Abstract
Substantial effort has been placed in developing efficacious recombinant attenuated adenovirus-based vaccines. However induction of immunity to the vector is a significant obstacle to its repeated use. Here we demonstrate that skin-based delivery of an adenovirus-based malaria vaccine, HAdV5-PyMSP142, to mice using silicon microneedles induces equivalent or enhanced antibody responses to the encoded antigen, however it results in decreased anti-vector responses, compared to intradermal delivery. Microneedle-mediated vaccine priming and resultant induction of low anti-vector antibody titres permitted repeated use of the same adenovirus vaccine vector. This resulted in significantly increased antigen-specific antibody responses in these mice compared to ID-treated mice. Boosting with a heterologous vaccine; MVA-PyMSP142 also resulted in significantly greater antibody responses in mice primed with HAdV5-PyMSP142 using MN compared to the ID route. The highest protection against blood-stage malaria challenge was observed when a heterologous route of immunization (MN/ID) was used. Therefore, microneedle-mediated immunization has potential to both overcome some of the logistic obstacles surrounding needle-and-syringe-based immunization as well as to facilitate the repeated use of the same adenovirus vaccine thereby potentially reducing manufacturing costs of multiple vaccines. This could have important benefits in the clinical ease of use of adenovirus-based immunization strategies.
Similar content being viewed by others
Introduction
Immunization is the most successful strategy to combat infectious diseases. The creation of an effective Plasmodium falciparum malaria vaccine has been a much sought after goal for the vaccine community, however development of an efficacious malaria vaccine has been clinically challenging1. Recombinant replication-defective adenoviral vectored vaccines were initially developed as candidate vaccines for induction of T cell responses against HIV-1, liver-stage malaria parasites and other intercellular pathogens2. More recently, heterologous prime-boost immunization regimens, involving adenoviruses (AdV) or the poxvirus modified vaccinia virus Ankara (MVA), have shown particular promise in antibody, as well as T cell, induction in pre-clinical animal models of blood-stage malaria vaccines3. Furthermore, in mice, vaccination with an AdV-MVA regimen can protect against a lethal challenge with blood-stage and liver-stage P. yoelii, a significant proof-of-concept that multi-stage malaria immunity can be achieved by rational choice of antigen and of vaccine platform4. Promising levels of immunogenicity have been reported in proof-of-concept Phase I/II clinical trials using replication defective recombinant viral vectored vaccines based on AdV and MVA5,6,7. Therefore recombinant adenovirus-based vaccines have now become one of the leading candidate delivery platforms in the malaria vaccine field.
In the HIV field, despite acceptable safety and tolerability in early human vaccine trials, a 3000-volunteer Phase II test-of-concept study of an adenovirus human serotype 5 (HAdV5)-based vaccine was prematurely stopped due to lack of efficacy. This led to renewed focus on understanding host immunity to adenovirus vectors and how the anti-adenovirus response affects vaccine potency and efficacy. It is accepted that pre-existing humoral and cellular immunity to HAdV5 prevents repeated administration of this virus vector for booster immunizations as observed by a failure to increase immunity to the encoded antigen on repeated immunization8. Clinical development efforts to overcome this obstacle have focussed on the use of simian or rare human AdV serotypes with low seroprevalence in humans5,6,7,9. However, anti-vector immunity prevents repeated use of the same serotype within a short time-frame and even heterologous AdV vectors have shown disappointing clinical results when used together in a prime-boost regimen against hepatitis C virus10. Most clinical development programmes have thus focussed on the use of AdV-MVA regimens as the leading approach for malaria vaccines3. Consequently, although new immunogenic adenovirus serotypes are being developed and are demonstrating clinical potential, the cost and logistic problem of requiring heterologous vaccines remains to be addressed. It has been demonstrated that pre-existing immunity can be overcome by vaccinating by mucosal routes11,12,13,14, however, there has been little, if any, investigation into whether this immunogenicity can be skewed in favour of the encoded antigen (and away from the vector) by altering the method of primary immunization.
Microneedles are micron-scale needles that penetrate the stratum corneum, creating temporary conduits for drug or vaccine administration to desired depths in the skin or underlying tissue, which cannot passively permeate into the skin due to their large molecular size and hydrophilic nature. We previously demonstrated that equivalent or significantly higher immune responses can be induced by silicon microneedle patches (MN) using only approximately 3% of the vaccine dose used by the intradermal (ID) route15. Various types of microneedle systems are being developed in an effort to eliminate the need for hypodermic needles (solid or hollow microneedles) or also to stabilize vaccine out of cold chain conditions (coated or dissolvable microneedles)16. Use of a simpler vaccine delivery device that eliminates sharps waste and reduces the requirements for training in correct vaccination technique as well as in appropriate waste handling would have a positive impact on the cost-effectiveness and success of immunization programmes17.
Here, we demonstrate that, compared to ID injection with a needle and syringe, administering a recombinant HAdV5 blood-stage malaria vaccine using silicon microneedle patches decreases the anti-vector antibody response, while the humoral response to the encoded antigen is equivalent to the response induced by the ID route. The decreased anti-vector immune response induced by vaccination using silicon MN permitted successful repeated immunization with the same recombinant adenovirus vaccine. In challenge studies, the highest levels of protection against blood-stage malaria challenge were observed when a heterologous route (AdV delivered by MN then ID) was used for priming and boosting. To our knowledge, this is the first study to demonstrate that the balance in immune responses to the virus vector compared to the encoded antigen can be favourably modulated by transcutaneous immunization.
Results
Microneedle array design does not influence the magnitude of vaccine-induced antibody responses
We previously demonstrated that the dimensions of solid silicon microneedle arrays affects vaccine-induced CD8+ T cell responses15. Here, we wished to initially determine if the design of the microneedle array also impacted on the induction of serum antibody responses. A range of silicon microneedle arrays were fabricated that differed in the area of the patch, the density of the microneedles, the height of each microneedle and the total pore volume of each patch. The total pore volume of the microneedle array refers to the maximum volume of the conduits that can be created in the skin after application of a microneedle array and has been arbitrarily defined as small, intermediate or large. Eight array types with increasing total pore volume that differ in microneedle density or height were fabricated (Table I).
