Abstract
A systematic review of the literature was conducted to identify randomized trials involving continuous dopaminergic stimulation (CDS) in PD patients with motor complications. Difference between n groups was assessed by partitioning heterogeneity and using the χ2 distribution with n-1 degrees of freedom, where n equals the number of groups. We looked for publication bias using funnel plotting, Egger's test and Begg's test. Twenty Randomized Controlled Trials (RCTs) were included. The results showed that CDS could evidently improve the Unified Parkinson's Disease Rating Scale (UPDRS) Part II (p < 0.0001), part III (P < 0.00001) and UPDRS total score (p < 0.00001). There was also a statistical discrepancy in off time reduction (p < 0.00001) and prolongation of on time (p < 0.00001) by the CDS therapy compared with control groups. Meanwhile, the results of this study showed obvious side effects in the CDS therapy compared with the placebo, especially at the expense of increased dyskinesia (23.4% vs 11.7%). The present study showed that CDS was beneficial in the treatment of PD patients with motor complications. But the incidence of the side events is more common than placebo.
Similar content being viewed by others
Introduction
Parkinson's disease (PD) is a progressive central nervous system disorder affecting at least six million people worldwide, making it being the second common neurodegenerative disease after Alzheimer's disease1. At the same time, with a growing elderly population and a reduction in other causes of mortality, the prevalence of PD is likely to increase2. The burden of PD is speculated to grow substantially over the next few decades as the size of the elderly population grows. Such projections give impetus to the need for innovative new treatments to prevent, delay onset, or alleviate symptoms of PD3. To our knowledge, the gold standard treatment for PD still is oral L-dopa because of its excellent efficacy and tolerability4. However, long-term use with oral L-dopa administration leads to various motor complications, including the “wearing-off” phenomenon and involuntary movements5. Motor complications occur in up to 50% of patients taking L-dopa more than 5 years and in as many as 100% of patients with young-onset PD and are a dominating cause of disability. This presents a particular challenge to managing advanced PD patients. The motor complications are supposed to be the result of a combination of progressive denervation of the striatum and long-term abnormal, intermittent drug administration leading to pulsatile stimulation of dopaminergic neurons6,7. Over time, this pulsatile stimulation results in gene and protein changes in striatal dopaminergic neurons, affecting firing patterns in basal ganglia output neurons and ultimately leads to motor complications8. Moreover, in animal models of PD, intermittent but not continuous administration of L-dopa can lead to the wearing-off phenomenon and dyskinesia9. All of these results provide the rationale for treating patients with continuous dopaminergic stimulation (CDS) approach.
The concept of CDS has received considerable attention as a therapeutic approach to the treatment of Parkinson's disease10. Long-acting dopaminergic agents matched to provide comparable motor improvement to L-dopa treated animal models, but experience an evidently reduced frequency and severity of motor complications11. What is more, clinical studies of L-dopa/carbidopa intraduodenal gel infusion revealed marked reductions in ‘off’ time along with improvement in ‘on’ time when compared with conventional treatment12. Recent numerous evidences suggest wide-ranging symptoms improvement with CDS in patients with severe motor complication, which has beneficial implications for the management of patients with advanced PD in clinical practice13. This theory proposes that CDS can provide more continuous stimulation of brain dopaminergic receptors than intermittent doses of oral L-dopa, which is indicate dyskinesia and motor fluctuations could be delayed even though prevented by realizing continuous stimulation of striatal dopamine receptors using long-acting medicines such as dopamine agonists14.
Nevertheless, the CDS theory has fallen out of favor for a variety of reasons. Some studies reported the incidence of dyskinesia had not been proven to be directly correlated with drug half-life15. In additional, many of these studies are of small sample size and thus cannot fully elucidate the efficacy and safety implications of the therapy. Hence, there is uncertainty whether CDS is more effective than another in the PD patients with motor complications. We therefore conducted the present meta-analysis by pooling the results of these trials in order to provide more useful information about efficacy and safety of CDS in the PD patients with motor complications.
Results
This Meta-analysis is conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses16.
