Abstract
Few therapeutic options are available for non-small cell lung cancer (NSCLC) after failure to primary epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs). Since TKI rechallenge is one of the most common salvage approaches in clinical practice, we sought to identify the independent factors that associated with 2nd progression progression-free survival (PFS) and overall survival (OS). Seventy-two consecutive EGFR-mutated NSCLC patients with TKI retreatment after initial failure were retrospectively analyzed in this study. Univariate survival analysis and Cox proportional hazards regression model was used to determine if EGFR-TKIs readministration is tolerable as well as efficacious for a certain group of patients.
Similar content being viewed by others
Introduction
The utility of epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) in metastatic non-small cell lung cancer (NSCLC) had been confirmed through several trails1,2. However, patients who initially responded to EGFR-TKIs would eventually have progressive disease (PD) within a year3. Albeit several resistant mechanisms like secondarily EGFR mutations (e.g. T790M) or activation of alternate bypass pathways were reported4, these patients still have limited options for treatment.
TKI rechallenge is one of the most common therapeutic approaches in current practice though the progression-free survival (PFS) varies among studies and most of the results are barely beyond 2 months5. Therefore, we designed this retrospective study to investigate factors that affect the benefit from TKI readministration.
Results
Patients' characteristics
Seventy-two patients that met entry criteria were analyzed finally. Baseline information were showed in Table 1. All patients finished the second round of EGFR-TKIs therapy until a PD was documented. Only one patient with negative EGFR T790M mutation had a rebiopsy after resistance.
In total, 43 patients (59.7%) harbored deletion mutation in exon 19 and 29 patients (40.3%) had a point mutation of L858R in exon 21.
Treatment details and drug switch
In detail, 26 (36.1%), 37 (51.4%), 7 (9.7%) and 2 (2.8%) patients received the 1st EGFR-TKIs in the first, second, third and fourth line respectively. As for the 2nd EGFR-TKIs treatment, 22 (30.6%), 30 (41.7%), 16 (22.2%), 3 (4.2%) and 1 (1.4%) patients were in the second, third, fourth, fifth and sixth line respectively. In addition, 57 (79.2%) and 15 (20.8%) patients received gefitinib and erlotinib in the 1st EGFR-TKI treatment. As for the 2nd EGFR-TKI therapy, 11 patients (15.3%) had switched the TKI regimen (all switched from gefitinib to erlotinib).
Of all patients that enrolled, 19 patients (26.4%) had an EGFR-TKI holiday (“wash out” time) between treatments and the median “wash out” time was 1.51 months. 20 patients (27.8%) received radiotherapy and 15 of them (15/72, 20.8%) were used after acquired resistance to 1st EGFR-TKIs, especially in ten patients (10/19, 52.6%) whose site of disease progression was new metastases in the brain.
Types of progression following 1st EGFR-TKIs
Three types of progression to 1st EGFR-TKIs were defined according to previous study6: A. local progression: (1) disease control lasting ≥3 months with EGFR-TKI treatment (2) PD due to solitary extracranial lesion or limitation in intracranial lesions (covered by a radiation field) (3) symptom scored ≤1; B. minimally/slowly progression: (1) disease control lasting ≥6 months with EGFR-TKI treatment (2) compared with the previous assessment, no significant increment of tumor burden and progressive involvement of non-target lesions (3) symptom scored ≤1; C. rapid progression: (1) disease control lasting ≥3 months with EGFR-TKI treatment (2) compared with the previous assessment, rapid progression of multiple target lesions, or progressive involvement of non-target lesions (3) symptom scored 2.
Symptom scores 0, 1 and 2 was quantified in accordance with the asymptomatic status, stability of pre-existing status and deterioration of any pre-existing or new symptom7.
In total, 19 patients (26.4%) exhibited local progression, 31 patients (43.1%) showed minimally/slowly progression and 22 patients (30.6%) had rapid progression. As is demonstrated in Table 1, lung (35/72, 48.6%), brain (19/72, 26.4%) and bone (9/72, 12.5%) were the the three leading sites of progression, respectively. As for treatments upon progression, 9 patients (9/35, 25.7%) received chemotherapy immediately after the pulmonary progression (8 patients received a platinum doublet chemotherapy while the other 9 patients received a single agent chemotherapy), while the remaining group (26/35, 74.3%) kept receiving a TKI regimen; 10 of 19 (52.6%) patients that with brain progression received intracranial radiation and 4 of them (21.1%) received chemotherapy as salvage treatment after resistance to first TKI treatment; for patients with bone metastases progression, the majority of them (8/9, 88.9%) continued to be treated with EGFR TKI rather than chemotherapy.