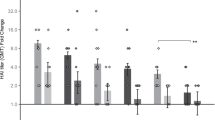
Female C57BL/6 mice were immunized with human adenovirus serotype 5 expressing P. yoelii MSP142 (HAdV5-PyMSP142) by the intradermal (ID) route or using these silicon microneedle patches. The ID route was chosen as it has been repeatedly used in clinical studies and, similar to microneedles, the vaccine is delivered to skin. Serum total IgG antibody responses, examined 8 weeks after priming, demonstrated that vaccination using any microneedle patch design induced a humoral response to the 19 kDa C-terminal region of the encoded blood-stage malaria antigen (PyMSP119), that was not significantly different to ID delivery (Fig. 1A). Vaccine delivery using patch A or F resulted in a trend for lower serum antibody responses compared to other patches. Of interest, patches A and F possess the smallest total pore volume (Table I). We propose that these patch designs deliver the lowest dose of HAdV5-PyMSP142 that results in a weaker serum antibody response compared to all other forms of delivery tested here. Therefore, in contrast to CD8+ T cell responses15, we demonstrate that, apart from small total pore volumes (A and F), the design of the microneedle array does not impact on the magnitude of the antibody response induced by a live adenoviral vaccine. This initial study also demonstrates that microneedle patches with pore volumes in the intermediate and large range are more suitable for the delivery of antibody-inducing virus vectored vaccines.
Influence of the microneedle patch design on the induction of antibody responses by a recombinant adenovirus vaccine.
C57BL/6 mice were immunized with 1 × 1010 vp HAdV5-PyMSP142 by the intradermal (ID) route or using a silicon microneedle patch of differing design, as outlined in Table I. All mice were boosted at 8 weeks with MVA-PyMSP142 by the ID route. Total IgG titers in blood were measured to P. yoelii MSP119 at 8 weeks after the primary immunization and 2 weeks after the boost. (A) Individual responses at 8 weeks post-prime. (B) Antigen-specific total IgG responses at 8 weeks post-prime (light grey bars) and 2 weeks post-boost (dark grey bars). * p < 0.05, ** p < 0.01, ***p < 0.001 compared to post-prime antibody responses, by 2way ANOVA with Bonferroni post-test.; ★ p < 0.05, ★★★ p < 0.001 compared to ID immunization by one way ANOVA using Dunn's multiple comparison test. IgG1(light grey bars) and IgG2a (dark grey bars) antibody responses to the antigen were measured in the serum (C) 8 weeks post-prime and (D) 2 weeks after MVA-PyMSP142 boosting. The mean and standard error of the mean (SEM) (n = 5 to 17 mice) are shown in all panels.
The effect of the mode of priming on antibody responses after a heterologous boosting, with MVA, was next investigated. All groups were boosted in an identical manner, with MVA-PyMSP142 by the ID route so that post-boost immunity reflected differences in the existing primary response. A significant boost in the anti-PyMSP119 IgG response was seen in all groups, however, priming using intermediate or large pore volume microneedle arrays resulted in significantly higher post-boost serum antibody responses compared to ID immunization (Fig. 1B). Interestingly, despite the weaker responses noted above, priming with small pore volume microneedle arrays (arrays A and F) resulted in equivalent post-boost antibody responses to ID priming (Fig. 1B). A significant increase in the post-boost antibody response when using microneedle-priming was unexpected, given post-prime responses were equivalent (Fig. 1A) and previous work demonstrated that adenovirus vaccine-induced antibody responses were boosted to very similar extents by a range of different vaccines18.
We also determined the PyMSP119-specific isotype profile in these immunized C57BL/6 mice. Vaccine administration using any of the microneedle arrays did not significantly change the ratio of IgG1:IgG2a eight weeks post-prime, however there was a trend towards higher IgG2a in most of the microneedle groups compared to ID immunization (Fig. 1C). A similar trend towards increased IgG2a was also observed two weeks after the MVA-PyMSP142 ID boost (Fig. 1D).
Overall, these results demonstrate that the humoral response induced by silicon microneedle arrays and ID primary immunization are quantitatively and qualitatively similar in terms of serum IgG antibody induction. However, silicon microneedle-mediated priming induces an antibody response that is significantly different with respect to responding to antigen re-exposure during secondary immunization with MVA-PyMSP142.
Microneedle-mediated immunization significantly skews the antigen-to-vector antibody response towards the antigen
We examined the effect of microneedle-mediated adenovirus delivery on the induction of anti-vector antibody responses. Surprisingly, we determined that the antibody response to the adenovirus vector was decreased when microneedle arrays were used to administer the recombinant HAdV5-PyMSP142 vaccine compared to ID immunization (Fig. 2A). Intradermal immunization with HAdV5-PyMSP142 primed a humoral response whereby the anti-vector response was equivalent to or higher than the anti-transgene antibody response, as evidenced by an equivalent or higher relative level of vector-specific antibodies compared to antigen-specific antibodies (median ratio: 0.42; 95% CI 0.163 to 1.761). In contrast, immunization with microneedle arrays induced a response whereby the antibody response was significantly skewed by approximately 30-fold to the PyMSP119 antigen compared to the anti-vector antibody response (median ratio of all MN-treated animals: 12.90, 95% CI: 17.31 to 33.69) (Fig. 2B). This skewed response to the antigen and away from the vector also occurred when microneedle arrays A and F were used to administer the vaccine (despite lower overall immunogenicity). Finally, to determine if the kinetic of the antibody response to the transgene and to the vector was different in mice vaccinated via the ID or MN route, we examined the ratio of the anti-PyMSP119 to anti-vector response at 21, 35 or 54 days after priming (Fig. 2C). This ratio did not significantly change over 8 weeks in the ID treated group. However, in the microneedle group the ratio of the antibody response to the antigen compared to the vector steadily increased to a level where it was significantly higher compared to ID treated animals at days 35 and 54. This suggests that antibody responses develop at different rates in mice primed using microneedle patches compared to ID administration. These results further demonstrate that there is a fundamental difference in the induction of humoral immunity to the virus vector when a recombinant HAdV5-based vaccine is administered by silicon microneedle patches compared to needle-and-syringe ID delivery.
Microneedle-mediated immunization alters the ratio of the antigen-to-vector antibody response.