Study inclusion
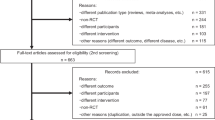
We identified 652 potentially relevant articles from six databases. After removal of duplicates, 279 records remained. After going through the titles and abstracts, 162 were excluded because they failed to meet the inclusion criteria. By reading the full text of the remaining 117 articles as possibly reporting the CDS for the PD patients with motor complications, 49 studies were excluded because a result of without control groups, 37 were excluded because of the subjects did not have developed motor complications, 11 studies were excluded due to not compared with the placebo. Ultimately, 20 eligible studies satisfied the pre-established inclusion criteria17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36 (Figure 1).
Characteristics of studies
Twenty studies with 3234 PD patients with motor complications were retrieved based on the search criteria17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36. Among them, only 2 studies belong to L-dopa drug class, remaining 18 studies were dopamine agonist's drug class. The number of participants randomised into the 20 trials included in this meta-analysis ranged from 2428 to 39332 participants. The mean age of the participants in the trials was approximately 64 years. Meanwhile, 64% of the participants were male and they had PD for approximately 8 years. The duration of treatment was varied from 6 weeks28 to 32 weeks22. UPDRS as the outcome measure was observed in 15 studies, the change of the on/off time was observed in 12 studies, adverse effects were reported in 19 studies. Of the L-dopa drug class, L-dopa intraduode infusion and intrajejunal infusion of levodopa/carbidopa intestinal gel were administrated in 1 study, respectively28,36. In the dopamine agonists drug class, pramipexole were administrated in 8 studies (8/18, 44.4%), ropinirole were used in 3 studies (3/18, 16.7%), cabegoline and bromocriptine as the intervention therapy in 2 studies. What is more, lisuride infusion27, rotigotine transdermal system30 and subcutaneous apomorphine infusion34 in 1 study, respectively. There were 3 three-arm trials comparing with the placebo. The basic characteristics of each case control study were listed in Table 1.
Risk of bias in included studies
We utilized the criteria recommended by Cochrane Handbook for Systematic Reviews to assess the risk of bias in the 20 articles included. Although all included studies claimed randomization, 11 articles described the method of random sequences generation (for example, random number generator, computer generated). But only 5 trials gave information that allowed the assessment of whether an adequate concealment of allocation procedure was used. All the studies reported the blinding of participants except two trials27,34. Non-selective reporting was found in 14 trials. Three studies existed certain degree other potential threats to validity. Therefore, all of the included trials were deemed to have a low risk of bias. The methodological quality of each study was summarized in Table 2.
Meta analysis results
UPDRS
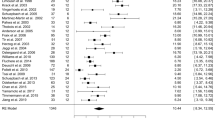
Data on the clinician-rated UPDRS Part II scores were available from 8 trials with 1 trials of L-dopa drug class and 7 trials of dopamine agonist's drug class. For the UPDRS Part II score, there were 2034 participants included in the analysis. We pooled the whole data to process and found significant difference when CDS treatment compared with placebo {p < 0.0001, standard mean difference (SMD) = −3.04, 95% CI: −4.51 to −1.57, Figure 2A}. Nevertheless, there was severe heterogeneity for the analysis of UPDRS Part II between studies (Tau2 = 4.46, Chi2 = 960.54, p < 0.00001, I2 = 99%, Figure 2A). Consequently, we should interpret the pool result prudently. The remaining three studies failed to pool analysis due to the raw data demonstrated in the form of percentage improvement (%) or missing, but all of them reported the significant effects of CDS for ameliorating the UPDRS Part II compared with the placebo group (p < 0.05 or p < 0.01). Compared to placebo treatment, ten independent studies showed significant effects of CDS therapy for improving the UPDRS Part III scores (P < 0.00001, SMD = −2.16, 95% CI −3.10 to −1.23; heterogeneity Tau2 = 2.17, Chi2 = 632.53, p < 0.00001, I2 = 99%, Figure 2B), suggesting that CDS treatment could contribute to improving complications of treatment in patients with PD with motor complications, but the remaining two studies did not provide raw data and thus failed for meta-analysis. There were 3 studies included in the meta-analysis to comparing CDS with placebo according to the UPDRS total score as the outcome index. We pooled the trials and found significant difference between two groups (p < 0.00001, SMD = −0.63, 95%CI: −0.83 to −0.44, Figure 2C) without heterogeneity (Tau2 = 0.00, Chi2 = 2.13, p = 0.34, I2 = 6%, Figure 2C), indicating CDS could apparent ameliorate the symptoms of the PD patients with motor complications. The funnel plot was roughly symmetric for the effect of CDS on UPDRS score. Thus, funnel plots did not suggest an obvious publication bias (Figure 3A). Meanwhile, Egger's weighted regression (p = 0.246 > 0.05, Figure 3B) and Begg's test (p = 0.755 > 0.05, Figure 3B) both suggested low likelihood of publication bias for all analysis.