Efficacy and tolerance of the second round TKI
The last follow-up time was May 2014 and median follow-up duration was 46.61 months from the initial TKI therapy (range: 15.93–118.17 months). Sixty-five patients (90.3%) exhibited PD and forty-seven (65.3%) confirmed death in the last date. Response data of initial and readministrated TKI are demonstrated in Table 2. The median 2nd PFS and overall survival (OS) were 4.00 months (95% confidence interval [CI], 3.48–4.51 months) and 18.20 months (95% CI, 14.10–22.30 months), respectively.
Two patients received an intercalated treatment beyond 1st EGFR-TKI treatment (one in the local progression group and the other one in the minimally/slowly progression group), both of them took 2nd EGFR-TKI as maintenance treatment after the salvage chemotherapy to 1st EGFR-TKI. The 2nd PFS of these two patients were 19.27 and 6.87 months.
When using smoking history, pathological subtypes, pathological differentiation, brain metastases detail (brain metastases in 1st TKI, progression to 1st TKI in brain, Brain treatment models in 2nd TKI: local control group [radiotherapy + surgery] vs. no local control group [chemotherapy + TKI only] vs. without brain metastases group), initial TKI efficacy, types of progression to 1st TKI, cessation of TKI after initial failure, inserted chemotherapy before readministration, TKI regimen change and EGFR mutational site as variables, the univariate analysis demonstrated that patients without brain metastasis in 1st TKI treatment, had no brain metastases or with local control of brain metastases in the 2nd TKI period, favored a disease control longer than 6 months in initial therapy, with local or minimally progression and had no chemotherapy before a TKI retreatment could benefit more from the TKI readministration (Table 3). Whereas none of these factors affected the OS significantly.
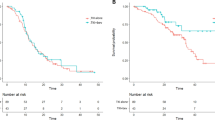
Nevertheless, only types of progression to 1st TKI was significantly associated with 2nd PFS (Figure 1) under multivariate Cox proportional hazards regression model (hazard ratio [HR] for local progression vs. rapid progression, .317; 95% CI, .160 to .627, P = .001; HR for minimally/slowly progression vs. rapid progression, .222, 95% CI, .118 to .417, P< .001).(Table 3)
Additionally, we found a phenomenon that patients that with rapid progression to 1st TKI tended to receive chemotherapy first after resistance (8/22, 36.4% vs. 4/19, 21.1% in local progression and 5/31, 16.1% in minimally/slowly progression, P = .096).
The most common adverse event was grade 1 or 2 rash, which affected 15 patients (20.8%), whereas no grade 3 skin rash was observed. Besides, no dose reduction or discontinuation of TKI due to unbearable TKI-associated toxicity was required.
Discussion
Salvage treatment for patients harboring EGFR mutation with NSCLC after initial failure to EGFR-TKIs remains controversial even though a set of plausible mechanisms to resistance has been reported8. Theoretically, several options to overcome EGFR TKI resistance are available (re-administration of TKIs; second-generation TKIs-eg, afatinib or dacomitinib; anti-EGFR combinations-eg, EGFR TKI combined with anti-EGFR antibody). Recent report indicated that TKI-retreatment might be useful for ex-responders following a drug holiday9. Therefore, it is postulated that certain proportional “oncogene-addicted” cells might still remain even when a resistance was occurred.
Several studies10,11,12 reported the clinical outcomes of readministrated EGFR-TKIs after acquired resistance and the PFS and OS of these trials varied from 2.0 months to 3.4 months and 11.4 months to 12.0 months, respectively. While these differences may be partly explained by the various enrolled criteria among trials (eg, patients with clinical benefit >6 months of initial EGFR-TKIs were enrolled in Koizumi's11 study but >3 months in Oh, I.J's10 trial and not all patients harbored EGFR mutation), a significant better response to TKI retreatment was observed in those who had a PFS more than 6 months during the initial TKI treatment5. In 2010, a clinical definition of acquired resistance to EGFR-TKIs in NSCLC13 was proposed for those who responded (> = 6 months) to initial gefitinib or erlotinib treatment with a drug sensitivity associated mutation site. Considering one of the principal findings in this study is that patients with local or minimally/slowly progression to initial TKI benefited more from the readministrated treatment than those rapidly progressed, it seems that the definition above is quite reasonable since our research also confirm patients who had part of the characteristic mentioned above gain a better disease control with 2nd TKI.