Total IgG titers to a non-recombinant HAdV5 vector (HAdV5-mCherry) in the serum of mice used in Figure 1 were measured 8 weeks after the primary immunization. (A) Individual responses at 8 weeks post-prime. (B) The ratio of antigen-specific total IgG responses to the anti-vector IgG antibody response at 8 weeks post-prime. Lines represent the median. * p < 0.05, ** p < 0.01, ***p < 0.001 compared to the ID group by one way ANOVA with Dunn's multiple comparison test. (C) The ratio of total IgG responses to the antigen and to the vector were measured at 21, 35 or 54 days after the primary immunization by the ID route or using microneedle patch type D. The mean and SEM (n = 7 to 15 mice) are shown. * p < 0.05, ***p < 0.001 by Mann Whitney test.
Microneedle-mediated primary immunization enhances antigen-specific immunity induced by repeated adenovirus vaccination
Based on the finding that microneedle-mediated immunization increased the ratio of anti- PyMSP119 to anti-vector antibody responses, we next determined if primary immunization using microneedles enhanced the responses that could be induced by repeated, homologous adenovirus immunization. Mice were thus primed with HAdV5-PyMSP142 by the ID route or using microneedle patch type D. Mice were then boosted with HAdV5-PyMSP142 by the ID route, using a microneedle array on day 56 or were not boosted (“ID/−”). Serum was examined post-boost on day 74. No significant increase in anti-PyMSP119 antibody responses was observed post-boost in any animal primed by the ID route, regardless of whether the boost was performed using a microneedle patch or by the ID route (Fig. 3A). In contrast, the anti-PyMSP119 antibody response in mice that were primed with a microneedle patch was significantly increased by a HAdV5-PyMSP142 boost administered by either ID or microneedle array (Fig. 3A). Repeated immunization with HAdV5-PyMSP142 where the prime was administered with a microneedle patch and the boost administered by the ID route (MN/ID) induced the highest anti-PyMSP119 serum IgG response. With respect to anti-vector antibody induction, repeated adenovirus immunization by the ID route induced the highest anti-vector antibody titers (Fig. 3B). In contrast, the anti-vector antibody response was not significantly boosted when the delivery route was changed, i.e. ID/MN or MN/ID, to repeatedly deliver the adenovirus-based vaccine. Homologous adenovirus immunization using a microneedle array in the prime and the boost induced the lowest anti-vector antibody response. An examination of the ratio of the antibody responses to the antigen compared to the vector demonstrated that repeated delivery by ID of HAdV5-PyMSP142 immunization skewed the ratio towards the anti-vector response, compared to mice that were not boosted (Fig. 3C). In contrast, repeated vaccination using microneedle arrays induced a humoral response that was significantly skewed towards the antigen and away from the vector. These data thus demonstrate that the anti-PyMSP119 antibody response induced by recombinant adenovirus priming by the ID route is refractory to repeated adenovirus immunization, however this inability to homologously boost antigen-specific antibody response induced by recombinant adenovirus can be overcome by microneedle-mediated primary immunization.
Microneedle-mediated immunization permits effective boosting by the adenovirus vaccine.
Mice were immunized with 1 × 1010 vp HAdV5-PyMSP142 by the intradermal (ID) route or using a silicon microneedle patch type D (MN) at day 0 and day 56, using the same (ID/ID or MN/MN) or alternative route (ID/MN, MN/ID) at each immunization. One group of mice received one immunization by the ID route (ID/−). Total IgG titers to (A) the encoded malaria antigen (PyMSP119) or (B) a non-recombinant HAdV5 vector in the serum at 35 or 54 days after the first immunization or 2 weeks post-boost at day 74. (C) The ratio of antigen-specific total IgG responses to the anti-vector antibody response at 2 weeks post-boost. The mean and SEM (n = 5 mice per group) are shown. * p < 0.05, ** p < 0.01, *** p < 0.001 by one way ANOVA with Bonferroni's multiple comparison posttest.
Microneedle-mediated HAdV5-PyMSP142 immunization alters the induction of pro-inflammatory cytokines
Having observed these differences in the ability of needle-and-syringe versus microneedle delivery to prime humoral immune responses by AdV immunization, we proceeded to investigate the inflammatory profile of each platform at the site of injection and in the draining lymph nodes. We have previously demonstrated that silicon microneedle patch delivery of a recombinant MVA vaccine does not induce a pro-inflammatory response, in contrast to ID immunization15. Here, we determined if this was also true when silicon microneedle arrays were used to administer a recombinant adenovirus vaccine. We chose to examine four cytokines that have previously been demonstrated to be induced by AdV administration or have been shown to affect the induction of immunity by this vector, namely IL-1, TNF-α IL-10 and type I IFN19,20,21. As we previously demonstrated that the analysis of cytokine proteins in skin and in lymph node homogenates was at the limit of detection15, we analyzed changes in mRNA for these cytokines at 6, 18 and 48 hours after administration of HAdV5-PyMSP142 by the ID route or using silicon microneedle array D (Fig. 4). In the skin IL-1 mRNA was elevated in both ID- and MN-treated mice at 6 and 18 hours. At the latest time point (48 h), IL-1 in the skin of ID treated mice was significantly higher compared to MN-treated animals, which had returned to baseline. The induction of TNF-α was significantly greater in microneedle- compared to ID-treated animals at 6 h in the skin but returned to a level seen in untreated mice by 18 h. In contrast, TNF-α mRNA in the skin remained significantly greater in ID- compared to microneedle-treated animals at 18 and 48 h after HAdV5-PyMSP142 administration (Fig. 4A). For both IL-10 and type I IFN, significant increases in mRNA levels were observed in ID- but not in MN-treated mice. In the lymph nodes of microneedle-treated mice, a different response was observed compared to the skin, as no induction of these cytokine transcripts above naïve levels was observed. In contrast in the lymph nodes of ID immunized mice, at 6 h post-immunization, all cytokines were induced to a significantly higher level, compared to microneedle-treated mice. The pro-inflammatory cytokines IL-1, TNF-α and type I IFN remained significantly higher in lymph nodes of ID- compared to microneedle-treated mice at 18 h post-immunization (Fig. 4B). Overall, these results demonstrate that, when it occurs, cytokine induction in microneedle-mediated immunization is early but transient and is only observed at the site of administration, whereas it is more sustained at the local administration site and is significantly higher in the lymph nodes of mice immunized by the ID route.
Induction of cytokine mRNA in the skin and draining lymph node following ID or MN vaccine delivery.