(A) change from baseline to endpoint in UPDRS Part II of CDS vs Placebo in PD patients with motor complications; (B) change from baseline to endpoint in UPDRS Part III of CDS vs Placebo in PD patients with motor complications; (C) change from baseline to endpoint in UPDRS total score of CDS vs Placebo in PD patients with motor complications.
Prolongation of on time and off time reduction
A total of 3 trials evaluated CDS treatment versus placebo in patients of on time increase. Patients had greater response to CDS than placebo as evidenced by obvious significant effects of CDS for prolongation of on time compared with control groups (p < 0.00001, SMD = 2.59, 95%CI: 2.35 to 2.83, Figure 4A). The average on time increased by 2.3 hours for CDS treatment and by 0.7 hours for the placebo group, which was apparent statistical difference between CDS and placebo. Data on off-time reduction were available from 6 trials including 930 participants. Compared to placebo, CDS significant reduced off-time by approximately 2.0 hour a day (p < 0.00001, SMD = −4.34, 95%CI: −5.82 to −2.86, Figure 3C). Nevertheless, there was severe heterogeneity between trials (Tau2 = 3.08, Chi2 = 157.33, p < 0.00001, I2 = 97%, Figure 4B). Some studies failed to pool analysis due to the aforementioned reasons.
Adverse effects
Adverse effects (AE) were reported in 20 studies. CDS in general showed statistically significant higher rates of AE than placebo (Data not showed). There was significant difference of adverse events including incidences of dyskinesia (23.4% vs 11.7%), nausea (14.7% vs 9.3%), dizziness (6.53% vs 4.5%), diarrhea (3.44% vs 1.6%) between the CDS and placebo. However, there was no obvious discrepancy of insomnia (4.7% vs 4.1%).
Discussion
Main findings
Our meta-analysis found that CDS could evidently improve the UPDRS Part II, part III and UPDRS total score compared with placebo in PD patients with motor complications. There was also a statistical significance in off time reduction and prolongation of on time by the CDS therapy. These results displayed that patients receive CDS experienced significant improvement in both “off” and “on” time, as well as in improving the UPDRS subscale score in PD patients with motor complications. However, the results of this study showed obvious side effects in the CDS therapy compared to the placebo, especially at the expense of increased dyskinesia. Hence, it was necessary to detect the optimal therapeutic efficacy to balance the incidence of adverse events. To our best knowledge, it was no question that CDS was beneficial in the treatment of PD patients with motor complications. But it was less clear which class of drug therapy was more effective between L-dopa drug class and dopamine agonist drug class. We failed to draw conclusion which was more effective based on the present meta-analysis due to only two studies focused on the L-dopa drug class. In terms of AE, two kinds of drugs have a nearly similar incidence of AE, both higher rates than placebo.
Interpretation of the results
CDS has been influential in recent years and has lead to increased prevalence of dopamine agonists for PD37. Under normal physiologic condition, the dopamine concentration in the striatum is maintained at constant levels38. Therefore, continuous stimulation of postsynaptic receptors is thought to be essential for normal basal ganglia function39. Meanwhile, motor complications are associated with non-physiologic intermittent or pulsatile stimulation of dopamine receptors40. Based on such theory, CDS has been shown to reduce motor complications in advanced PD patients and has been hypothesized to prevent their incidence when given as early therapy in PD13. Indeed, some basic researches showed treatment with intermittent injections of a short-acting dopamine agonist induced motor complications while continuous infusion of the same agent did not11. But, whether CDS can reduce the development of dyskinesia has yet to be studied. STRIDE-PD study reported initial therapy with carbidopa-levodopa-entacapone result in an increased frequency of dyskinesia compared to standard carbidopa/levodopa41. Moreover, laboratory and clinical studies have indicated that although dyskinesias are closely linked to the short serum half-life of levodopa, other factors may also play a pivotal role in the pathogenesis7. Consequently, CDS is no doubt effective to manage the PD patients with motor complications, but it may cause serious side effects, especially at the expense of increased dyskinesia.