Even though EGFR-TKIs have shown certain utility in patients with brain metastases14, these patients still yield a shorter PFS than those without cerebral metastases in our study, which could be explained by the devastating consequence of disease progression in these patients. In addition, the 2nd PFS of local control group and without brain metastases group tended to be longer than that in no local control group regarding to the treating models of brain in 2nd TKI: 5.80 and 4.13 months vs. 2.13 months, P = .013 in univariate analysis). Similarly, as another significant factor that affected 2nd EGFR-TKIs' efficacy in univariate analysis that those did not received chemotherapy before the second round EGFR-TKIs favor a longer PFS, the findings may be interpreted by the hypothesis that– patients with biologically more aggressive disease were more inclined to receive chemotherapy rather than continue EGFR-TKIs or local control treatment.
Although a better outcome was reported for patients receiving 2nd round TKI after a EGFR-TKI free holiday15, we did not observe this difference as another pilot study11 had indicated. This might be due to a limited number of patients that had a EGFR-TKI holiday between treatments in our study(19/72, 26.4%).
As only one patient had a rebiopsy after resistance, the frequency of EGFR T790M mutation or other resistant mechanisms (mainly-activation of EGFR signaling pathways via other aberrant molecules-eg, c-MET amplification or PIK3CA mutations) is unclear in the current study. Therefore limitations remained as patients harboring EGFR T790M mutations are more likely to benefit from prolonged TKI administration16. Furthermore, the conclusions of this study contains uncertainty and future studies designed to testify the utility of a readministrated EGFR-TKIs in former responders (with local or minimally/slowly progression) according to the type of resistance mechanisms are warranted.
Methods
Eligibility criteria
The current research is a retrospective study, 335 consecutive patients with histologically confirmed recurrent or metastatic NSCLC in Sun Yat-Sen University Cancer Center from May 2003 to September 2011 were reviewed. Patients were categorized as never-smoker (less than 100 cigarettes in a life-time), ex-smoker (quit more than 1 year ago) or current smoker(currently smoking or quit less than 1 year ago).
The entry criteria are as follows: (1). Patients that with drug sensitivity associated mutation site(e.g.: G719X, exon 19 deletion, L858R, L861Q); (2). Patients had treated with a EGFR-TKI: gefitinib 250 mg/qd (Iressa™, Astra Zeneca, London, Britain) or erlotinib 150 mg/qd (Tarceva™, Roche Pharma, Basel, Switzerland) with a disease control that more than 3 months; (3). Patients received re-administrated EGFR-TKI after progression to 1st EGFR-TKIs; (4). Cessation of the 2nd EGFR-TKIs therapy was permitted only when the patients incurred unbearable toxicity or disease progression according to the guidelines set out by Response Evaluation Criteria in Solid Tumors (RECIST) Committee17,18.
Monitoring and ethics review
A RECIST evaluating committee formed by experienced radiologists that took charge of evaluating tumor shrinkage or progression in our institute. Evaluation of treatment response by computer tomography was performed after the first month and repeated every 2 months. The version of RECIST 1.017 or 1.118 was used regarding to the year of evaluation (ex. patients before 2010 were evaluated by RECIST 1.0 and those after were assessed by RECIST 1.1).
Study protocol was approved by the institutional review boards of Sun Yat-Sen University Cancer Center. Written informed consent was obtained from each patient: including signed consent for tissue analysis as well as consent to be recorded for potential medical research at the time of sample acquisition. All experiments were performed in accordance with relevant guidelines and regulations.
EGFR mutational status detection
The methodology of mutation detection has been previously described in detail19. After the extraction of DNA from tumor tissues, qualitative detection of mt-EGFR was done using a fluorescence based, real-time detection method [ABI PRISM 7500 Sequence Detection System (Taqman); Perkin-Elmer Applied Biosystems, Foster City, CA.].
Statistical analysis
The initial progression free survival (1st PFS) was defined as the interval between the beginning of EGFR-TKI and the progression time. Likewise, 2nd PFS was defined as the period from the start of TKI retreatment to the date at which disease progression or death was noted. OS was defined as the period from the start of TKI retreatment to the date of death. Objective response rates (ORRs) were defined as (complete response [CR]+ PR)/whole patients; Disease control rates (DCRs) were defined as (CR+PR+SD)/whole patients. The comparison in different groups was performed using the χ2 test or Fisher's exact test as needed. PFS and OS were analyzed by the Kaplan-Meier method and the log-rank test was used to compare the difference within different groups. Multivariate Cox proportional hazards regression model was used to evaluate independent predictive factors associated with 2nd PFS. A two-sided P value of less than .05 was considered statistically significant. All analyses were conducted using PASW statistical software, version 18.0 (SPSS Inc., Chicago, IL).