Mice were immunized with HAdV5-PyMSP142 by the ID route or using a silicon microneedle patch type D (MN) at time 0. At 6, 18 and 48 hours after immunization, 5 mice per vaccinated group were culled and the skin site of immunization and the draining lymph nodes were harvested and snap frozen. Skin and lymph nodes from 2 naïve mice were harvested to determine background levels of expression. Gene expression values relative to GAPDH were calculated as 2−ΔCt. The mean (+/−SEM) values for each group are plotted; cytokine induction due to ID immunization is in red; due to MN-mediated immunization is in blue and samples from naïve mice are in black. * p < 0.05, ** p < 0.01, *** p < 0.001 by unpaired student t test of the two immunized groups.
Antigen expression levels by HAdV5 differ when delivered by a microneedle patch versus ID needle-and-syringe
Having examined the inflammatory profile induced by the two vaccine delivery platforms, we also sought to assess in vivo antigen expression levels. Traditional delivery of AdV vaccines by needle-and-syringe has shown a very clear dose response relationship with regard to serum IgG induction against the encoded transgene, in mice22, rabbits23 and human6,7 trials. Given the comparable priming of serum IgG against PyMSP119 by the HAdV5 vector administered via the ID route and most microneedle patches (Fig. 1A), we next examined the kinetics of antigen expression in ID- or microneedle-mediated delivery of a HAdV5 expressing luciferase. Substantially higher luciferase was detected at the site of immunization in ID- compared to microneedle-treated mice at day 2 and day 7 post-administration (Fig. 5A,B). The peak luciferase expression was observed 2 or 7 days after HAdV5 delivery by ID or microneedle respectively (Fig. 5B). In contrast to the sharp increase and decrease in ID-treated mice, luciferase expression was sustained at a constant level in microneedle-treated animals (Fig. 5B). Thus overall, the ID route delivers a much larger burst of antigen in a pro-inflammatory environment, in comparison to microneedles which deliver a lower quantity of transgene product under less inflammatory conditions. However, despite these differences, the priming of serum IgG against the transgene is comparable.
Antigen expression levels by HAdV5 when delivered by ID or MN route.
(A) In vivo imaging of luciferase expression following delivery of HAdV5 expressing luciferase to BALB/c mice by the ID (n = 3, left panels) or microneedle array type D (n = 4, right panels). Photon emissions in each group are shown at day 2 or day 10 (5 minute exposure); the emissions were measured using an RLU scale. (B) Total flux (as defined by photons emitted per second) was assessed by injection of D-luciferin at day 2, 7, 10 and 14 in each animal and is graphed on a log scale.
Repeated HAdV5 delivered by a heterologous method induces the highest vaccine efficacy against P. yoelii blood-stage challenge
Based on the favourable antibody responses we hypothesized that protective efficacy induced by HAdV5/HAdV5 prime/boost immunization could be enhanced if a microneedle array was used to deliver the priming vaccine. Female C57BL/6 mice were immunized using a homologous vaccine regimen (AdV/AdV); using the same or an alternate route (ID or microneedle patch) in the prime and boost. A group of mice were also immunized with the heterologous prime/boost regimen of AdV/MVA; both vaccines were given by the ID route. This group is similar to previous studies4,24, except a lower dose of HAdV5-PyMSP142 and C57BL/6 mice were used. All immunized animals were challenged two weeks post-boost, at the same time as a group of naïve mice, with 10,000 red blood cells infected with lethal P. yoelii24 (Fig. 6A). No protection was observed in the naïve animals, who were culled from day 6 to day 12. The delivery of Ad//MVA by ID/ID gave a high level of survival in C57BL/6 mice (83%), in agreement with previous studies (although slightly lower vaccine doses were used here). The use of the same vaccine and the same route in the prime and the boost (AdV/AdV MN/MN, or AdV/AdV ID/ID) induced a low level of survival (50%) with variable levels of parasitemia in surviving mice. However, administering HAdV5-PyMSP142 twice, using a microneedle patch in the prime and the ID route to boost (AdV/AdV MN/ID) induced complete survival. Five of 6 of these mice controlled the parasitemia to under 20%. The sixth animal in this group displayed a sharp increase in parasitemia on day 15 that was resolved by day 20. Therefore, complete survival and good control of parasite growth can be obtained by homologous adenovirus immunization using a heterologous regimen of microneedle-prime, ID-boost in a stringent blood-stage malaria challenge.
The route of immunization impacts on vaccine efficacy.
C57BL/6 mice were immunized twice (day 0 and 56) with 1 × 1010 vp HAdV5-PyMSP142 by the ID route or using a silicon microneedle patch type D (MN) (Ad/Ad) using the same (ID/ID, MN/MN) route in both immunizations or using a MN patch in the prime and boosting by the ID route (MN/ID). Alternatively, mice primed with HAdV5-PyMSP142 were boosted with 1 × 106 pfu MVA-PyMSP142 by the ID route (Ad/MVA). (A) Two weeks post-boost all immunized mice or naïve controls were challenged i.v. with 1 × 104 pRBCs. Parasitemia was measured as described in Materials and Methods from day 2 after challenge. The number of surviving mice, of a total of 6 mice per group, is indicated. Crosses indicate when mice were sacrificed.
Discussion
Recombinant attenuated adenovirus vector-based vaccines are demonstrating strong clinical potential for malaria, hepatitis and HIV vaccines2,3. However, the induction of anti-vector responses during immunization limits the repeated use of the same adenovirus vector in short time frames generally used in immunization schedules. Previous studies have examined the induction of transgene-specific immunity by mucosal routes to bypass pre-existing systemic adenovirus-specific immunity11,12,13,14. In this study, we demonstrate that transcutaneous delivery of an adenovirus-based priming vaccine modulates the humoral response to one that can be boosted more effectively by re-exposure to antigen. The use of silicon microneedle transcutaneous patches to deliver the initial adenovirus-based malaria vaccine primed a humoral response that was significantly greater than intradermal-mediated immunization when animals were boosted with recombinant MVA- or adenovirus-based vaccines. Consequently, the highest levels of protection against blood-stage malaria infection was achieved in mice repeatedly immunized with HAdV5-PyMSP142 when microneedle patches were used to deliver the primary immunization and the ID route was used in the boost. These data indicate that high levels of protection can be achieved by homologous AdV immunization if utilizing a heterologous form of skin delivery.
