Abstract
Sex hormones may have a role in the pathophysiology of substance use disorders, as demonstrated by the association between testosterone and addictive behaviour in opioid dependence. Although opioid use has been found to suppress testosterone levels in men and women, the extent of this effect and how it relates to methadone treatment for opioid dependence is unclear. The present multi-centre cross-sectional study consecutively recruited 231 patients with opioid dependence from methadone clinics across Ontario, Canada between June and December of 2011. We obtained demographic details, substance use, psychiatric history and blood and urine samples from enrolled subjects. The control group included 783 non-opioid using adults recruited from a primary care setting in Ontario, Canada. Average testosterone level in men receiving methadone treatment was significantly lower than controls. No effect of opioids including methadone on testosterone level in women was found and testosterone did not fluctuate significantly between menstrual cycle phases. In methadone patients, testosterone level was significantly associated with methadone dose in men only. We recommend that testosterone levels be checked in men prior and during methadone and other opioid therapy, in order to detect and treat testosterone deficiency associated with opioids and lead to successful methadone treatment outcomes.
Similar content being viewed by others
Introduction
Opioid dependence has previously been observed in men1,2, however the increased prevalence of prescription opioid drug abuse has led to an increase in opioid use and dependence in women3. This trend has sparked interest in the sex-related aspects of the disorder. To date, sex differences have been reported in many aspects of opioid dependence and treatment4,5,6,7,8,9,10 leading to the need for separate addiction treatment profiles for men and women.
Sex hormones are often studied as the biological basis for sex differences due to their role in central nervous system regulation, implicating the endocrine system in the pathophysiology of substance use disorders and addictive behaviour11. The emerging research on sex hormones in addiction has shed light on the association between testosterone and specific addictive behaviours in men and women, including impulsivity, aggression, risk-taking and sensation-seeking12,13. This provides evidence for the importance of testosterone in substance use disorders including opioid dependence.
Reviews of the literature on testosterone in chronic opioid use report an opioid-induced deficiency in androgen function14,15,16, significantly lower than normative levels of testosterone seen in the clinical literature (average range: 300–1000 ng/dL or 10–35 nmol/L for men and 20–85 ng/dL or 0.7–3 nmol/L for women)17. Opioids exert inhibitory effects on the hypothalamus, the area responsible for production of gonadotropin-releasing-hormone (GnRH). GnRH normally acts on the pituitary gland to stimulate the release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH); when GnRH is inhibited, this leads to low LH and FSH causing suppression of sex hormone secretion from the gonads18. Although these findings are supported in samples of men, levels of sex hormones including testosterone in opioid-dependent women have not been extensively studied to date19. Due to the increase of chronic opioid use in women, a re-examination of the literature is necessary.
Objectives
The purpose of this study is to examine serum total testosterone level in men and women with opioid dependence receiving methadone treatment. We aim to (1) determine what effect opioids including methadone have on testosterone in this sample and if this effect is present in both men and women; (2) identify other methadone-related treatment factors that are associated with testosterone level; and (3) examine the variability of testosterone level across menstrual cycle phases in women.
Results
Sample characteristics
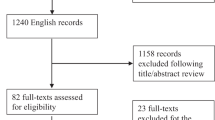
Of the initial 260 participants undergoing methadone treatment that were recruited, 29 participants were excluded from the study (duplicate entries = 5, buprenorphine treatment = 3, undetectable or out-of-range testosterone levels = 15, hormone replacement therapy = 2, using prescription opioids for chronic pain = 4). Therefore, 231 participants in total were included in the analysis (Figure 1). The sample consisted of 56.7% men with mean age 38.3 (standard deviation [SD] 11.0) and 43.3% women with mean age 35.2 (SD 9.4). The majority of the sample population (84.4%) was of European ethnicity. Refer to Table 1 for additional information on demographics, substance use history and treatment outcomes. Control participants included 287 (36.7%) men and 496 (63.3%) women who were not using opioids. Mean age was 46.2 (SD 13.1) and 44.6 (SD 12.6) years for men and women, respectively.