Limitations
Several limitations of this study should be considered. Firstly, a large proportion of the studies included in this review are of six months or less in duration. Among the 20 studies, a very large proportion of studies treatment duration are less than one years, suggesting that there are insufficient data on the comparative efficacy and tolerability of these drugs beyond six months. In such a chronic long-term disease as PD, it would inevitable be valuable to know whether the drug actions persist for a much longer period. Secondly, The role of potentially useful CDS such as levodopa-carbidopa intestinal gel infusion, continuous duodenal infusion, subcutaneous apomorphine or lisuride infusion, continuous transdermal delivery of rotigotine were very inadequate in this meta-analysis. Although the fact that an extensive search for such studies was performed, most of them were not RCTs. Therefore, it is necessary to carry out more RCTs to assess the efficacy of such CDS in the PD patients with motor complications. Thirdly, patients included in the trials were not always on behalf of patients in clinic. Patients with Mini-Mental State Examination score less than 24, atypical parkinsonian syndromes, any history of deep brain stimulation, psychiatric or clinically significant hypotension were excluded. Thus, the results cannot be generalized to the entire population of PD patients with motor complications. Finally, high heterogeneity occurred several times when comparing CDS with placebo treatment. Inevitably, studies brought together in a systematic review will differ. Any kind of variability among studies in a systematic review or meta-analysis may be termed heterogeneity42. Variability in the participants, interventions, study design and risk of bias all can result in heterogeneity. To solve this problem, in this meta-analysis, random-effects model may be used to incorporate heterogeneity among studies. However, random-effects model is not a substitute for a thorough investigation of heterogeneity. It is intended primarily for heterogeneity that cannot be explained43.
Implications for Practice
This meta-analysis of RCTs mainly focuses on the efficacy and safety of CDS therapy in PD patients with motor complications. According to our meta-analysis, patients receiving CDS therapy exhibit significant ameliorate in their PD symptoms as evidenced by improvements in their UPDRS scores, as well as off time reduction compared with placebo. In terms of the safety assessment of this meta-analysis, CDS therapy in generally showed significant higher rates of adverse events than placebo, especially at the expense of increased dyskinesia. Although acknowledging the limitations of this meta-analysis, our findings present a majority of high-quality trials and provide hopefully evidences that CDS therapy can additionally benefit relieve symptoms of PD patients with motor complications.
Implications for research
A number of implications for research arise from this paper. Firstly, improvement in the methodological quality of RCTs is critical for later trials and more methodologically rigorous studies are needed in this field and sample size calculation should be conducted before enrollment. Secondly, various kinds of different forms of CDS were administered in the 21 studies included. Thus, it is necessary to identify which form of the CDS displays the most effective anti-Parkinsonian action. Thirdly, a new theory points out standard oral administration of dopamine agonists does not result in constant plasma drug levels, therefore, more continuous drug delivery (CDD) may result in both prolonged reversal of motor deficits and reduced levels of dyskinesia. So, we need carry out more well-designed, randomized, double-blind, placebo-controlled trials and report in detail about continuous duodenal or intravenous L-dopa infusion, subcutaneous apomorphine or lisuride infusion, continuous transdermal delivery of rotigotine in future.
Methods
Inclusion and exclusion criteria
Only Randomized Controlled Trials (RCTs) which evaluate CDS for PD patients with motor complications were included in this review. CDS is a therapeutic concept for the management of PD that proposes continuous, as opposed to intermittent or pulsatile, stimulation of striatal dopamine receptors. However, the form of the CDS is multitudinous. In this meta-analysis, we determine in advance the method to achieve CDS are as follow: (1) levodopa-carbidopa intestinal gel infusion; (2) long half-time dopamine agonists (e.g. ropinorole, bromocriptine and pramipexole); (3) subcutaneous apomorphine or lisuride infusion or continuous transdermal delivery of rotigotine. The former one kind belongs to L-dopa class and the later two kinds were ascribed to dopamine agonist's class. Moreover, eligible studies had to meet all of the following criteria: (1) published in a peer-reviewed journal; (2) conducted to evaluate the CDS versus placebo, both on a background of L-dopa therapy, with all other aspects of treatment being the same in both arms; (3) had original data being independent from other studies; (4) had adequate data such as absolutely change on/off time or UPDRS score as the main outcome measures.