An efficacious malaria vaccine is urgently needed to reduce the morbidity and mortality caused by this disease. However, the costs and logistics of immunization programmes must also be considered during the vaccine development process. Here we assessed if an alternative route of immunization would permit repeated immunization with the same vaccine. This would eliminate the cost of manufacturing two different vaccines required in a heterologous prime-boost vaccine regimen. Furthermore removal of hypodermic needles and syringes would have a substantial impact on the overall cost of immunization17. Thus, our objectives in this study was firstly to determine if repeated microneedle-mediated immunization would induce at least equivalent humoral immunity compared to repeated immunization using a needle and syringe by the intradermal route and secondly to determine if repeated microneedle-mediated delivery of adenovirus in the prime and the boost was immunogenic and efficacious.
The ID route has been shown to be a highly immunogenic route for both adenovirus and MVA and induces similar immune responses to the more clinically utilized intramuscular route25,26,27. Although the ID route is clinically more technically difficult, it was used here as a conventional method of delivering vaccine using a needle and syringe into the same organ used by microneedle-mediated immunization. Human adenovirus serotype 5 was chosen in this study as a model adenovirus due to lack of intellectual property restrictions and its induction of comparable immunogenicity in pre-clinical models to clinically-tested simian adenoviruses9,28. Our data from previous murine studies using a different transgene expressed from HAdV5 (and from human clinical trials with a related adenoviral malaria vaccine expressing the antigen METRAP7), demonstrated that parenteral administration of lower doses of adenovirus-based vaccines (ID in mouse, IM in human) induced lower immune responses to the transgene and to the adenovirus vector26,28. Adenovirus-naïve animals were used to model immunization of individuals that would be naïve to simian or other rare human adenovirus-based vaccines that are being developed for clinical use. We initially determined if the design of the microneedle patch would impact on the magnitude and isotype of the antigen-specific antibody response. In contrast to microneedle-mediated vaccine-induced CD8+ T cell responses15, no correlation between microneedle design and antibody response was observed. There was a trend for the smallest pore volume patches (designs A and F) to induce lower antibody responses. Our data demonstrate that, apart from these two patch designs, sufficient vaccine dose was delivered to the skin to induce antibody responses to the encoded antigen of an equivalent magnitude to that seen by immunization by the ID route. We previously demonstrated that the delivery efficiency of these silicon microneedle patches is approximately 3% of the applied vaccine15. Given that IgG induction by recombinant adenovirus vaccines is very dose responsive6,7,22,23, the induction of equivalent transgene-specific antibody titers after the priming immunization by MN compared to ID immunization was a surprising finding. Furthermore, the significant increase in MN- compared to ID-treated mice subsequent to MVA-PyMSP142 boosting suggests that the priming immunization using microneedle patches likely induced a memory B cell response that led to stronger plasma cell responses and higher serum IgG post-boost.
We next aimed to determine if a transcutaneous route of immunization could be used to reduce the induction of anti-vector immunity during the primary immunization. Using in vivo imaging, we discovered that the total transgene expression was lower to that observed when the ID route was used (Fig. 5B). This is likely due to the lower dose of vaccine administered by microneedles compared to ID15. The kinetics of transgene expression was also different in the two groups; transgene expression peaked at a high level early after ID administration, whereas microneedle-mediated delivery resulted in a steady but low expression over the course of the study. We previously demonstrated that biodistribution is affected by the delivery method. Intradermal injection permits efficient antigen drainage into lymphatic capillaries. In contrast, silicon microneedle delivery did not result in effective drainage to lymph15. This lack of lymphatic drainage in microneedle treated animals may also underlie the lack of cytokine induction in draining lymph nodes (Fig. 4). We propose that, although a lower dose of vaccine is delivered by silicon microneedle patches, the adenovirus that is delivered predominantly remains at the site of delivery in the skin, infects cells and produces antigen over the duration of at least 2 weeks (Fig. 5), which results in the induction of an improved humoral response to the transgene, with a comparatively lower anti-vector response (due to the lower dose delivered by the microneedles). It is also known that a proportion of a delivered adenovirus dose consists of non-infectious virus particles29, which can be viewed as a subunit anti-adenovirus vaccine. We propose that a significantly lower dose of non-infectious particles was delivered by silicon microneedle patches. Thus, although an adequate dose of viable adenovirus was delivered by the microneedle patches for de novo production of a sufficient dose of the encoded antigen, resulting in the induction of strong anti-PyMSP119 antibody responses, the 3% dose of non-infectious adenovirus particles was below a threshold required to induce strong anti-vector immune responses. In addition to the effect of the dose of non-viable virus particles, we propose that the lack of inflammatory responses and drainage of vector antigens to the lymph nodes are integral to the enhanced transgene-specific and reduced anti-vector responses induced by microneedle-mediated delivery of recombinant adenovirus vaccines. In effect, microneedle patches permit sufficient viable adenovirus infection to induce strong humoral immunity to the encoded antigen but insufficient delivery of non-viable adenovirus particles and proteins, in the context of a non-immune stimulated environment. Other mechanisms may also be involved in driving this response, for example, different kinetics or level of induction of germinal centres, or altered induction of plasma or memory B cells. In conclusion, we propose that, in comparison to systemic needle-and-syringe ID-mediated immunization, microneedle-mediated delivery of a priming recombinant adenovirus vaccine results in more favourable induction of antibody responses to the encoded antigen and lower anti-vector immunity due to these combinations of dose, biodistribution and inflammatory factors.
We subsequently assessed the ability of a homologous HAdV5 immunization to boost these responses. In agreement with the above, effective boosting was observed when the HAdV5 was delivered by ID route – confirming the minimal impact of anti-vector responses after the microneedle prime, but suggesting ID delivery is better for AdV boosting (possibly due to dose, inflammatory environment and/or antigen expression levels). Following challenge, despite the induction of antibody responses dominated by antigen-specific responses by repeated Ad immunization with MN, repeated use of microneedle patches or of the ID route to deliver HAdV-PyMSP142 resulted in a poor survival rate – consistent with poor boosting of the transgene-specific responses and low overall antibody titers. In contrast, using a heterologous delivery regimen (MN/ID) resulted in complete survival and good control of parasitemia. This MN/ID regimen also induced the highest anti-PyMSP119 IgG antibody titers in this study, suggesting that the Ad/Ad MN/ID vaccine regimen provides protection against this stringent blood-stage malaria challenge model almost certainly by inducing high IgG antibody responses30. This study therefore demonstrates that repeated adenovirus immunization can protect against blood-stage P. yoelii challenge when the microneedle route of immunization is used in the prime and ID is used in the boost.