Effect of opioid use and methadone treatment on testosterone serum level
Men with opioid dependence undergoing methadone treatment had significantly suppressed testosterone levels (mean = 100.10 ng/dL, SD 72.21, or 3.47 nmol/L, SD 2.51) compared to controls (mean = 414.74 ng/dL, SD 141.81, or 14.39 nmol/L, SD 4.92) (estimated β = −1.661; 95% confidence interval [CI] −1.793, −1.529; p < 0.0001). Testosterone levels for women on MMT did not differ significantly compared to controls (mean = 36.61 ng/dL, SD 23.19, or 1.27 nmol/L, SD 0.81; and mean = 25.93 ng/dL, SD 15.20, or 0.90 nmol/L, SD 0.52, respectively) (estimated β = 0.063; 95% CI −0.098, 0.224; p = 0.441). Table 2 presents a statistical summary of testosterone level in both samples by sex.
Factors associated with testosterone level in methadone treatment
Sex was positively associated with testosterone in this model as expected, with men having a higher testosterone level than women (estimated β = 1.034; 95% CI 0.857, 1.211; p < 0.0001). Testosterone level was found to be inversely associated with methadone dose (estimated β = −0.002; 95% CI −0.003, −0.000; p = 0.018) (Table 3), indicating that a higher methadone dose is correlated with lowered testosterone levels. In the subgroup analysis by sex, testosterone level was inversely associated with methadone dose (estimated β = −0.003; 95% CI −0.005, −0.001; p = 0.003) (Figure 2) and positively associated with the number of cigarettes smoked per day (estimated β = 0.011; 95% CI 0.000, 0.021; p = 0.046) in men. Although no significant correlations were found in the sample of women, polysubstance use showed a positive trend of association with testosterone level (estimated β = 0.244; 95% CI −0.004, 0.493; p = 0.054) (Table 3).
Variability of testosterone level across menstrual cycles phases in women
We employed a linear regression to determine whether serum total testosterone level differs between menstrual cycle phases (follicular and luteal) and the menopause phase in our control sample of women (n = 419). Results demonstrated no difference in testosterone level between all three phases (estimated β = −0.992; 95% CI −21.263, 19.279; p = 0.923) when controlling for age and smoking status (Figure 3). This suggests that testosterone does not fluctuate significantly across phases of the menstrual cycle or during menopause.
Discussion
The objectives of this study were to examine the overall effect of opioids including methadone on serum testosterone level in men and women, determine what factors are associated with testosterone level in this sample and examine the variability in testosterone level across menstrual cycle phases in women.
Our results have confirmed the suppressive effect of methadone on testosterone in men undergoing methadone treatment, however they also demonstrate that methadone does not suppress testosterone levels in women. There is limited information on testosterone levels in women with opioid dependence who are currently undergoing methadone treatment and this study has aimed to add to the scant literature.
This sex-specific difference in methadone effects on testosterone is indicative of a distinct biological mechanism between men and women. Opioids including methadone exert their effects on the gonads through the HPG axis and suppress the release of sex hormones20. In women, β-estradiol is the primary sex hormone and opioids may act to primarily suppress β-estradiol and target testosterone as a secondary androgen. This is supported by studies looking at the role of opioids in estrogen release. Findings from these studies demonstrate that estradiol was suppressed in opioid-dependent women21 and after methadone consumption19. Studies also report the effect of opioids on prolactin release22,23. Prolactin is a hormone responsible primarily for milk production during pregnancy, however it also has a role in sexual behaviours. Prolactin may act to mediate this relationship by inhibiting GnRH secretion, causing a decrease of estrogen in women and testosterone in men24.
The second objective of this study was to investigate the association between testosterone and methadone-related factors. In MMT patients, we found that methadone dose was inversely associated with testosterone level, indicating that the relationship between methadone and testosterone is dose-dependent. Our sub-group analysis by sex showed that this dose-dependent association was present in men only. This is consistent with previous studies in the literature that looked at the effect of morphine and heroin dosing on testosterone level12,25,26, however these studies did not control for other relevant factors such as duration on treatment and continued illicit opioid use. Bolelli et al.25 measured heroin plasma concentration in association with testosterone level in men and also found suppressed testosterone levels, however their study was limited by a very small sample size as well as a lack of an accurate heroin concentration measure; they were unable to control for varying rates of heroin metabolism between participants or by which route the heroin entered the blood. Dev et al.26 focused on a small sample of cancer patients using morphine for pain management. They found a similar inverse relationship between morphine dose and testosterone level in men. Our study has confirmed this relationship in a large sample of patients specifically using methadone for the treatment of opioid addiction and we have incorporated an analysis to test this effect in women.