Literature search
We electronically searched six databases of Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, EMBASE database up to February 2014 for all English language publications. Reference lists from the resulting publications and reviews were used to identify further relevant publications. The following search strategy, using the grouped terms, was used for MEDLINE and was modified to suit other databases.
Medline (Pubmed) search strategy.
-
1
Motor complications
-
2
Dyskinesia
-
3
Fluctuation
-
4
On/off time
-
5
Wearing-off.
-
6
or/1–5
-
7
Parkinson's disease
-
8
Parkinson disease
-
9
PD
-
10
or/7–9
-
11
6 and 10
Data extraction
For each study, information was carefully collected from all eligible publications, including first author, year of publication, number of cases and controls, sex ratio, mean age of cases and controls, duration of PD disease, mean L-dopa dosage, intervention of the trial groups and main outcome measures. For studies including subjects of different arms, data were extracted separately for each arm whenever possible. If more than one paper with the same study was found, the one that contained the most detailed was reviewed. If the data for meta-analysis were missing or only expressed graphically, we tried to contact authors to inquire for further information or calculated by ourselves if available or excluded.
Quality assessment of RCTs
The methodological quality of RCTs was assessed independently using the Cochrane Handbook for Systematic Reviews of Interventions. Two investigators independently evaluated the methodological quality of the included articles. Disagreements were resolved through consensus or discussed with a third author.
Statistical analysis
We considered the main outcome measures as continuous data and then were given an estimate of the combined overall effect sizes utilizing SMD with a random effects model. We used a random effects rather than a fixed effects model because of this takes into account heterogeneity between multi-studies. We looked for publication bias using funnel plotting, Egger's test and Begg's test. SMD with its 95% CI was used to assess the strength of efficacy of CDS in PD patients with motor complications. If any trials with two or more treatment arms were included, then we predetermine two methods to resolve the matter: (1) if the study was comparing two different drugs within the same drug class versus control, then the data for those drugs were combined to give one comparison of adjuvant therapy versus control; (2) if the study was comparing the same drug but at different dosages versus control, then the arm using generally recommended dose was chosen for inclusion in the analysis. Difference between n groups was assessed by partitioning heterogeneity and using the χ2 distribution with n-1 degrees of freedom (df), where n equals the number of groups. All analysis was performed with Revman version 5.1. Probability value p < 0.05 were considered significant.
References
Muangpaisan, W., Mathews, A., Hori, H. & Seidel, D. A systematic review of the worldwide prevalence and incidence of Parkinson's disease. J Med Assoc Thai 94, 749–755 (2011).
Akushevich, I., Kravchenko, J., Ukraintseva, S., Arbeev, K. & Yashin, A. I. Age patterns of incidence of geriatric disease in the U.S. elderly population: Medicare-based analysis. J Am Geriatr Soc 60, 323–327 (2012).
Kowal, S. L., Dall, T. M., Chakrabarti, R., Storm, M. V. & Jain, A. The current and projected economic burden of Parkinson's disease in the United States. Mov disord 28, 311–318 (2013).
Lewitt, P. A. Levodopa for the treatment of Parkinson's disease. N Engl J Med 359, 2468–2476 (2008).
Vlaar, A., Hovestadt, A., van Laar, T. & Bloem, B. R. The treatment of early Parkinson's disease: levodopa rehabilitated. Pract Neurol 11, 145–152 (2011).
Olanow, C. W. & Obeso, J. A. Pulsatile stimulation of dopamine receptors and levodopa-induced motor complications in Parkinson's disease: implications for the early use of COMT inhibitors. Neurology 55, 72–77 (2000).
Warren Olanow, C. et al. Factors predictive of the development of Levodopa-induced dyskinesia and wearing-off in Parkinson's disease. Mov disord 28, 1064–1071 (2013).