In conclusion, this proof-of-concept study to determine how MN modulate humoral immunity induced by repeated adenovirus demonstrates that a heterologous route induces a higher level of efficacy against a stringent blood-stage malaria challenge in mice. Given the equivalent responses to the transgene and reduced anti-vector immunity, silicon microneedle-mediated immunization demonstrates strong potential for future development of repeatedly using the same adenovirus for immunization purposes. A limitation of this study is that it was conducted in a mouse model, which has significant differences in anatomical and physiological characteristics compared to humans. However, based on our previous studies, we have a high level of confidence that the microneedle patches used in this study penetrate human skin31,32. We have also tested delivery of vaccines and drugs to ex vivo pig skin with these silicon microneedles33,34 using accepted models of transcutaneous drug delivery; this gives us an initial level of confidence that these silicon microneedle patches will deliver vaccine in vivo to humans. Furthermore, we6,35,36 and others11,37 have also previously demonstrated that immunogenicity findings in mice for adenovirus-based vaccines can translate to humans. However, in the absence of clinical testing, we cannot predict if adenovirus-based vaccine administration using solid microneedle patches would result in the same effect on immunogenicity and protection in humans as was determined in this study. Future developments where the AdV is coated onto or into MN38,39,40 would maximize the potential of this form of vaccine delivery as a feasible needle-free approach to vaccination, that aims to overcome several cost and logistic obstacles of immunization programmes in addition to enhancing vaccine efficacy.
Methods
Silicon microneedle patch design
Silicon microneedles were fabricated using wet-etch technology as previously described41. The area of each microneedle patch and the length and number of microneedles per patch were designed to produce a microneedle patch that created specific total pore volumes when inserted into skin. Each microneedle array patch was either 5.4 mm × 5.4 mm or 7.4 mm × 7.4 mm in size. The number of microneedles per patch design ranged from 16 to 100. The pore volume of these pyramidal silicon microneedles was determined using the formula to calculate the volume of a right circular cone (V = πr2h/3), the effect of the length of the microneedle is therefore encompassed within the parameter of pore volume. The total pore volume per array is the sum of the volume of each pore on the array. The design and total pore volume created by each patch is detailed in Table I.
Vaccines
The construction, design and preparation of HAdV5 and MVA vaccines expressing the 42 kDa C-terminal region of P. yoelii strain YM merozoite surface protein 1 (PyMSP142, amino acids 1394–1757) or the fluorescent marker mCherry has been previously described24,28. HAdV5 vectors were titered by UV spectroscopy as previously described to give viral particles (vp) per mL and the MVA vector was titered to give plaque forming units (pfu). HAdV5 expressing Renilla luciferase was a gift from Dr. Sean Tucker (Vaxart Inc, San Francisco USA). All viruses were resuspended in endotoxin-free phosphate buffered saline (PBS) (Sigma) for immunization.
Animals and immunization
Female C57BL/6 mice, 4–6 weeks old (Harlan, UK) were used in all experiments which were conducted in strict accordance with the terms of licences from the Irish Department of Health and Children, under the Cruelty to Animals Act (licence numbers B100/4034 and B100/3157) and according to the approval of the UCC AECC Committee. Mice were immunized with 1 × 1010 vp; the same dose that was used in other studies8,12,14,18,19,20. Vaccine was administered with a conventional 28G needle and syringe intradermally (ID) into the ear (50 μL of 1 × 1010 vp per mouse divided across both ears). Alternatively, 5 μL of vaccine was placed on the dorsal surface of each ear and administered to the anaesthetized mouse by pressing a microneedle array onto the ear, using a force of approximately 10–20N (1 × 1010 vp in 10 μL per mouse). Mice were primed on day 0 and then boosted by the ID route or using a microneedle array at 8 weeks after the priming immunization using the same vaccine dose or using 1 × 106 pfu of MVA-PyMSP142.
Immunogenicity studies
Antibody responses to the vector and to the antigen were determined by ELISA as previously described24,39. Briefly, antibody responses to PyMSP142 were assessed against the C-terminal moiety PyMSP119 (against which protective IgG responses are raised when using this antigen)42. Recombinant glutathione S-transferase (GST)-PyMSP119 fusion protein or GST control were produced as previously described4,24, adsorbed overnight at room temperature (RT) to 96 well Nunc-Immuno Maxisorp plates (Fisher Scientific) at 2 μg/mL in PBS. To detect antibody responses to the adenovirus vector, 3 × 109 vp/mL HAdV5-mCherry in PBS was adsorbed onto plates. Sera were typically diluted to 1:100, added in duplicate wells and serially diluted. Bound antibodies were detected using horseradish peroxidase-conjugated goat anti-mouse total IgG (Sigma) or biotin-conjugated rat anti-mouse IgG1 or IgG2a (BD Biosciences, Oxford, UK) followed by incubation with ExtrAvidin horseradish peroxidase conjugate (Sigma). Plates were developed by adding TNB one component substrate (Cambridge Biosciences). Optical density was read at 595 nm (OD595). Endpoint titers were taken as the x-axis intercept of the dilution curve at an absorbance value 3× standard deviations greater than the OD595 for naïve mouse serum (typical cut-off OD595 for positive sera = 0.1). A standard serum sample was included in all assays as a reference control. All GST control ELISAs were negative (data not shown).
In vivo imaging studies
Delivery of replication-incompetent HAdV5 expressing luciferase was measured using an IVIS 100 charge-coupled device imaging system (Xenogen, Alameda, CA, USA). For luminescent measurements of luciferase expression, mice were imaged following intraperitoneal (i.p.) administration of D-luciferin (120 mg/kg body weight; BioThema AB, Sweden) and transferred to the imaging chamber following anaesthesia. Emission images were collected with 2, 5 or 10 min integration times. The total flux, defined by the number of photons per second, was processed using region of interest (ROI) analysis (ROIs were set to include the entire ear site) with background subtraction using Living Image 2.5 software (Caliper).