In order to estimate the magnitude of this effect, we used the exponentiated beta coefficient to reverse the logarithmic transformation and multiplied this by 10 so that methadone dose can be quantified in 10 mg increments. We found that for each 10 mg increase in methadone dose, there is a 0.97 ng/dL (0.03 nmol/L) decrease in testosterone level (estimated exp(β) = 0.969; 95% CI 0.950, 0.989; p = 0.003), suggesting that men with a higher methadone dose will be more likely to have more suppressed testosterone. In addition, we observed a positive association between the number of cigarettes smoked daily and serum testosterone level in men. Using the same calculation, we estimate that for each additional cigarette smoked per day, there is a 1.01 ng/dL (0.04 nmol/L) increase in testosterone level in men (estimated exp(β) = 1.011; 95% CI 1.000, 1.021; p = 0.046). This may be explained by the effect of smoking on methadone metabolism, where smoking is an enzyme inducer in the liver accelerating the metabolism of methadone and hence reduces methadone blood level and its inhibition of testosterone27. This association may also be related to addictive behaviour, where smoking as an addictive behaviour is associated with the risk-taking behavioural profile of testosterone28. We speculate that this may also be a reason for why male methadone patients have a difficult time with smoking cessation29.
Low testosterone in men has been associated with poor quality of life, as well as erectile dysfunction, hypogonadism, symptoms of fatigue, weakness and mood disturbances14,30. Improvement in self-reported quality of life assessed across multiple dimensions has been shown to improve treatment outcomes such as retention and overall health in methadone patients after one year of stabilization on MMT31. By treating testosterone deficiency, it is suspected that patients will experience improvements in quality of life and therefore demonstrate successful treatment outcomes. Healthcare providers should be aware of the effect of opioids including methadone on testosterone and that symptoms related to testosterone deficiency can be actively managed by testosterone therapy. Health care providers should also ensure that patients are being prescribed the lowest possible dose for effective substitution opioid treatment to minimize testosterone suppression.
These findings are applicable to the larger population of methadone patients attending clinics across Ontario and across North America as well. Our patients were recruited from multiple sites of varying geographic locations, all of which follow a standardized treatment regimen, therefore making our sample representative of the overall methadone patient population.
Our final objective was to examine the variability of testosterone levels across menstrual cycle phases in women. In pre-menopausal women, testosterone level does not vary between follicular and luteal phases and it also does not differ significantly among post-menopausal women. A few studies have found that total testosterone differed between phases, with it being the highest in the luteal32,33 or mid-cycle phase34,35. However, studies also report no significant differences between cycle phases36,37,38. The variability in these findings may be explained by different methods of measuring testosterone (i.e. free, bound, or total; plasma vs. serum; diluted vs. non-diluted, etc.), by the use of different tests (i.e. hormone assays, mass spectrometry), or by differences in defining cycle phases (follicular, mid-cycle, or luteal). Our study has tested this effect in the largest sample of women to date and confirms that testosterone is not sensitive to menstrual cycle changes. Measurement of serum total testosterone level in future investigations does not need to account for menstrual cycle phase, as testosterone levels at any given time are generally representative of the average testosterone level in women.
One of the main limitations of the study is the small sample size of MMT patients when the analysis is divided by sex. There is adequate power to support the associations found in men (86%) however the power for the women sample is much lower (43%), which is not enough to detect any significant associations. Although our sample size for women is one of the largest among studies investigating testosterone in methadone treatment among women, it remains inadequate to draw any significant conclusions on the impact of opioids on testosterone in women, although it may provide some information regarding the magnitude and direction of effect. In addition, some variables included in this study, for example smoking, were based on self-report and therefore may not be entirely accurate or reliable.
Investigation into additional sex hormones may provide insight into the biological basis of methadone treatment and potentially decipher the effect that opioids have on women. Larger sample sizes with more sex hormones investigated would be ideal for future study, as well as implementing a prospective follow-up study design to observe whether testosterone levels change throughout the course of treatment with methadone. Future directions may include studying the association between testosterone and risk of relapse, as well as observing methadone treatment response and retention after treating low testosterone to determine whether these outcomes have improved.