Olanow, C. W., Obeso, J. A. & Stocchi, F. Continuous dopamine-receptor treatment of Parkinson's disease: scientific rationale and clinical implications. Lancet neurol 5, 677–687 (2006).
Picconi, B. et al. Loss of bidirectional striatal synaptic plasticity in L-DOPA-induced dyskinesia. Nature neuroscience 6, 501–506 (2003).
Olanow, C. W., Obeso, J. A. & Stocchi, F. Drug insight: Continuous dopaminergic stimulation in the treatment of Parkinson's disease. Nat Clin Pract Neurol 2, 382–392 (2006).
Bibbiani, F., Costantini, L. C., Patel, R. & Chase, T. N. Continuous dopaminergic stimulation reduces risk of motor complications in parkinsonian primates. Exp Neurol 192, 73–78 (2005).
Antonini, A. et al. Effect and safety of duodenal levodopa infusion in advanced Parkinson's disease: a retrospective multicenter outcome assessment in patient routine care. J Neural Transm 120, 1553–1558 (2013).
Wright, B. A. & Waters, C. H. Continuous dopaminergic delivery to minimize motor complications in Parkinson's disease. Expert Rev Neurother 13, 719–729 (2013).
Stocchi, F. The therapeutic concept of continuous dopaminergic stimulation (CDS) in the treatment of Parkinson's disease. Parkinsonism Relat Disord 3, S68–71 (2009).
Maratos, E. C., Jackson, M. J., Pearce, R. K., Cannizzaro, C. & Jenner, P. Both Short-and Long-Acting D-1/D-2 Dopamine Agonists Induce Less Dyskinesia than l-DOPA in the MPTP-Lesioned Common Marmoset (Callithrix jacchus). Exp Neurol 179, 90–102 (2003).
Moher, D., Liberati, A., Tetzlaff, J. & Altman, D. G. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6, e1000097 (2009).
Nyholm, D. et al. Duodenal levodopa infusion monotherapy vs oral polypharmacy in advanced Parkinson disease. Neurology 64, 216–223 (2005).
Toyokura, Y. et al. Effects of bromocriptine on parkinsonism. A nation-wide collaborative double-blind study. Acta Neurol Scand 72, 157–70 (1985).
Olanow, C. W. et al. A multicenter double-blind placebo-controlled trial of pergolide as an adjunct to Sinemet in Parkinson's disease. Mov disord 9, 40–47 (1994).
Steiger, M. J., El-Debas, T., Anderson, T., Findley, L. J. & Marsden, C. D. Double-blind study of the activity and tolerability of cabergoline versus placebo in parkinsonians with motor fluctuations. J Neurol 243, 68–72 (1996).
Hutton, J. T. et al. Multicenter, placebo-controlled trial of cabergoline taken once daily in the treatment of Parkinson's disease. Neurology 46, 1062–1065 (1996).
Rascol, O. et al. Ropinirole in the treatment of levodopa-induced motor fluctuations in patients with Parkinson's disease. Clin Neuropharmacol 19, 234–245 (1996).
Lieberman, A., Ranhosky, A. & Korts, D. Clinical evaluation of pramipexole in advanced Parkinson's disease: results of a double-blind, placebo-controlled, parallel-group study. Neurology 49, 162–168 (1997).
Guttman, M. Double-blind comparison of pramipexole and bromocriptine treatment with placebo in advanced Parkinson's disease. International Pramipexole Bromocriptine Study Group. Neurology 49, 1060–1065 (1997).
Wermuth, L. A double-blind, placebo-controlled, randomized, multi-center study of pramipexole in advanced Parkinson's disease. Eur J Neurol 5, 235–242 (1998).
Lieberman, A. et al. A multicenter trial of ropinirole as adjunct treatment for Parkinson's disease. Ropinirole Study Group. Neurology 51, 1057–1062 (1998).
Pinter, M. M., Pogarell, O. & Oertel, W. H. Efficacy, safety and tolerance of the non-ergoline dopamine agonist pramipexole in the treatment of advanced Parkinson's disease: a double blind, placebo controlled, randomised, multicentre study. J Neurol Neurosurg Psychiatry 66, 436–441 (1999).