RT-PCR assessment of cytokine and chemokine induction
Ears and lymph nodes were snap-frozen in liquid nitrogen immediately after harvesting. Tissues were then disrupted using MagNA Lyser Green Beads and total RNA purified using High Pure RNA tissue kit following the manufacturer's protocol (Roche, Germany). cDNA was prepared from isolated RNA using The High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, USA). Real-time quantitative RT-PCR analysis of cDNA samples, prepared from isolated RNA, for selected genes was performed using the TaqMan® Gene Expression Assays and ABI7300 Real-time PCR System instrument and software (Applied Biosystems) following the manufacturer's protocols. The relative expression of the following genes was measured: TNF-α (ID: Mm00443258_m1), IL1-β (ID: Mm00439620_m1), IL-1α (ID: Mm00434228_m1, IL-10 (ID: Mm00439616_m1), IFN-α (ID: Mm00439552_s1). The housekeeping gene used was GAPDH (ID: 4352932E). Real-time RT-PCR data were analyzed as follows: cycle numbers at threshold crossing (Ct) values were subtracted from Ct values for a control housekeeping gene, GAPDH, to generate ΔCt values. Gene expression values relative to GAPDH were calculated as 2−ΔCt.
P. yoelii pRBC challenge
Blood-stage challenge studies were performed as previously described24. Briefly, C57BL/6 mice were infected with 1 × 104 red blood cells (pRBCs) parasitized with P. yoelii YM by the intravenous (i.v.) route. Blood-stage parasitemia was monitored from day two post-challenge by microscopic examination of Giemsa-stained thin blood smears. Mice were deemed uninfected in the absence of patent parasitemia in 50 fields of view. Infection was considered lethal when parasitemia exceeded 80%, at which point animals were euthanized.
Statistical analysis
Data were analyzed using GraphPad Prism version 6 for Windows (GraphPad Software, San Diego, California, USA). Normality of distribution was assessed by Kolmogorov-Smirnov test. Unpaired two-tailed Student's t-test or one way ANOVA were performed, as appropriate, to compare the responses between groups.
References
Hill, A. V. Vaccines against malaria. Philos Trans R Soc Lond B Biol Sci 366, 2806–2814 (2011).
Draper, S. J. & Heeney, J. L. Viruses as vaccine vectors for infectious diseases and cancer. Nat Rev Microbiol 8, 62–73 (2010).
de Cassan, S. C. & Draper, S. J. Recent advances in antibody-inducing poxviral and adenoviral vectored vaccine delivery platforms for difficult disease targets. Expert Rev Vaccines 12, 365–378 (2013).
Draper, S. J. et al. Recombinant viral vaccines expressing merozoite surface protein-1 induce antibody- and T cell-mediated multistage protection against malaria. Cell Host Microbe 5, 95–105 (2009).
Ewer, K. J. et al. Protective CD8(+) T-cell immunity to human malaria induced by chimpanzee adenovirus-MVA immunisation. Nature communications 4, 2836 (2013).
Sheehy, S. H. et al. ChAd63-MVA-vectored blood-stage malaria vaccines targeting MSP1 and AMA1: assessment of efficacy against mosquito bite challenge in humans. Mol Ther 20, 2355–2368 (2012).
Sheehy, S. H. et al. Phase Ia clinical evaluation of the safety and immunogenicity of the Plasmodium falciparum blood-stage antigen AMA1 in ChAd63 and MVA vaccine vectors. PLoS One 7, e31208 (2012).
Sumida, S. M. et al. Neutralizing antibodies and CD8+ T lymphocytes both contribute to immunity to adenovirus serotype 5 vaccine vectors. J Virol 78, 2666–2673 (2004).
Reyes-Sandoval, A. et al. Single-dose immunogenicity and protective efficacy of simian adenoviral vectors against Plasmodium berghei. Eur J Immunol 38, 732–741 (2008).
Barnes, E. et al. Novel adenovirus-based vaccines induce broad and sustained T cell responses to HCV in man. Sci Transl Med 4, 115ra111 (2012).
Scallan, C. D., Tingley, D. W., Lindbloom, J. D., Toomey, J. S. & Tucker, S. N. An adenovirus-based vaccine with a double-stranded RNA adjuvant protects mice and ferrets against H5N1 avian influenza in oral delivery models. Clin Vaccine Immunol 20, 85–94 (2013).
Croyle, M. A. et al. Nasal delivery of an adenovirus-based vaccine bypasses pre-existing immunity to the vaccine carrier and improves the immune response in mice. PLoS One 3, e3548 (2008).
Xiang, Z. Q. et al. Oral vaccination of mice with adenoviral vectors is not impaired by preexisting immunity to the vaccine carrier. J Virol 77, 10780–10789 (2003).
Appledorn, D. M., Aldhamen, Y. A., Godbehere, S., Seregin, S. S. & Amalfitano, A. Sublingual administration of an adenovirus serotype 5 (Ad5)-based vaccine confirms Toll-like receptor agonist activity in the oral cavity and elicits improved mucosal and systemic cell-mediated responses against HIV antigens despite preexisting Ad5 immunity. Clin Vaccine Immunol 18, 150–160 (2011).
Carey, J. B. et al. Microneedle Array Design Determines the Induction of Protective Memory CD8+ T cell Responses Induced by a Recombinant Live Malaria Vaccine in Mice. PLoS One 6, e22442 (2011).
van der Maaden, K., Jiskoot, W. & Bouwstra, J. Microneedle technologies for (trans)dermal drug and vaccine delivery. J Control Release 161, 645–655 (2012).
Gandhi, G. et al. Projections of costs, financing and additional resource requirements for low- and lower middle-income country immunization programs over the decade, 2011-2020. Vaccine 31 Suppl 2, B137–148 (2013).
de Cassan, S. C. et al. The requirement for potent adjuvants to enhance the immunogenicity and protective efficacy of protein vaccines can be overcome by prior immunization with a recombinant adenovirus. J Immunol 187, 2602–2616 (2011).
Lindsay, R. W. et al. CD8+ T cell responses following replication-defective adenovirus serotype 5 immunization are dependent on CD11c+ dendritic cells but show redundancy in their requirement of TLR and nucleotide-binding oligomerization domain-like receptor signaling. J Immunol 185, 1513–1521 (2010).
Hensley, S. E. et al. Type I interferon inhibits antibody responses induced by a chimpanzee adenovirus vector. Mol Ther 15, 393–403 (2007).
Pine, S. O. et al. Pre-existing adenovirus immunity modifies a complex mixed Th1 and Th2 cytokine response to an Ad5/HIV-1 vaccine candidate in humans. PLoS One 6, e18526 (2011).