In this study, we provided an investigation of the influence of opioid dependence and methadone treatment on testosterone. We demonstrated that methadone has a dose-dependent suppressive effect on testosterone in men and that testosterone is not sensitive to menstrual cycle changes in women. The results of this study can be used to guide the decision-making process for men and encourage them to seek treatment for opioid dependence. We also recommend that testosterone levels be checked in men prior to undergoing any opioid therapy and at regular intervals thereafter, in order to treat testosterone deficiency associated with opioids.
Methods
Study design
We collected cross-sectional data from the Genetics of Opioid Addiction (GENOA) research program39, a collaboration between Ontario Addiction Treatment Centres (OATC) and the Population Genomics Program (PGP), Chanchlani Research Centre at McMaster University. Data were collected for the GENOA study from four OATC outpatient methadone clinics specializing in Opiate Agonist Therapy (OAT) across Southern Ontario, Canada between June and December of 2011. Recruitment consisted of a structured interview conducted on site by a trained OATC clinical staff member and completion of study-specific case report forms. Demographics, anthropometric measurements and history of current and past substance use, psychiatric diagnoses and medical conditions were obtained, in addition to urine and blood samples. This study was carried out in accordance with ethical guidelines and approval by the Hamilton Integrated Research Ethics Board (HIREB) and written informed consent was obtained from each study participant.
Study participants
We recruited men and women aged 18 years and older consecutively from OATC clinics. Inclusion criteria consisted of current enrolment in MMT for a diagnosis of opioid dependence according to DSM-IV criteria and having the ability to provide written informed consent. Exclusion criteria were the use of opioid substitution therapy other than methadone for opioid dependence, inability to communicate in English and refusal to provide biological samples. Patients received supervised daily methadone doses, addiction counseling (including methods for coping with stress, reacting to environmental stressors, developing constructive social networks, etc.) and regular medical follow up as per usual clinical care.
The control group was a sample of adults aged 18–74 years who were screened for DSM-IV dysthymic disorder in a primary care university-affiliated Health Services Organization (HSO) located in Southern Ontario, Canada. This population was an English-speaking, middle class, suburban family community, which consisted of individuals without opioid dependence40,41,42.
Outcome measures
The primary outcome is serum total testosterone level. Covariates include continued opioid use (use of illicit opioids detected by weekly or bi-weekly urine screens, measured as the percentage of positive opioid urine screens per total number of urine screens available), methadone treatment duration (length of time in months between the methadone start date and date of most recent methadone dose reported by the patient or obtained from clinic records), methadone dose (current daily dose of methadone at time of interview), polysubstance use (use of a minimum of two substances of abuse in addition to opioids, which include stimulants, hallucinogens, inhalants, cannabis, barbiturates, benzodiazepines, performance-enhancing drugs, or diet pills within the last 12 months; these data were acquired through interviews using the Mini International Neuropsychiatric Interview (M.I.N.I.) Version 6: Drug and Alcohol Modules)43 and smoking (self-reported average number of cigarettes smoked daily). We also collected age of initial opioid use (self-reported age at which participant began using opioids regularly).
Laboratory analysis
We measured serum total testosterone level in the MMT sample using enzyme-linked immunosorbent assay (ELISA) technique (Enzo Life Sciences, Plymouth Meeting, PA, USA); intra-assay variation is 3.3%, while inter-assay variation is 9.8%, with sensitivity of 2.6%. Serum testosterone in the control sample was measured with the Coat-A-Count total testosterone solid phase radioimmunoassay (RIA) kit (Diagnostic Products Corp., Los Angeles, CA, USA); intra-assay variation is 7.2%, inter-assay variation is 9.4% and sensitivity is 3.6%. We used different assays for testosterone measurement between MMT and control groups because the hormone assay method used in the past was RIA, which was used for our control group, whereas ELISA is currently the preferred method of hormone analysis. Standard curves of both assays showed a comparable detectable range and appropriate sensitivities, therefore the methods are unlikely to lead to discrepancies in testosterone levels between samples. We conducted qualitative and seminquantitative urine analysis for opioids using iMDx™ Prep Assay [NOVX Systems Inc, Richmond Hill, Ontario, Canada] and performed these weekly or bi-weekly throughout the study period as part of routine clinical care.