References
Zhou, C. et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 12, 735–742 (2011).
Rosell, R. et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 13, 239–246 (2012).
Cataldo, V. D., Gibbons, D. L., Perez-Soler, R. & Quintas-Cardama, A. Treatment of non-small-cell lung cancer with erlotinib or gefitinib. N Engl J Med. 364, 947–955 (2011).
Yano, S. et al. Hepatocyte growth factor induces gefitinib resistance of lung adenocarcinoma with epidermal growth factor receptor-activating mutations. Cancer Res. 68, 9479–9487(2008).
Kaira, K. et al. Pooled analysis of the reports of erlotinib after failure of gefitinib for non-small cell lung cancer. Lung Cancer. 68, 99–104 (2010).
Yang, J. J. et al. Clinical modes of EGFR tyrosine kinase inhibitor failure and subsequent management in advanced non-small cell lung cancer. Lung Cancer. 79, 33–39 (2013).
van Puijenbroek, R. et al. Gefitinib monotherapy in advanced nonsmall cell lung cancer: a large Western community implementation study. Eur Respir J. 29, 128–133 (2007).
Ohashi, K., Maruvka, Y. E., Michor, F. & Pao, W. Epidermal growth factor receptor tyrosine kinase inhibitor-resistant disease. J Clin Oncol. 31, 1070–1080 (2013).
Watanabe, S. et al. Clinical responses to EGFR-tyrosine kinase inhibitor retreatment in non-small cell lung cancer patients who benefited from prior effective gefitinib therapy: a retrospective analysis. BMC Cancer. 11, 1 (2011).
Oh, I. J., Ban, H. J., Kim, K. S. & Kim, Y. C. Retreatment of gefitinib in patients with non-small-cell lung cancer who previously controlled to gefitinib: a single-arm, open-label, phase II study. Lung Cancer. 77, 121–127 (2012).
Koizumi, T. et al. Prospective study of gefitinib readministration after chemotherapy in patients with advanced non-small-cell lung cancer who previously responded to gefitinib. Clin Lung Cancer. 13, 458–463 (2012).
Asahina, H. et al. Phase II study of gefitinib readministration in patients with advanced non-small cell lung cancer and previous response to gefitinib. Oncology. 79, 423–429 (2010).
Jackman, D. et al. Clinical definition of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. J Clin Oncol. 28, 357–360 (2010).
Jamal-Hanjani, M. & Spicer, J. Epidermal growth factor receptor tyrosine kinase inhibitors in the treatment of epidermal growth factor receptor-mutant non-small cell lung cancer metastatic to the brain. Clin Cancer Res. 18, 938–944 (2012).
Hata, A. et al. Erlotinib after gefitinib failure in relapsed non-small cell lung cancer: clinical benefit with optimal patient selection. Lung Cancer. 74, 268–273 (2011).
Hata, A. et al. Rebiopsy of non-small cell lung cancer patients with acquired resistance to epidermal growth factor receptor-tyrosine kinase inhibitor: Comparison between T790M mutation-positive and mutation-negative populations. Cancer. 119, 4325–4332 (2013).
Therasse, P. et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 92, 205–216 (2000).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 45, 228–247 (2009).
Zhang, L. J. et al. Relationship between epidermal growth factor receptor gene mutation and copy number in Chinese patients with non-small cell lung cancer. Chin J Cancer. 31, 491–499 (2012).
Author information
Authors and Affiliations
Contributions
H.L. is the guarantor of the manuscript and takes responsibility for the integrity of the data and the accuracy of the data analysis. Z.R.Z. and W.L. contributed to the concept and design; analysis and interpretation of the data; and the drafting, writing, review, revision and approval of the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Zhao, ZR., li, W. & Long, H. Readministration of EGFR Tyrosine Kinase Inhibitor in Non-small Cell Lung Cancer Patients after Initial Failure, What Affects its Efficacy?. Sci Rep 4, 5996 (2014). https://doi.org/10.1038/srep05996
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep05996
This article is cited by
-
The salvage therapy in lung adenocarcinoma initially harbored susceptible EGFR mutation and acquired resistance occurred to the first-line gefitinib and second-line cytotoxic chemotherapy
BMC Pharmacology and Toxicology (2017)
-
Impact of clinical parameters and systemic inflammatory status on epidermal growth factor receptor-mutant non-small cell lung cancer patients readministration with epidermal growth factor receptor tyrosine kinase inhibitors
BMC Cancer (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.