Ophorst, O. J. et al. Increased immunogenicity of recombinant Ad35-based malaria vaccine through formulation with aluminium phosphate adjuvant. Vaccine 25, 6501–6510 (2007).
Forbes, E. K. et al. T cell responses induced by adenoviral vectored vaccines can be adjuvanted by fusion of antigen to the oligomerization domain of C4b-binding protein. PLoS One 7, e44943 (2012).
Draper, S. J. et al. Effective induction of high-titer antibodies by viral vector vaccines. Nat Med 14, 819–821 (2008).
Meyer, J. et al. Comparing the safety and immunogenicity of a candidate TB vaccine MVA85A administered by intramuscular and intradermal delivery. Vaccine 31, 1026–1033 (2013).
O'Hara, G. A. et al. Clinical assessment of a recombinant simian adenovirus ChAd63: a potent new vaccine vector. J Infect Dis 205, 772–781 (2012).
Douglas, A. D. et al. Tailoring subunit vaccine immunogenicity: maximizing antibody and T cell responses by using combinations of adenovirus, poxvirus and protein-adjuvant vaccines against Plasmodium falciparum MSP1. Vaccine 28, 7167–7178 (2010).
Goodman, A. L. et al. New candidate vaccines against blood-stage Plasmodium falciparum malaria: prime-boost immunization regimens incorporating human and simian adenoviral vectors and poxviral vectors expressing an optimized antigen based on merozoite surface protein 1. Infect Immun 78, 4601–4612 (2010).
Dicks, M. D. et al. A novel chimpanzee adenovirus vector with low human seroprevalence: improved systems for vector derivation and comparative immunogenicity. PLoS One 7, e40385 (2012).
Hirunpetcharat, C. et al. Complete protective immunity induced in mice by immunization with the 19-kilodalton carboxyl-terminal fragment of the merozoite surface protein-1 (MSP1[19]) of Plasmodium yoelii expressed in Saccharomyces cerevisiae: correlation of protection with antigen-specific antibody titer, but not with effector CD4+ T cells. J Immunol 159, 3400–3411 (1997).
Enfield, J. et al. In vivo dynamic characterization of microneedle skin penetration using optical coherence tomography (OCT). J. Biomedical Optics 15, 046001 (2010).
Haq, M. I. et al. Clinical administration of microneedles: skin puncture, pain and sensation. Biomed Microdevices 11, 35–47 (2008).
Paleco, R., Vucen, S. R., Crean, A. M., Moore, A. & Scalia, S. Enhancement of the in vitro penetration of quercetin through pig skin by combined microneedles and lipid microparticles. Int J Pharm 472, 206–213 (2014).
Vucen, S. R. et al. Improved percutaneous delivery of ketoprofen using combined application of nanocarriers and silicon microneedles. J Pharm Pharmacol 65, 1451–1462 (2013).
Biswas, S. et al. Transgene optimization, immunogenicity and in vitro efficacy of viral vectored vaccines expressing two alleles of Plasmodium falciparum AMA1. PLoS One 6, e20977 (2011).
Sheehy, S. H. et al. Phase Ia Clinical Evaluation of the Plasmodium falciparum Blood-stage Antigen MSP1 in ChAd63 and MVA Vaccine Vectors. Mol Ther (2011).
Peters, W. et al. Oral administration of an adenovirus vector encoding both an avian influenza A hemagglutinin and a TLR3 ligand induces antigen specific granzyme B and IFN-gamma T cell responses in humans. Vaccine 31, 1752–1758 (2013).
Bachy, V. et al. Langerin negative dendritic cells promote potent CD8+ T-cell priming by skin delivery of live adenovirus vaccine microneedle arrays. Proc Natl Acad Sci U S A 110, 3041–3046 (2013).
Vrdoljak, A. et al. Coated microneedle arrays for transcutaneous delivery of live virus vaccines. J Control Release 159, 34–42 (2012).
DeMuth, P. C. et al. Vaccine delivery with microneedle skin patches in nonhuman primates. Nat Biotechnol 31, 1082–1085 (2013).
Wilke, N., Mulcahy, A., Ye, S. R. & Morrissey, A. Process optimization and characterization of silicon microneedles fabricated by wet etch technology. Microelectronics Journal 36, 650–656 (2005).
Ahlborg, N., Ling, I. T., Howard, W., Holder, A. A. & Riley, E. M. Protective immune responses to the 42-kilodalton (kDa) region of Plasmodium yoelii merozoite surface protein 1 are induced by the C-terminal 19-kDa region but not by the adjacent 33-kDa region. Infect Immun 70, 820–825 (2002).
Acknowledgements
The authors wish to thank Sean Tucker and Jonathan D. Lindbloom at Vaxart, Inc., (San Francisco, USA) for the adenovirus expressing luciferase and Pat Ford and Pat Casey at the Cork Cancer Research Centre for assistance with the IVIS imaging; and the Viral Vector Core Facility (Jenner Institute, University of Oxford) for assistance. This work was supported by Enterprise Ireland (Commercialisation Fund, CFTD07/117) and Science Foundation Ireland (National Access Programme 70 and 170). AVSH and SJD are Jenner Investigators; and SJD is a UK MRC Career Development Fellow [G1000527] and Lister Institute Research Prize Fellow.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: J.B.C., S.J.D. and A.C.M. Performed the experiments: J.B.C., A.V. and A.C.M. Analyzed the data: J.B.C., A.V. and A.C.M. Contributed reagents/materials/analysis tools: C.O.M. and A.V.S.H. Wrote the paper: S.J.D. and A.C.M. All authors reviewed the final version of manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests. AVSH and SJD are named inventors on patent applications covering malaria vectored vaccines and immunization regimes. JBC, AV, COM, AVSH, ACM are named inventors on patent applications covering microneedle-mediated vaccine delivery.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Carey, J., Vrdoljak, A., O'Mahony, C. et al. Microneedle-mediated immunization of an adenovirus-based malaria vaccine enhances antigen-specific antibody immunity and reduces anti-vector responses compared to the intradermal route. Sci Rep 4, 6154 (2014). https://doi.org/10.1038/srep06154
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep06154
This article is cited by
-
STING-pathway modulation to enhance the immunogenicity of adenoviral-vectored vaccines
Scientific Reports (2022)
-
Improved immunogenicity of individual influenza vaccine components delivered with a novel dissolving microneedle patch stable at room temperature
Drug Delivery and Translational Research (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.