Statistical analysis
Continuous variables are presented as a mean and standard deviation and dichotomous variables are presented as a proportion of the sample population. Data for testosterone level showed a skewed distribution on a normal probability plot in the MMT and control groups; these distributions were transformed with the natural logarithm before inclusion in the multivariable regression analysis. Extreme outliers were removed based on the maximum and minimum detectable limit of the hormone assays in the laboratory. We used multiple imputation methods for missing data.
We conducted multivariable linear regression analyses to determine differences in mean log-transformed testosterone levels between men and women MMT participants and controls (n = 1014) and to determine which methadone-related factors are associated with testosterone level (n = 231), with the following covariates included in the model: age, sex, age of initial opioid use, number of cigarettes smoked per day, methadone dose, duration on methadone treatment, polysubstance use and continued illicit opioid use (based on urine test results). Sub-group analysis by sex was decided a priori. We also performed a linear regression to test if there was a significant difference in serum total testosterone level across follicular and luteal phases of the menstrual cycle and the post-menopausal phase in control women (n = 419) while accounting for age and smoking status. Patients on hormonal medication including birth control, hormone replacement therapy and thyroid medications were removed from the sample.
The study is reported in adherence to the Strengthening of Reporting of Observational Studies in Epidemiology (STROBE) statement for observational studies44. The results are reported as an estimate of the association expressed as a mean difference or model coefficient, corresponding 95% confidence interval and associated p-value. All statistical analyses were performed using STATA Version 11.
References
Fischer, B., Medved, W., Gliksman, L. & Rehm, J. Illicit opiate users in Toronto: a profile of current users. Addict Res 7, 377–415 (1999).
Fischer, B., Rehm, J., Patra, J. & Cruz, M. F. Changes in illicit opioid use across Canada. CMAJ 175, 1385, 10.1503/cmaj.060729 (2006).
United States Department of Health and Human Services. Substance Abuse and Mental Health Services Administration. Office of Applied Studies. National Household Survey on Drug Abuse, 2000. Ann Arbor, MI: Inter-university Consortium for Political and Social Research, 10.3886/ICPSR03262.v5 (2013). Date of access: 25/02/2014.
Back, S. E. et al. Comparative profiles of men and women with opioid dependence: Results from a national multisite effectiveness trial. Am J Drug Alcohol Ab 37, 313–323 (2011).
Maremmani, I. et al. Differential substance abuse patterns distribute according to gender in heroin addicts. J Psychoactive Drugs 42, 89–95 (2010).
Chen, C. K., Shu, L. W., Liang, P. L., Hung, T. M. & Lin, S. K. Drug use patterns and gender differences among heroin addicts hospitalized for detoxification. Changgeng Yi Xue Za Zhi 21, 172–178 (1998).
Lin, H. C. et al. Gender differences in heroin users receiving methadone maintenance therapy in Taiwan. J Addict Dis 32, 140–149, 10.1080/10550887.2013.795466 (2013).
Back, S. E., Lawson, K. M., Singleton, L. M. & Brady, K. T. Characteristics and correlates of men and women with prescription opioid dependence. Addict Behav 36, 829–834, 10.1016/j.addbeh.2011.03.013 (2011).
Chatham, L. R., Hiller, M. L., Rowan-Szal, G. A., Joe, G. W. & Simpson, D. D. Gender differences at admission and follow-up in a sample of methadone maintenance clients. Subst Use Misuse 34, 1137–1165 (1999).
Fischer, B., Cruz, M. F. & Rehm, J. Illicit opioid use and its key characteristics: a select overview and evidence from a Canadian multisite cohort of illicit opioid users (OPICAN). Can J Psychiat 51, 624–634 (2006).
Stumpf, W. E. & Sar, M. Steroid hormone target sites in the brain: the differential distribution of estrogin, progestin, androgen and glucocorticosteroid. J Steroid Biochem 7, 1163–1170 (1976).
Mendelson, J. H., Mendelson, J. E. & Patch, V. D. Plasma testosterone levels in heroin addiction and during methadone maintenance. J Pharmacol Exp Ther 192, 211–217 (1975).
Kosten, T. A. & Ambrosio, E. HPA axis function and drug addictive behaviors: insights from studies with Lewis and Fischer 344 inbred rats. Psychoneuroendocrino 27, 35–69 (2002).