Stocchi, F., Ruggieri, S., Vacca, L. & Olanow, C. W. Prospective randomized trial of lisuride infusion versus oral levodopa in patients with Parkinson's disease. Brain 125, 2058–2066 (2002).
Moller, J. C., Oertel, W. H., Koster, J., Pezzoli, G. & Provinciali, L. Long-term efficacy and safety of pramipexole in advanced Parkinson's disease: results from a European multicenter trial. Mov disord 20, 602–610 (2005).
LeWitt, P. A., Lyons, K. E. & Pahwa, R. Advanced Parkinson disease treated with rotigotine transdermal system: PREFER Study. Neurology 68, 1262–1267 (2007).
Poewe, W. H. et al. Efficacy of pramipexole and transdermal rotigotine in advanced Parkinson's disease: a double-blind, double-dummy, randomised controlled trial. Lancet Neurol 6, 513–520 (2007).
Pahwa, R. et al. Ropinirole 24-hour prolonged release: randomized, controlled study in advanced Parkinson disease. Neurology 68, 1108–1115 (2007).
Hauser, R. A. et al. Safety and tolerability of pardoprunox, a new partial dopamine agonist, in a randomized, controlled study of patients with advanced Parkinson's disease. Eur Neurol 62, 40–48 (2009).
Martinez-Martin, P. et al. Chronic subcutaneous infusion therapy with apomorphine in advanced Parkinson's disease compared to conventional therapy: a real life study of non motor effect. J Parkinsons Dis 1, 197–203 (2011).
Schapira, A. H. et al. Extended-release pramipexole in advanced Parkinson disease: a randomized controlled trial. Neurology 77, 767–774 (2011).
Olanow, C. W. et al. Continuous intrajejunal infusion of levodopa-carbidopa intestinal gel for patients with advanced Parkinson's disease: a randomised, controlled, double-blind, double-dummy study. Lancet Neurol 13, 141–149 (2014).
Stocchi, F. Continuous dopaminergic stimulation and novel formulations of dopamine agonists. J Neurol 258, S316–322 (2011).
Grace, A. A. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience 41, 1–24 (1991).
Jenner, P., McCreary, A. C. & Scheller, D. K. Continuous drug delivery in early- and late-stage Parkinson's disease as a strategy for avoiding dyskinesia induction and expression. J Neural Transm 118, 1691–1702 (2011).
Kishore, A. et al. Acute dopamine boost has a negative effect on plasticity of the primary motor cortex in advanced Parkinson's disease. Brain 135, 2074–2088 (2012).
Stocchi, F. et al. Initiating levodopa/carbidopa therapy with and without entacapone in early Parkinson disease: the STRIDE-PD study. Ann Neurol 68, 18–27 (2010).
Zhao, J. G. Identifying and measuring heterogeneity across the studies in meta-analysis. J Hand Surg Am 38, 1449–50 (2013).
Shuster, J. J., Jones, L. S. & Salmon, D. A. Fixed vs random effects meta-analysis in rare event studies: the rosiglitazone link with myocardial infarction and cardiac death. Stat Med 26, 4375–4385 (2007).
Acknowledgements
We gratefully acknowledge Professor Zhen-Guo Liu for his help in guiding and revising the manuscript. We also thank all the study participants.
Author information
Authors and Affiliations
Contributions
C.L.X. and W.W.W. conceived and participated in its design, searched databases, extracted and assessed studies and helped to draft the manuscript. J.G. and S.F.Z. carried out the statistical analysis and interpretation of data. Z.G.L. participated in the conceptualization and design of the review, performed the selection of studies, data extraction and analysis and drafted the review. All authors read and approved the final manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Xie, Cl., Wang, WW., Zhang, Sf. et al. Continuous dopaminergic stimulation (CDS)-based treatment in Parkinson's disease patients with motor complications: A systematic review and meta-analysis. Sci Rep 4, 6027 (2014). https://doi.org/10.1038/srep06027
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep06027
This article is cited by
-
Infusion Therapies for Parkinson’s Disease
Current Neurology and Neuroscience Reports (2020)
-
Combined Diffusion Tensor Imaging and Arterial Spin Labeling as Markers of Early Parkinson’s disease
Scientific Reports (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.