithium batteries. Sci Rep 4, 6272 (2014). https://doi.org/10.1038/srep06272
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep06272
This article is cited by
-
Stable operation of polymer electrolyte-solid-state batteries via lone-pair electron fillers
Nano Research (2023)
-
Advances in Nonwoven-Based Separators for Lithium-Ion Batteries
Advanced Fiber Materials (2023)
-
Preparation and characterisation of chitosan extracted from shrimp shell (Penaeus monodon) and chitosan-based blended solid polymer electrolyte for lithium-ion batteries
Polymer Bulletin (2022)
-
Promoted ion conductivity of sodium salt–poly(ethylene oxide) polymer electrolyte induced by adding conductive beta-alumina and application in all-solid-state sodium batteries
Journal of Materials Science (2021)
-
Properties enhancement of carboxymethyl cellulose with thermo-responsive polymer as solid polymer electrolyte for zinc ion battery
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.