Smith, H. S. & Elliott, J. A. Opioid-induced androgen deficiency (OPIAD). Pain Physician 15, Es145–156 (2012).
Wahlstrom, J. T. & Dobs, A. S. Acute and long-term effects of AIDS and injection drug use on gonadal function. J Acquir Immune Defic Syndr 25 Suppl 1, S27–36 (2000).
Katz, N. & Mazer, N. A. The impact of opioids on the endocrine system. Clin J Pain 25, 170–175, 10.1097/AJP.0b013e3181850df6 (2009).
Bhasin, S. et al. Testosterone therapy in adult men with androgen deficiency syndromes: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 91, 1995–2010, 10.1210/jc.2005-2847 (2006).
Cicero, T. J. Effects of exogenous and endogenous opiates on the hypothalamic--pituitary--gonadal axis in the male. Fed Proc 39, 2551–2554 (1980).
Daniell, H. W. Opioid endocrinopathy in women consuming prescribed sustained-action opioids for control of nonmalignant pain. J Pain 9, 28–36, 10.1016/j.jpain.2007.08.005 (2008).
Kalra, S. P. & Simpkins, J. W. Evidence for noradrenergic mediation of opioid effects on luteinizing hormone secretion. Endocrinology 109, 776–782 (1981).
Woody, G. et al. Hormone secretion in methadone-dependent and abstinent patients. NIDA Res Monogr 81, 216–223 (1988).
Rajagopal, A., Vassilopoulou-Sellin, R., Palmer, J. L., Kaur, G. & Bruera, E. Hypogonadism and sexual dysfunction in male cancer survivors receiving chronic opioid therapy. J Pain Symptom Manage 26, 1055–1061 (2003).
Paice, J. A., Penn, R. D. & Ryan, W. G. Altered sexual function and decreased testosterone in patients receiving intraspinal opioids. J Pain Symptom Manage 9, 126–131 (1994).
Hemmings, R., Fox, G. & Tolis, G. Effect of morphine on the hypothalamic-pituitary axis in postmenopausal women. Fertil Steril 37, 389–391 (1982).
Bolelli, G. et al. Heroin addiction: relationship between the plasma levels of testosterone, dihydrotestosterone, androstenedione, LH, FSH and the plasma concentration of heroin. Toxicology 15, 19–29 (1979).
Dev, R. et al. Association between serum cortisol and testosterone levels, opioid therapy and symptom distress in patients with advanced cancer. J Pain Symptom Manage 41, 788–795, 10.1016/j.jpainsymman.2010.06.021 (2011).
Elkader, A. K., Brands, B., Selby, P. & Sproule, B. A. Methadone-nicotine interactions in methadone maintenance treatment patients. J Clin Psychopharmacol 29, 231–238, 10.1097/JCP.0b013e3181a39113 (2009).
Burt, R. D., Dinh, K. T., Peterson, A. V., Jr & Sarason, I. G. Predicting adolescent smoking: a prospective study of personality variables. Prev Med 30, 115–125, 10.1006/pmed.1999.0605 (2000).
Richter, K. P., Gibson, C. A., Ahluwalia, J. S. & Schmelzle, K. H. Tobacco use and quit attempts among methadone maintenance clients. Am J Public Health 91, 296–299 (2001).
Borjesson, G., Martensson, A., Holmer, H. I. & Westerling, D. Low testosterone levels in men with long-term opioid treatment. Eur J Pain Suppl 5, 178 (2011).
Dazord, A., Mino, A., Page, D. & Broers, B. Patients on methadone maintenance treatment in Geneva. Eur Psychiat 13, 235–241, 10.1016/s0924-9338(98)80011-4 (1998).
Anttila, L., Koskinen, P., Irjala, K. & Kaihola, H. L. Reference intervals for serum sex steroids and gonadotropins in regularly menstruating women. Acta Obstet Gynecol Scand 70, 475–481 (1991).
Mathor, M. B., Achado, S. S., Wajchenberg, B. L. & Germek, O. A. Free plasma testosterone levels during the normal menstrual cycle. J Endocrinol Invest 8, 437–441 (1985).
Rothman, M. S. et al. in Steroids 76, 177–182 (2010 Elsevier Inc, 2011).
Stahl, F., Dorner, G., Rohde, W. & Schott, G. Total and free testosterone and total and free 17 beta-oestradiol in normally menstruating women. Endokrinologie 68, 112–114 (1976).
Braunstein, G. D., Reitz, R. E., Buch, A., Schnell, D. & Caulfield, M. P. Testosterone reference ranges in normally cycling healthy premenopausal women. J Sex Med 8, 2924–2934, 10.1111/j.1743-6109.2011.02380.x (2011).
Elliott, K. J., Cable, N. T., Reilly, T. & Diver, M. J. Effect of menstrual cycle phase on the concentration of bioavailable 17-beta oestradiol and testosterone and muscle strength. Clin Sci (Lond) 105, 663–669, 10.1042/cs20020360 (2003).
Haring, R. et al. Age-Specific Reference Ranges for Serum Testosterone and Androstenedione Concentrations in Women Measured by Liquid Chromatography-Tandem Mass Spectrometry. J Clin Endocr Metab 97, 408–415, 10.1210/jc.2011-2134 (2012).
Samaan, Z. et al. Genetic influence on methadone treatment outcomes in patients undergoing Methadone Maintenance Treatment (MMT) for opioid addiction: A pilot study. In press. Neuropsychiatr Dis Treat 10, 1–6 (2014).
Bell, B. et al. Burden of dysthymia and comorbid illness in adults in a Canadian primary care setting: high rates of psychiatric illness in the offspring. J Affect Disord 78, 73–80 (2004).
Browne, G. et al. Sertraline and/or interpersonal psychotherapy for patients with dysthymic disorder in primary care: 6-month comparison with longitudinal 2-year follow-up of effectiveness and costs. J Affect Disord 68, 317–330 (2002).
Steiner, M. et al. Prevalence of dysthymic disorder in primary care. J Affect Disord 54, 303–308 (1999).
Sheehan, D. V. et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiat 59 Suppl 20, 22–33; quiz 34–57 (1998).
von Elm, E. et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370, 1453–1457, 10.1016/s0140-6736(07)61602-x (2007).
Acknowledgements
We would like to thank Jacqueline Hudson, administrative research assistant at the Population Genomics Program at McMaster University, for her efforts in the collaboration between research and clinic staff as well as handling the administrative aspects of the study. We would also like to acknowledge the OATC for their partnership and their invaluable help in recruitment and data collection. This work was supported by CIHR Drug Safety and Effectiveness Network (DSEN) grant (Grant number: 126639) from Ottawa, Canada and by The Department of Psychiatry and Behavioural Neurosciences, McMaster University, Innovation Award (Grant number: 2-15311) from Hamilton, Canada. The funding sources have no role in the study design or reporting of the results.
Author information
Authors and Affiliations
Contributions
M.B. and Z.S. were responsible for the development of the research question, interpretation of data, manuscript writing and critical revision of the manuscript. M.B. and B.D. performed statistical analyses and B.D. also contributed to manuscript writing and critical revision. M.C.S. contributed to data interpretation and organization and critical revision of the manuscript. C.P., A.W., M.V., J.D., D.M., D.D. and G.P. were all jointly responsible for data collection from OATC clinics, as well as clinical interpretation of results and critical revision of the manuscript. R.A. was involved in interpretation of data and critical revision of manuscript. M.S. was responsible for data collection of the control sample and critical revision of the manuscript. M.C. performed all laboratory analyses for testosterone and assisted with interpretation of data and critical revision of manuscript. L.T. assisted with statistical analysis, interpretation of data and revision of manuscript. All authors have reviewed and approved the final manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Bawor, M., Dennis, B., Samaan, M. et al. Methadone induces testosterone suppression in patients with opioid addiction. Sci Rep 4, 6189 (2014). https://doi.org/10.1038/srep06189
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep06189
This article is cited by
-
The effect of methadone, buprenorphine, and shift of methadone to buprenorphine on sperm parameters and antioxidant activity in a male rat model
Comparative Clinical Pathology (2020)
-
Bone mineral density and its determinants in men with opioid dependence
Journal of Bone and Mineral Metabolism (2017)
-
Sex differences in substance use, health, and social functioning among opioid users receiving methadone treatment: a multicenter cohort study
Biology of Sex Differences (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.